1. Introduction
The accumulation of fluid in the corneal stroma [
1,
2] is a defining feature of corneal edema, leading to significant visual compromise due to reduced corneal transparency. It originates primarily from pathological states, such as Fuchs endothelial corneal dystrophy (FECD) [
1,
2,
3], post-procedural complications, and traumatic ocular injury. This pathological condition exhibits heterogeneous severity, compromising visual function to variable degrees. The corneal endothelium assumes a fundamental role in preserving stromal dehydration and maintaining optical transparency [
1,
4].
Endothelial cell count (ECC), expressed as the number of endothelial cells per mm
2, serves as a vital indicator of posterior corneal integrity [
1,
4]. In individuals aged 20–39, ECC typically averages around 3000 cells/mm
2 and declines steadily at a rate of 0.3%–0.6% annually. By the age of 60–79, average values decrease to approximately 2600 cells/mm
2, with counts below 1000–1200 cells/mm
2 associated with increased risk of corneal decompensation [
5,
6]. A reduction in ECC is often accompanied by an increase in central corneal thickness (CCT), due to impaired endothelial function and fluid imbalance [
5,
7]. CCT refers to the distance between the anterior corneal epithelium and the posterior endothelial surface at the corneal apex. It does not represent the total corneal thickness, which gradually increases toward the periphery. In healthy adults, the average CCT is approximately 550 μm, with peripheral values up to 23% greater due to natural corneal geometry [
7]. Interindividual variation has been reported in the range of 520–570 μm [
7].
Although corneal transplantation remains the primary treatment approach for advanced corneal edema, alternative strategies such as therapeutic contact lenses [
8], pharmacological agents [
1,
9,
10], and minimally invasive procedures like collagen cross-linking are being adopted with growing frequency [
11,
12]. These evolving modalities focus on maintaining corneal deturgescence, with treatments like topical hypertonic saline often employed, despite mixed evidence regarding their efficacy. Advances in surgical technique and postoperative care have reduced the incidence of corneal edema following cataract surgery, a cornerstone of ophthalmic practice [
13,
14,
15]. Nevertheless, complications can still arise, including mechanical trauma, inflammation, chemical injuries, and worsening of underlying conditions [
13,
14,
15]. The variable incidence of postoperative corneal edema underscores the importance of tailored patient assessment and management [
14,
16,
17,
18]. Ripasudil, a Rho-associated protein kinase (ROCK) inhibitor approved in Japan for the treatment of glaucoma and ocular hypertension, enhances aqueous outflow via the trabecular meshwork and has shown additional potential in supporting corneal endothelial function [
19,
20]. Since this pharmacological approach focuses on reducing intraocular pressure (IOP), its mechanism has prompted growing interest in its potential use for corneal edema, where it may support endothelial function and promote cytoskeletal remodeling to facilitate fluid resolution [
21,
22].
Alternative ROCK inhibitors such as Netarsudil, which was approved in the US and Europe, and Fasudil, which was approved in Japan, offer distinct therapeutic alternatives, each possessing unique characteristics and regulatory approvals across various geographical areas [
21,
22]. Current clinical investigations continue to examine the therapeutic potential of novel ROCK inhibitors for treating multiple ocular disorders. While Ripasudil shows promise due to its pharmacological mechanism of action, comprehensive clinical evidence confirming its efficacy across various forms of corneal edemas in real-world settings is still limited [
19,
20]. Standard dosing typically requires administration three times daily in the first week and twice daily in the following weeks and may lead to adverse effects such as blepharitis.
This study aims to assess the efficacy of Ripasudil in the management of diverse corneal edema etiologies through a retrospective analysis conducted at a single tertiary center. Given that endothelial keratoplasty remains the current standard of care, the study further explores the urgent need for effective non-surgical treatment alternatives for corneal edema.
4. Discussion
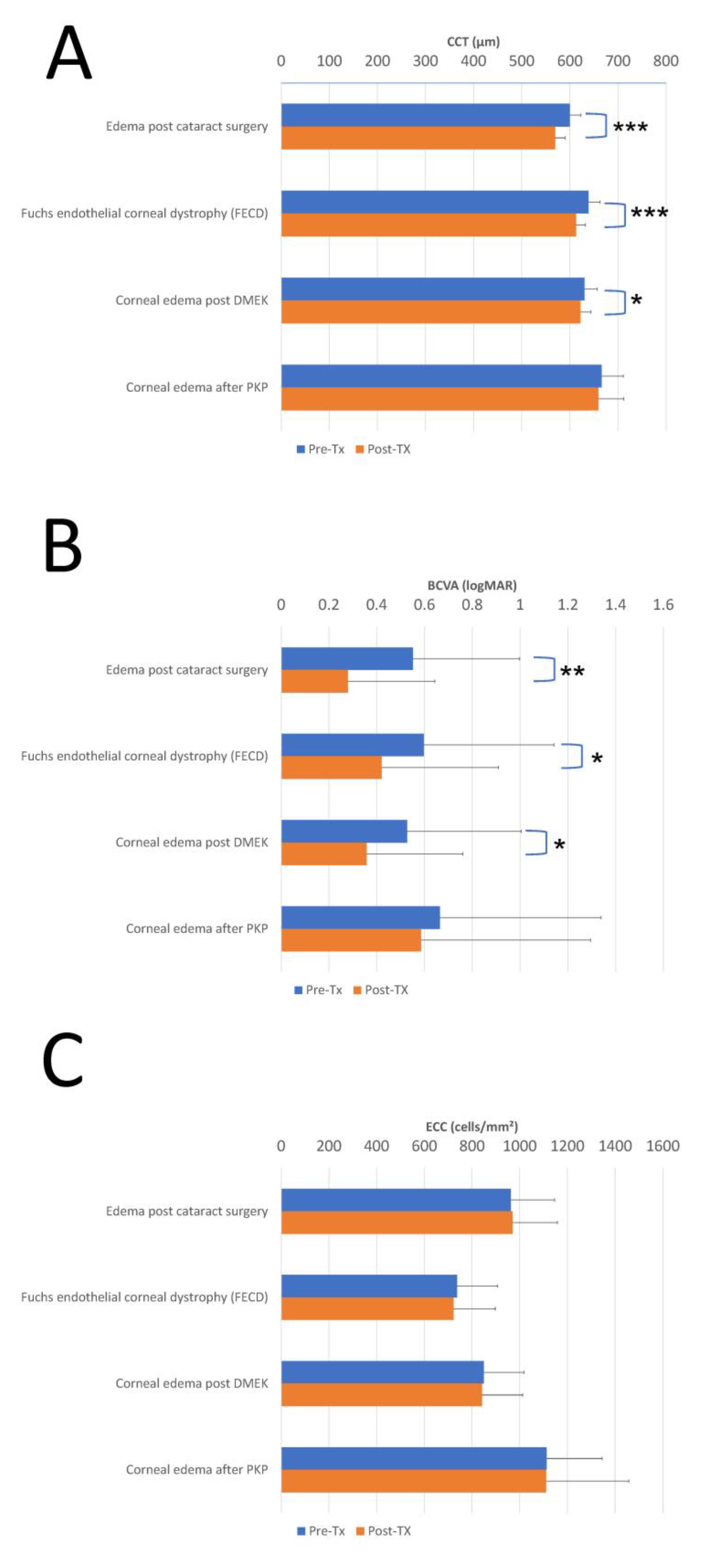
This study demonstrates the variable therapeutic efficacy of Ripasudil across different causes of corneal edema, with greatest therapeutic gains seen in patients with post-cataract surgery corneal edema. The differential response patterns observed across diagnostic groups provide important insights into the potential mechanisms of action and optimal clinical applications of this ROCK inhibitor. As mentioned, the post-cataract group showed the most significant response, suggesting greater efficacy in inflammation-related edema. The degree of reduction in corneal thickness and enhancement in visual acuity represents clinically meaningful changes that would be readily apparent to both patients and clinicians. Similarly, the FECD group demonstrated significant corneal thickness reduction along with significant visual acuity improvement, highlighting the therapeutic potential of Ripasudil in reducing corneal edema in patients with endothelial dysfunction and providing meaningful visual benefits despite the chronic nature of the underlying pathology. Multivariable regression analyses further support Ripasudil’s efficacy, with significant CCT and BCVA improvements in the post-cataract surgery (β = −28.12 µm,
p < 0.001; β = −0.24 logMAR,
p = 0.002) and FECD groups (β = −23.45 µm,
p < 0.001; β = −0.16 logMAR,
p = 0.018) after adjusting for age, gender, and edema duration, reinforcing the robustness of these findings (
Supplementary Table S3).
The reductions in CCT and improvements in BCVA observed in this study have notable clinical significance. These values exceed commonly accepted thresholds for clinical significance, typically defined as a ≥20–30 μm reduction in CCT or ≥0.1 logMAR gain in BCVA. In the post-cataract surgery group, a mean CCT reduction of 30.44 μm (
p < 0.001) likely contributes to improved corneal transparency and patient comfort, reducing symptoms such as glare and blurred vision. Similarly, the 0.27 logMAR improvement in BCVA (
p = 0.001) corresponds to a gain of approximately 2–3 lines on a Snellen chart. In the FECD group, a 25.56 μm reduction in CCT and 0.18 logMAR improvement in BCVA suggest that Ripasudil can provide functional benefits, despite the chronic nature of endothelial dysfunction. Responder analyses further confirm clinical significance, with 71.9% (23/32) and 68.8% (22/32) of post-cataract surgery patients and 65.5% (19/29) and 58.6% (17/29) of FECD patients achieving ≥20 µm CCT reduction and ≥0.1 logMAR BCVA improvement, respectively, potentially delaying surgical interventions (
Supplementary Table S3). These improvements may delay the need for surgical interventions, such as corneal transplantation, in selected patients, enhancing quality of life and reducing treatment burden. However, interpretation of these results requires careful consideration of potential confounding factors and sources of bias that may influence the observed therapeutic response across diagnostic groups. In the post-cataract surgery group, the natural resolution of postoperative inflammation, dry eye, and edema may have contributed to the observed improvements in CCT and BCVA, potentially confounding the therapeutic effect of Ripasudil, since cataract surgery-induced corneal edema often demonstrates spontaneous improvement over weeks to months as inflammatory mediators subside. Similarly, in the post-DMEK group, the healing process following endothelial keratoplasty likely played a role in corneal deturgescence, making it challenging to isolate Ripasudil’s specific contribution given the close temporal proximity between the procedure and treatment initiation. Additional confounding factors include baseline differences in endothelial function, variations in treatment adherence, and differences in the duration of corneal edema prior to treatment. Although no multivariable adjustment was performed, the influence of these confounding variables was considered in the interpretation of outcomes. The retrospective design introduces selection bias, particularly due to the lack of a control group, and the smaller sample size in the PKP group may limit statistical power and generalizability. These factors underscore the need for cautious interpretation of the results and highlight the importance of prospective, controlled studies to confirm Ripasudil’s efficacy.
Posterior stromal ripples have emerged as a clinically relevant biomarker for predicting visual recovery and graft stability following DMEK [
23,
24]. Posterior stromal ripple undulations in the posterior stroma seen in imaging are thought to reflect structural stress at the graft–host interface, potentially impairing optical quality and visual outcomes [
23,
25]. Recent studies have found that PSRs correlate with delayed visual recovery and a higher risk of graft detachment and re-bubbling [
23,
24,
26]. Ventura et al. showed that patients with preoperative posterior stromal ripples had significantly slower visual improvement and worse final acuity [
23]. Similarly, Lohmann et al. and Coco et al. identified posterior stromal ripples as risk indicators for detachment and poor graft adherence [
23,
25]. Structural analyses by Kilian et al. further supported this, linking ripples with topographic instability at the posterior surface [
26]. While some authors such as Parekh et al. and Levis et al. emphasized that not all ripples impair vision, some being transient or benign, they still advocate for better classification systems based on posterior stromal ripples severity and persistence [
24,
27].
From a mechanistic perspective, the preservation of ECC across all treatment groups is encouraging and indicates that Ripasudil does not exert deleterious effects on corneal endothelial cells, which reinforces the drug’s safety profile while suggesting that its primary mechanism of action likely involves enhancement of existing endothelial pump function and cytoskeletal activity rather than cellular regeneration or proliferation. Sensitivity analyses excluding extreme values (BCVA > 2 logMAR or CCT > 700 µm) corroborate these findings, with consistent CCT reductions (29.12 µm,
p < 0.001) and BCVA improvements (0.25 logMAR,
p = 0.001) in the post-cataract surgery group, enhancing confidence in Ripasudil’s efficacy across diverse etiologies, though the small PKP group size limits statistical power (
Supplementary Table S3). However, it is important to note that while ECC stability may imply preserved endothelial function, it should not be interpreted as evidence of cellular proliferation. The regenerative implications should therefore be viewed cautiously, particularly in the absence of statistically significant ECC increases. The ROCK inhibition pathway may enhance endothelial barrier function and pump activity through effects on cytoskeletal dynamics and intercellular junction integrity, which would explain the observed reduction in corneal thickness without corresponding changes in cell count. From a clinical standpoint, the variable response across diagnostic groups indicates that Ripasudil may be most beneficial as an adjunctive therapy in acute or subacute corneal edema conditions, notably those with an inflammatory component. The limited response in the PKP group, though possibly impacted by the small sample size, may also reflect the chronic nature of post-keratoplasty edema and the presence of more severe endothelial dysfunction.
Therapeutics for corneal edema have advanced over time, incorporating therapeutic contact lenses [
8], with pharmacological modalities gaining increasing prominence alongside surgical interventions such as corneal transplantation. The evolving interest in ROCK inhibitors such as Ripasudil for the management of corneal edema is grounded in the historical limitations of conventional topical therapies. As reviewed by several studies, medical treatments for corneal edema, including hypertonic saline, corticosteroids, and bandage contact lenses, have traditionally offered only modest and often transient symptomatic relief, primarily targeting epithelial hydration rather than addressing the underlying endothelial dysfunction [
1,
10]. The limited efficacy of these approaches, particularly in cases of chronic or endothelial-derived edema, underscores the unmet therapeutic need that has prompted investigation into agents capable of modulating cellular function at a deeper level. ROCK inhibitors, by enhancing endothelial barrier integrity and pump activity, represent a paradigm shift in this regard, offering a mechanism-based intervention aligned with the core pathophysiology of stromal swelling and endothelial compromise. Topical hypertonic saline has traditionally been used to manage corneal edema, although its effectiveness remains weakly supported by empirical evidence. Its mechanism relies on increasing tear film osmolality to facilitate corneal dehydration [
1,
10]. Several factors, including endothelial integrity and IOP regulation, contribute to sustaining optimal corneal hydration homeostasis. Disruption of these mechanisms can lead to the onset of corneal edema [
9].
Cataract extraction is a fundamental ophthalmologic intervention that significantly improves quality of life for millions worldwide [
13]. Recent advances in operative techniques and postoperative management have improved visual outcomes and corneal integrity [
14,
15]. The prevalence of corneal edema or decompensation after cataract surgery ranges from 0.2% to 2.4% [
14]. Corneal edema subsequent to intracapsular cataract extraction with anterior chamber or iris-fixated intraocular lens insertion occurs at significantly higher rates compared to intracapsular cataract extraction (ICCE) without intraocular lens (IOL) placement. Post-cataract surgery corneal edema develops from four primary causes [
14,
15]. First, mechanical trauma during the procedure is a predominant factor, often attributable to ultrasonic energy or instrument contact, underscoring the intrinsic procedural risks [
14,
15]. Second, inflammation or infection-related complications may play a role, often triggered by retained nuclear material or inadequately treated infections, requiring immediate therapeutic intervention [
14,
16,
17,
18]. Third, chemical injuries may result from intraoperative substances, emphasizing the importance of meticulous surgical preparation. Finally, pre-existing pathologies such as FECD may potentiate postoperative edema, necessitating comprehensive preoperative assessment for optimal patient management [
16,
17,
18].
Ripasudil is a selective Rho-associated protein kinase inhibitor developed by Kowa Company and approved in Japan for glaucoma and ocular hypertension. Its mechanism involves enhancement of aqueous humor efflux through the trabecular meshwork, receiving regulatory approval for use in treatment-resistant cases [
19,
20]. The primary therapeutic goal in glaucoma and ocular hypertension management is IOP normalization [
19,
20]. Ripasudil has recently demonstrated potential as a therapeutic option for ocular pathologies, including corneal edema, by modulating cellular contractility and enhancing endothelial cell functionality to support resolution of edema. This activity is mediated through inhibition of the Rho/ROCK signaling pathway, which regulates actomyosin contraction, tight junction formation, and cytoskeletal organization in endothelial and trabecular meshwork cells. Ripasudil acts downstream of various G protein-coupled receptors, inhibiting the Rho/ROCK signaling cascade that mediates cytoskeletal tension, cell adhesion, and endothelial permeability [
19,
28,
29]. By suppressing Rho kinase activity, Ripasudil reduces actin stress fiber formation and cellular stiffness, facilitating aqueous outflow and promoting a more permissive environment for endothelial repair [
19,
20]. However, clinical evidence validating its therapeutic effectiveness across diverse corneal edema manifestations, particularly in real-world clinical settings, remains limited [
19,
20]. The treatment protocol involves thrice-daily administration and may lead to adverse reactions, such as blepharitis [
29].
Two additional ROCK inhibitors are commercially available: Netarsudil in the United States and Europe and Fasudil in Japan. Each agent is indicated for distinct therapeutic applications [
21,
22]. Netarsudil acts by selective inhibition of both ROCK1 and ROCK2 isoforms, enhancing trabecular meshwork drainage and reducing episcleral venous pressure. In preclinical studies, it has also shown neuroprotective effects on axons in rat models. Authorized in the United States in 2017 and Europe in 2019 for managing elevated IOP in primary open-angle glaucoma or ocular hypertension, Netarsudil is administered once daily [
22,
30,
31]. The drug has demonstrated consistent IOP reduction throughout circadian cycles and maintained acceptable safety profiles across numerous clinical studies. Ripasudil and Netarsudil lack regulatory authorization in the United Kingdom, Canada, or Australia. Fasudil, marketed since 1995 in Japan, is primarily used for the treatment of cerebral vasospasm and has also been assessed for potential use in diabetic macular edema [
22,
29,
30,
31]. Other ROCK inhibitors, such as SNJ-1656 and Y-27632, are still undergoing clinical evaluation but have not yet been approved for commercial use [
22,
30,
31]. Although Ripasudil, Netarsudil, and Fasudil share the same general mechanism as Rho kinase inhibitors, Ripasudil primarily enhances aqueous outflow through the trabecular meshwork, Netarsudil combines this effect with a reduction in episcleral venous pressure, and Fasudil exerts broader systemic vasodilatory actions, originally developed for cerebral vasospasm [
32]. Importantly, differences in molecular selectivity, ocular penetration, and receptor affinity among these agents may explain their distinct clinical indications and dosing regimens [
22,
30,
31].
The enhancement in BCVA observed in our study aligns with prior reports demonstrating notable visual gains in patients with FECD following Ripasudil therapy [
20,
33,
34]. With regard to CCT, our results are consistent with those of a preceding case series in which Ripasudil was used for the treatment of segmental corneal edema, leading to partial or complete resolution of the edema in the majority of cases [
35]. This receives further reinforcement from findings in a study that examined the incidence of persistent corneal edema post-cataract surgery, which emphasizes the therapeutic potential of Ripasudil for this indication [
19,
36]. Although the modest rise in ECC did not reach statistical significance, it still holds clinical significance. Prior research indicates that Ripasudil can upregulate gene and protein expressions involved in cell cycle regulation, cell–matrix adhesion, and cellular migration [
19,
36]. In addition, another study strongly advocated the use of ROCK inhibitors as adjunctive therapy in cataract surgery, particularly in more complex scenarios, such as cases involving FECD [
19,
36]. Lastly, in our cohort, the average duration of Ripasudil treatment was approximately 4.9 months, during which 29% of patients eventually required DMEK. This trend suggests that Ripasudil may contribute to postponing more invasive interventions like DMEK, a notion supported by earlier evidence in the literature.
A published case series described four instances of persistent corneal edema treated successfully with topical Ripasudil following anterior segment surgeries [
35]. The underlying conditions included FECD, pseudophakic bullous keratopathy, and endothelial cell loss post-keratoplasty. Administered three times a day, Ripasudil induced both visual improvement and resolution of corneal edema, with no adverse effects reported [
35]. These findings support Ripasudil’s potential as a safe and effective treatment option while also highlighting the need for further investigation into optimal dosing regimens and treatment durations [
35].
An additional study explored Ripasudil’s regenerative effects on corneal endothelial cells in FECD patients, utilizing both ex vivo tissue and in vitro cell models. As mentioned, the results demonstrated that Ripasudil positively influences endothelial cell behavior, enhancing cell cycle activity, adhesion, and migration without altering the normal phenotype in either affected or unaffected endothelial cells [
34]. Moreover, Ripasudil was found to augment the expression of proteins essential for maintaining endothelial pump function and barrier integrity. Nevertheless, these in vitro and ex vivo findings do not directly translate to clinical evidence of proliferation in vivo, and the observed benefits in our cohort are more plausibly attributed to functional enhancement of existing cells. Collectively, these findings strengthen the rationale for using ROCK inhibitors like Ripasudil as regenerative agents in the management of patients with FECD [
34].
A clinical study evaluated the efficacy and safety of Descemet Stripping Only (DSO) combined with Ripasudil administration for managing FECD, including a total of 23 eyes [
33]. All cases underwent DSO, followed by postoperative treatment with Ripasudil. Remarkably, corneal clearance was achieved in 22 out of 23 eyes within an average of 4.1 weeks, accompanied by significant improvement in BCVA. The study applied stringent inclusion criteria, selecting participants with a minimum superior ECC of 1000 cells/mm
2 and central guttata as the primary cause of visual decline [
33]. Safety monitoring was thorough, including adverse event reporting, regular blood pressure checks, and routine blood tests. No serious adverse effects were observed, with the most frequently reported side effects being mild ocular surface discomfort and gastrointestinal symptoms. In terms of visual outcomes, the study demonstrated a meaningful gain in uncorrected visual acuity, with mean logMAR improving from 0.43 preoperatively to 0.24 at nine months post-treatment [
33].
Similarly, best spectacle-corrected BCVA showed a marked improvement, with the mean logMAR decreasing from 0.15 preoperatively to 0.002 at 12 months following surgery. ECC was assessed both before and after the procedure. Although a postoperative decline in superior ECC was noted, the comparison between the Ripasudil-treated group and the control group revealed no statistically significant difference [
33].
Another study evaluated the effectiveness of Ripasudil in enhancing BCVA among patients with FECD [
20]. A total of 30 eyes from 15 individuals were randomized to receive either Ripasudil treatment or serve as controls. The intervention group was administered 0.4% Ripasudil eye drops three times daily over an 18-month period [
20]. Patients receiving Ripasudil experienced significant improvements in both BCVA and corneal edema [
20]. Specular microscopy revealed a mean ECC of 727 ± 142 cells/mm
2 in the treatment group. A variety of diagnostic modalities, including anterior segment OCT, were utilized to support the findings. The authors concluded that Ripasudil represents a promising therapeutic option for managing FECD [
20].
In an additional case series, the combined use of Ripasudil therapy and femtosecond laser-assisted cataract surgery was examined in relation to corneal endothelial morphology [
36]. In the first case, improvements were noted in endothelial cell shape and size in the operated eye, indicating the potential benefit of Ripasudil as an intraoperative adjunct. The second case demonstrated a reduction in corneal decompensation, confirmed through both topographic and confocal imaging. Collectively, these cases underscore Ripasudil’s role in enhancing endothelial cell morphology and mitigating edema. These findings further support the application of ROCK inhibitors in treating endothelial dysfunction and reinforce the importance of ongoing research in this area [
36].
A recent study found that Ripasudil eye drops significantly reduced endothelial cell loss after cataract surgery, suggesting a protective effect on the corneal endothelium [
37]. The findings indicated a lower degree of endothelial cell loss among patients treated with Ripasudil [
19]. Additionally, no significant differences were observed between the groups with respect to age, sex, or preoperative corneal thickness [
19]. These results suggest that Ripasudil may serve as a viable therapeutic approach for addressing low ECC post-cataract surgery, highlighting the necessity for further research to validate its efficacy [
19].
This study found that Ripasudil did not promote an increase in ECC, implying that its therapeutic effect is more likely due to enhanced cellular function rather than stimulation of proliferation. These findings align with earlier studies that reported similar results [
33,
38,
39]. Accordingly, the discussion of regenerative potential should remain tempered by the understanding that functional improvement can occur in the absence of increased cell density. The resolution of corneal edema may be explained by one of two mechanisms: either Ripasudil promotes cytoskeletal remodeling and cellular migration, leading to structural changes and the reopening of a previously non-functional endothelial defect once treatment is withdrawn, or it improves the performance of cells within an already established endothelial monolayer [
33,
38,
39].
No statistically significant differences in IOP were observed between pre- and post-treatment in any of the groups. Despite Ripasudil’s known pharmacologic effect as an IOP-lowering agent, the absence of measurable change in this cohort suggests that the apparent IOP fluctuations during corneal edema and its resolution are more likely due to biomechanical alterations of the cornea rather than actual physiological changes [
40]. During edema, increased corneal thickness and pliability reduce applanation resistance, leading to underestimation of true IOP values [
41]. These findings support the hypothesis that tonometric variability, particularly under conditions of corneal edema, reflects measurement artifacts, rather than genuine changes in IOP.
Therefore, the observed IOP differences across groups should be interpreted with caution. For example, Neuburger et al. demonstrated that Goldmann tonometry systematically underestimates true intracameral pressure in edematous corneas, whereas rebound tonometers like iCare provide more accurate readings [
41]. Similarly, studies of post-phacoemulsification corneal edema reported that Goldmann tonometry readings were several mmHg lower than rebound tonometry values [
42,
43,
44]. As edema resolves and corneal structure normalizes, restoring thickness and rigidity, tonometric accuracy improves. Consequently, an apparent IOP rise following edema resolution often reflects restored measurement precision rather than a pathological increase in ocular pressure. Thus, variations in measured IOP may reflect biomechanical recovery rather than actual fluctuations. ROCK inhibition has also demonstrated vascular benefits in diabetic macular edema (DME), including reduction in central foveal thickness independent of IOP effects, likely through mechanisms such as stabilization of endothelial tight junctions and VEGF suppression [
45].
The gender-stratified analysis revealed no statistically significant differences in Ripasudil’s effectiveness between male and female patients across the four diagnostic groups for CCT, BCVA, or ECC. This finding suggests that Ripasudil’s therapeutic benefits are consistent, regardless of gender, supporting its broad applicability in treating corneal edema. However, slight variations were observed, such as a marginally greater CCT reduction in females in the post-cataract surgery group and in males in the FECD group, though these differences were not statistically significant. The absence of gender-specific effects may be attributed to the similar baseline characteristics and disease mechanisms across genders, as well as the relatively small sample sizes, particularly in the PKP group (n = 5 per gender), which may limit statistical power. Future studies with larger cohorts are needed to confirm these findings and explore potential gender-related differences in treatment response, particularly in less common etiologies like post-PKP edema.
While this study offers valuable preliminary insights into the use of Ripasudil for the treatment of corneal edema, several limitations must be acknowledged. The relatively short follow-up period limits the ability to evaluate long-term safety and sustained therapeutic efficacy. In addition, the distribution of patients across the clinical subgroups was not balanced. This was particularly notable in the PKP subgroup, which included only a small number of patients, potentially reducing the statistical strength and generalizability of the findings; as such, the results should be interpreted as exploratory and hypothesis-generating. Moreover, the retrospective nature of the study, which addressed corneal edemas of various etiologies, did not allow for the inclusion of a formal control group. Although this design reflects real-world clinical practice, the addition of a control cohort would have enhanced the interpretability of the outcomes. Another limitation was the absence of a direct comparison with other pharmacological agents that have been shown to reduce corneal edema, such as Netarsudil or Fasudil.
An additional consideration is the increased risk of type I error due to the multiple comparisons conducted across subgroups and clinical endpoints in this study. While Bonferroni correction was applied to account for multiplicity in between-group comparisons across diagnostic categories and primary outcomes (CCT, BCVA, ECC) and for gender-based subgroup comparisons, no formal correction was applied to the within-group pre- and post-treatment comparisons. This increases the likelihood of false-positive findings, particularly given the number of subgroup analyses and outcomes evaluated. Therefore, the observed statistically significant changes, especially those with marginal
p-values or small effect sizes, should be interpreted with caution. As previously noted, the application of Bonferroni correction (
p < 0.00417) for multiple comparisons across the four diagnostic groups and three primary outcomes (CCT, BCVA, ECC) ensures conservative interpretation, reducing type I error risk. Significant findings in the post-cataract surgery (CCT
p < 0.001, BCVA
p = 0.001) and FECD groups (CCT
p < 0.001) persist, while non-significant results in the post-DMEK group (CCT
p = 0.05, BCVA
p = 0.025) may reflect limited statistical power (
Supplementary Table S2). Global tests (ANOVA for CCT and ECC, Kruskal–Wallis for BCVA) confirm significant differences in treatment response across groups (CCT
p < 0.001, BCVA
p = 0.003), with post-cataract surgery and FECD groups showing superior outcomes compared to post-PKP, supporting targeted use of Ripasudil in acute and subacute edemas (
Supplementary Table S3).
Given the promising results of this study, further research is warranted to address its limitations and confirm Ripasudil’s efficacy. Prospective, randomized controlled trials with larger sample sizes, extended follow-up periods, and comparator arms (e.g., Netarsudil or placebo) are recommended to validate these findings. Such studies should include balanced subgroup sizes, particularly for post-keratoplasty edema, and control for confounding factors such as natural recovery and baseline endothelial function. These efforts will help establish Ripasudil’s role as a non-invasive treatment for corneal edema and its potential to delay or avoid surgical interventions.