Calcific Aortic Valve Stenosis: A Focal Disease in Older and Complex Patients—What Could Be the Best Time for an Appropriate Interventional Treatment?
Abstract
1. Introduction
Natural History and Outcomes of Calcific Aortic Stenosis in the Elderly
2. Calcific AS Is Not Only a Valve Disease: Phenotypes of Myocardial Damage and Heart Failure in the Natural History
- Aortic stenosis is not only a disease of the aortic valve but also involves the ventricle. HF decompensation remains the leading cause of cardiac rehospitalization and a major predictor of mortality in patients with AS, before or after AVR.
- Moderate AS may have a prognosis comparable to severe aortic stenosis if there is cardiac injury or dysfunction.
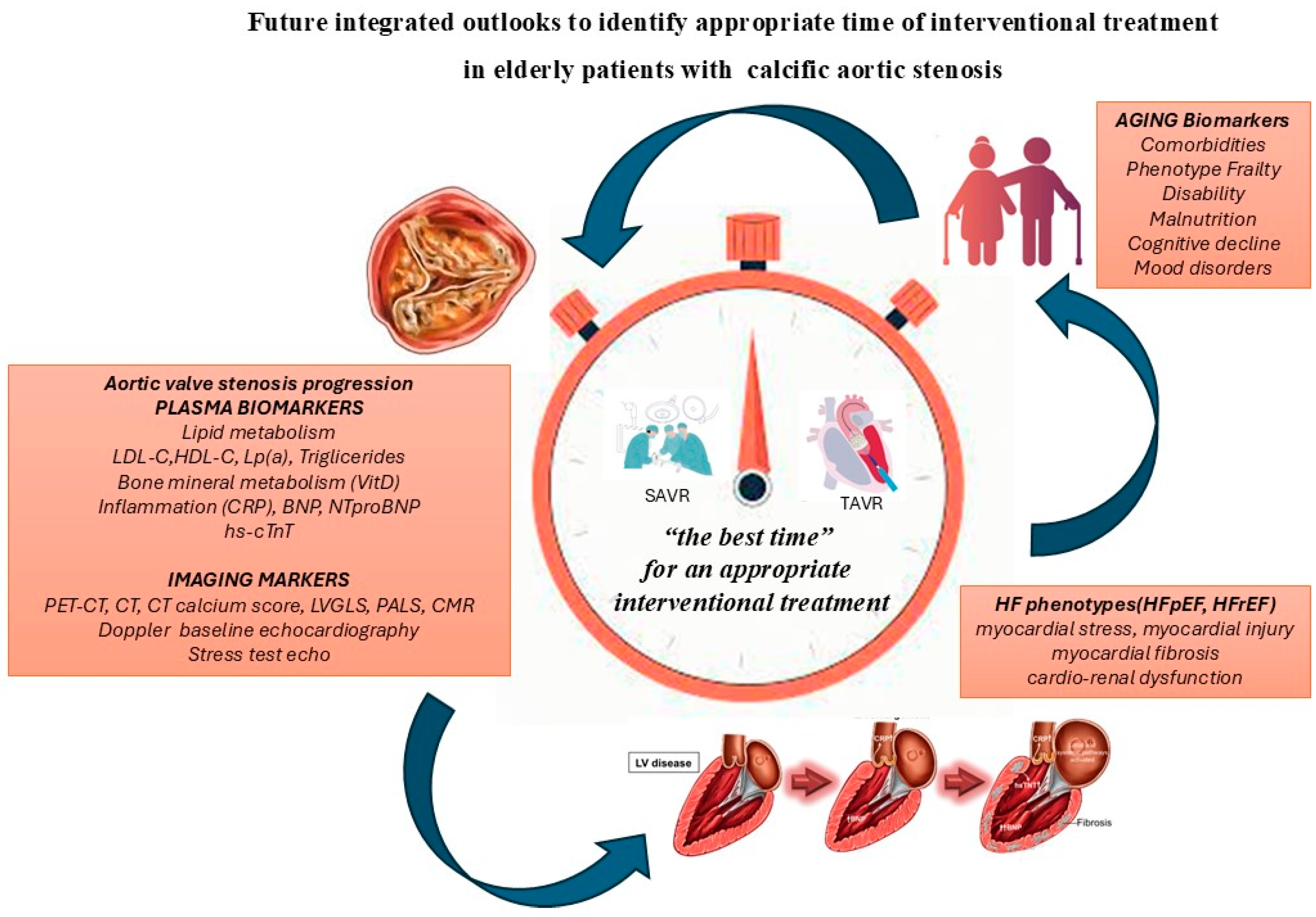
- Multimodality imaging techniques and biomarkers to assess cardiac damage and procedural factors that affect HF before and after AVR can facilitate timely intervention, reduce the risk of HF progression and have an impact of future recommendations.
3. Calcific AS in the Elderly Is Not Only a Cardiovascular Disease: The Weight of Frailty in the Trajectory of Aging
- Calcific aortic stenosis (CAS) is the most common valvular heart disease in the elderly, and elderly with severe CAS are heterogeneous and complex patients.
- 38.4% of older people with severe AS were also frail.
- In older adults, aortic valve stenosis, heart failure, comorbidities and frailty are strongly conditioned for the modality of expression, throughout life, by time and quality of aging.
- Differentiating biological aging from chronological aging plays a crucial role in older patients’ selection and aids in more accurately stratifying patients who are candidates for TAVR/SAVR.
What Could Be the Best Time for an Appropriate Interventional Treatment of Calcific Aortic Stenosis in the Elderly?
- It is useful to establish the best timing for an effective interventional treatment of the CAS in relation to the trends of the progression of the valvular disease, myocardial damage and biological age.
- The natural histories of aortic stenosis, heart failure, and frailty in older patient candidates for interventional treatment of CAS show a similar trend over time.
- A multidisciplinary integrated evaluation, during clinical surveillance of aortic stenosis, is crucial for appropriate tailored decision making and for choosing the best time for effective interventional treatment, also for asymptomatic severe aortic stenosis.
4. Severe Asymptomatic Aortic Stenosis: Early Intervention or Watchful Waiting
5. Integrated Use of Biomarkers as Indices for Risk Stratification in Severe Asymptomatic CAS Can Guide the Timing of Interventional Treatment
5.1. Blood Biomarkers
5.1.1. Lipoprotein a [Lp(a)]
5.1.2. Fetuin-A
5.1.3. Brain Natriuretic Peptide, NTproBNP, High-Sensitivity Cardiac Troponin T (hs-cTnT)
5.1.4. Systemic Inflammatory and Tissue Remodeling Biomarkers
5.2. Imaging Biomarkers
5.3. Biomarkers of Aging
6. New Perspective Features
7. Conclusions
Funding
Conflicts of Interest
References
- Nkomo, V.T.; Gardin, J.M.; Skelton, T.N.; Gottdiener, J.S.; Scott, C.G.; Enriquez-Sarano, M. Burden of valvular heart diseases: A population-based study. Lancet 2006, 368, 1005–1011. [Google Scholar] [CrossRef]
- Ross, J., Jr.; Braunwald, E. Aortic stenosis. Circulation 1968, 38 (Suppl. 1S5), 61–67. [Google Scholar] [CrossRef]
- Otto, C.M.; Newby, D.E.; Hillis, G.S. Calcific Aortic Stenosis: A Review. JAMA 2024, 332, 2014–2026. [Google Scholar] [CrossRef]
- Damluji, A.A.; Bernacki, G.; Afilalo, J.; Lyubarova, R.; Orkaby, A.R.; Kwak, M.J.; Hummel, S.; Kirkpatrick, J.N.; Maurer, M.S.; Wenger, N.; et al. TAVR in Older Adults: Mowing Towards a Comprehensive Geriatric Assessment and Away from Chronological Age: JACC Family Series. JACC Adv. 2024, 3, 100877. [Google Scholar] [CrossRef] [PubMed]
- Mazzone, A.; Del Turco, S.; Trianni, G.; Quadrelli, P.; Marotta, M.; Bastiani, L.; Gasbarri, T.; D’Agostino, A.; Mariani, M.; Basta, G.; et al. The Positive Impact of Early Frailty Levels on Mortality in Elderly Patients with Severe Aortic Stenosis Undergoing Transcatheter/Surgical Aortic Valve Replacement. J. Cardiovasc. Dev. Dis. 2023, 10, 212. [Google Scholar] [CrossRef] [PubMed]
- Lindman, B.R.; Sukul, D.; Dweck, M.R.; Madhavan, M.V.; Arsenault, B.J.; Coylewright, M.; Merryman, W.D.; Newby, D.E.; Lewis, J.; Harrell, F.E., Jr. Evaluating Medical Therapy for Calcific Aortic Stenosis: JACC State-of the-Art Review. J. Am. Coll. Cardiol. 2021, 78, 2354–2376. [Google Scholar] [CrossRef]
- Otto, C.M.; Nishimura, R.A.; Bonow, R.O.; Carabello, B.A.; Erwin, J.P., 3rd; Gentile, F.; Jneid, H.; Krieger, E.V.; Mack, M.; McLeod, C.; et al. 2020 ACC/AHA guideline for the management of patients with valvular heart disease: Executive summary: A report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J. Am. Coll. Cardiol. 2021, 77, 450–500. [Google Scholar] [CrossRef]
- Vahanian, A.; Beyersdorf, F.; Praz, F.; Milojevic, M.; Baldus, S.; Bauersachs, J.; Capodanno, D.; Conradi, L.; De Bonis, M.; De Paulis, R.; et al. 2021 ESC/EACTS guidelines for the management of valvular heart disease. Eur. Heart J. 2022, 43, 561–632. [Google Scholar] [CrossRef] [PubMed]
- Généreux, P.; Banovic, M.; Kang, D.H.; Giustino, G.; Prendergast, B.D.; Lindman, B.R.; Newby, D.E.; Pibarot, P.; Redfors, B.; Craig, N.J.; et al. Aortic Valve Replacement vs Clinical Sureveillance in Asymptomatic Severe Aortic Valve Replacement: A Systematic review and Meta-Analysis. J. Am. Coll. Cardiol. 2025, 85, 912–922. [Google Scholar] [CrossRef]
- Gilmanov, D.; Mazzone, A.; Berti, S.; Glauber, M. Is comorbidity equivalent to symptoms in asymptomatic AS? Nat. Rev. Cardiol. 2011, 8, 725. [Google Scholar] [CrossRef]
- Halapas, A.; Cokkinos, D.V. Aortic Stenosis Progression Is a New Cardiovascular Paradigm Coming of Age? J. Clin. Med. 2025, 14, 903. [Google Scholar] [CrossRef]
- Lindman, B.R.; Dweck, M.R.; Lancellotti, P.; Généreux, P.; Piérard, L.A.; O’Gara, P.T.; Bonow, R.O. Management of Asymptomatic Severe Aortic Stenosis: Evolving Concepts in Timing of Valve Replacement. Cardiovasc. Imaging 2020, 13, 481–493. [Google Scholar]
- Du, Y.; Gössl, M.; Garcia, S.; Enriquez-Sarano, M.; Cavalcante, J.L.; Bae, R.; Hashimoto, G.; Fukui, M.; Lopes, B.; Ahmed, A.; et al. Natural history observations in moderate aortic stenosis. BMC Cardiovasc. Disord. 2021, 21, 108. [Google Scholar]
- Shah, P.K. Should severe aortic stenosis be operated on before symptom onset? Severe aortic stenosis should not be operated on before symptom onset. Circulation 2012, 126, 118–125. [Google Scholar] [CrossRef]
- Jean, G.; Mogensen, N.S.B.; Clavel, M.A. Aortic Valvular Stenosis and Heart Failure: Advances in Diagnostic, Management, and Intervention. Heart Fail. Clin. 2023, 19, 273–283. [Google Scholar] [CrossRef]
- Mihatov, N.; Pibarot, P. Moderate Aortic Stenosis with Cardiac Damage: A New Type of Severe Aortic Stenosis. Struct. Heart 2024, 8, 100336. [Google Scholar] [CrossRef]
- Zilberszac, R.; Gabriel, H.; Schemper, M.; Laufer, G.; Maurer, G.; Rosenhek, R. Asymptomatic Severe Aortic Stenosis in the Elderly. Cardiovasc. Imaging 2017, 10, 43–50. [Google Scholar] [CrossRef]
- Greenhalgh, T.; A’Court, C.; Shaw, S. Understanding heart failure: Explaining telehealth—A hermeneutic systematic review. BMC Cardiovasc. Disord. 2017, 17, 156. [Google Scholar] [CrossRef] [PubMed]
- Kaewkes, D.; Ochiai, T.; Flint, N.; Patel, V.; Patel, J.; Kim, I.; Enta, Y.; Joseph, J.; Yoon, S.H.; Chakravarty, T.; et al. Transcatheter Aortic Valve Implantation in Patients with Acute Severe Aortic Stenosis Hospitalized with Acute Heart Failure. Am. J. Cardiol. 2021, 144, 100–110. [Google Scholar] [CrossRef] [PubMed]
- Esposito, A.; Foffa, I.; Vecoli, C.; Bastiani, L.; Berti, S.; Mazzone, A. The impact of Acute Heart Failure on Frailty Degree and Outcomes in Elderly Patients with Severe Aortic Stenosis and Chronic Heart Failure with Preserved Ejection Fraction. J. Cardiovasc. Dev. Dis. 2024, 11, 150. [Google Scholar] [CrossRef] [PubMed]
- Mengi, S.; Januzzi, J.L., Jr.; Cavalcante, J.L.; Avvedimento, M.; Galhardo, A.; Bernier, M.; Rodés-Cabau, J. Aortic Stenosis, Heart Failure, and Aortic Valve Replacement. JAMA Cardiol. 2024, 9, 1159–1168. [Google Scholar] [CrossRef]
- Rodríguez-Pascual, C.; Paredes-Galán, E.; Ferrero-Martínez, A.I.; Baz-Alonso, J.A.; Durán-Muñoz, D.; González-Babarro, E.; Sanmartín, M.; Parajes, T.; Torres-Torres, I.; Piñón-Esteban, M.; et al. The frailty syndrome and mortality among very old patients with symptomatic severe aortic stenosis under different treatments. Int. J. Cardiol. 2016, 224, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Ramos, M.; Quezada, M.; Ayala, R.; Gómez-Pavón, F.J.; Jaramillo, J.; Calderón-Domínguez, M.; Toro, R. Asymptomatic aortic stenosis in a geriatric population: The role of frailty and comorbidity in mortality. Rev. Esp. Cardiol. 2021, 74, 167–174. [Google Scholar] [CrossRef]
- Kim, D.H.; Rockwood, K. Frailty in Older Adults. N. Engl. J. Med. 2024, 391, 538–548. [Google Scholar] [CrossRef] [PubMed]
- Fukui, S.; Kawakami, M.; Otaka, Y.; Ishikawa, A.; Mizuno, K.; Tsuji, T.; Hayashida, K.; Rakisheva, A.; Soloveva, A.; Shchendrygina, A.; et al. Heart Failure with Preserved Ejection Fraction and Frailty: From Young to Superaged Coexisting HFpEF and Frailty. Int. J. Heart Fail. 2024, 6, 93–106. [Google Scholar]
- Zamudio-Rodríguez, A.; Avila-Funes, J.A.; Tabue-Teguo, M.; Dartigues, J.F.; Amieva, H.; Pérès, K. Towards an approach of disability along a continuum from robustness, pre-frailty, frailty to disability. Age Ageing 2022, 51, afac025. [Google Scholar] [CrossRef]
- Franceschi, C.; Garagnani, P.; Morsiani, C.; Conte, M.; Santoro, A.; Grignolio, A.; Monti, D.; Capri, M.; Salvioli, S. The Continuum of Aging and Age-Related Diseases:Common Mechanisms but Different Rates. Front. Med. 2018, 5, 61. [Google Scholar] [CrossRef] [PubMed]
- Ferrucci, L.; Levine, M.E.; Kuo, P.L.; Simonsick, E.M. Time and Metric of Aging. Circ. Res. 2018, 123, 740–744. [Google Scholar] [CrossRef]
- Damluji, A.A.; Nanna, M.G.; Rymer, J.; Kochar, A.; Lowenstern, A.; Baron, S.J.; Narins, C.R.; Alkhouli, M. Cronological vs Biological Age in Interventional Cardiology: A Comprehensive Approach to Care for Older Adults: JACC Family Series. JACC Cardiovasc. Interv. 2024, 17, 961–978. [Google Scholar] [CrossRef] [PubMed]
- Brunetti, E.; Lucà, F.; Presta, R.; Marchionni, N.; Boccanelli, A.; Ungar, A.; Rao, C.M.; Ingianni, N.; Lettino, M.; Del Sindaco, D. A Comprehensive Geriatric Workup and Frailty Assessment in Older Patients with Severe Aortic Stenosis. J. Clin. Med. 2024, 13, 4169. [Google Scholar] [CrossRef]
- Généreux, P.; Schwartz, A.; Oldemeyer, J.B.; Pibarot, P.; Cohen, D.J.; Blanke, P.; Lindman, B.R.; Babaliaros, V.; Fearon, W.F.; Daniels, D.V.; et al. Transcatheter Aortic-Valve Replacement for Asymptomatic Severe Aortic Stenosis. N. Engl. J. Med. 2025, 392, 217–227. [Google Scholar] [CrossRef]
- Loganath, K.; Craig, N.J.; Everett, R.J.; Bing, R.; Tsampasian, V.; Molek, P.; Botezatu, S.; Aslam, S.; Lewis, S.; Graham, C.; et al. Early Intervention in Patients with Asymptomatic Severe Aortic Stenosis and Myocardial Fibrosis: The EVOLVED Randomized Clinical Trial. JAMA 2025, 333, 213–221. [Google Scholar] [CrossRef] [PubMed]
- Banovic, M.; Putnik, S.; Penicka, M.; Doros, G.; Deja, M.A.; Kockova, R.; Kotrc, M.; Glaveckaite, S.; Gasparovic, H.; Pavlovic, N.; et al. Aortic Valve Replacement Versus Conservative Treatment in Asymptomatic Severe Aortic Stenosis: The AVATAR Trial. Circulation 2022, 145, 648–658. [Google Scholar] [CrossRef]
- Kang, D.H.; Park, S.J.; Lee, S.A.; Lee, S.; Kim, D.H.; Kim, H.K.; Yun, S.C.; Hong, G.R.; Song, J.M.; Chung, C.H.; et al. Early Surgery or Conservative Care for Asymptomatic Aortic Stenosis. N. Engl. J. Med. 2020, 382, 111–119. [Google Scholar] [CrossRef] [PubMed]
- Jacquemyn, X.; Sá, M.P.; Marín-Cuartas, M.; Bax, J.J.; Borger, M.A.; Clavel, M.A.; Pibarot, P.; Généreux, P.; Sultan, I. Early aortic valve replacement versus conservative management in asymptomatic severe aortic stenosis: Meta-analysis of time-to-event data of randomized controlled trials. Int. J. Cardiol. 2025, 432, 133269. [Google Scholar] [CrossRef] [PubMed]
- Kamstrup, P.R.; Tybjærg-Hansen, A.; Nordestgaard, B.G. Elevated lipoprotein(a) and risk of aortic valve stenosis in the general population. J. Am. Coll. Cardiol. 2014, 63, 470–477. [Google Scholar] [CrossRef]
- Ciofani, J.L.; Han, D.; Rao, K.; Gill, D.; Woolf, B.; Rahimi, K.; Allahwala, U.K.; Bhindi, R. Lipid-lowering therapies for aortic stenosis: A drug-target Mendelian randomization study. Eur. Heart J. Cardiovasc. Pharmacother. 2025, 11, 136–142. [Google Scholar] [CrossRef]
- Koos, R.; Brandenburg, V.; Mahnken, A.H.; Mühlenbruch, G.; Stanzel, S.; Günther, R.W.; Floege, J.; Jahnen-Dechent, W.; Kelm, M.; Kühl, H.P. Association of fetuin-A levels with the progression of aortic valve calcification in non-dialyzed patients. Eur. Heart J. 2009, 30, 2054–2061. [Google Scholar] [CrossRef]
- Nakatsuma, K.; Taniguchi, T.; Morimoto, T.; Shiomi, H.; Ands, K.; Kanamori, N.; Murata, K.; Kitai, T.; Kawase, Y.; Izumi, C.; et al. B-type natriuretic peptide in patients with asymptomatic severe aortic stenosis. Heart 2019, 105, 384–390. [Google Scholar] [CrossRef]
- Stein, E.J.; Fearon, W.F.; Elmariah, S.; Kim, J.B.; Kapadia, S.; Kumbhani, D.J.; Gillam, L.; Whisenant, B.; Quader, N.; Zajarias, A.; et al. Left Ventricular Hypertrophy and Biomarkers of Cardiac Damage and Stress in Aortic Stenosis. J. Am. Heart Assoc. 2022, 11, e023466. [Google Scholar] [CrossRef]
- Lindman, B.R.; Pibarot, P.; Schwartz, A.; Oldemeyer, J.B.; Su, Y.R.; Goel, K.; Cohen, D.J.; Fearon, W.F.; Babaliaros, V.; Daniels, D.; et al. Cardiac biomarkers in patients with asymptomatic severe aortic stenosis: Analysis from the EARLY TAVR trial. Circulation 2025, 151, 1550–1564. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, M.; Chen, H.; Li, H. Prognostic Value of Global Longitudinal Strain in Asymptomatic Aortic Stenosis: A Systematic Review and Meta-Analysis. Front. Cardiovasc. Med. 2022, 9, 778027. [Google Scholar] [CrossRef]
- Springhetti, P.; Tomaselli, M.; Benfari, G.; Milazzo, S.; Ciceri, L.; Penso, M.; Pilan, M.; Clement, A.; Rota, A.; Del Sole, P.A.; et al. Peak atrial longitudinal strain and risk stratification in moderate and severe aortic stenosis. Eur. Heart J. Cardiovasc. Imaging 2024, 25, 947–957. [Google Scholar] [CrossRef]
- Treibel, T.A.; Kozor, R.; Schofield, R.; Benedetti, G.; Fontana, M.; Bhuva, A.N.; Sheikh, A.; López, B.; González, A.; Manisty, C.; et al. Reverse Myocardial Remodeling Following Valve Replacement in Patients with Aortic Stenosis. J. Am. Coll. Cardiol. 2018, 71, 860–871. [Google Scholar] [CrossRef]
- Treibel, T.A.; López, B.; González, A.; Menacho, K.; Schofield, R.S.; Ravassa, S.; Fontana, M.; White, S.K.; DiSalvo, C.; Roberts, N.; et al. Reappraising myocardial fibrosis in severe aortic stenosis: An invasive and non-invasive study in 133 patients. Eur. Heart J. 2018, 39, 699–709. [Google Scholar] [CrossRef] [PubMed]
- Dweck, M.R.; Joshi, S.; Murigu, T.; Alpendurada, F.; Jabbour, A.; Melina, G.; Banya, W.; Gulati, A.; Roussin, I.; Raza, S.; et al. Midwall fibrosis is an independent predictor of mortality in patients with aortic stenosis. J. Am. Coll. Cardiol. 2011, 58, 1271–1279. [Google Scholar] [CrossRef] [PubMed]
- Raisi-Estabragh, Z.; Szabo, L.; Schuermans, A.; Salih, A.M.; Chin, C.W.L.; Vágó, H.; Altmann, A.; Ng, F.S.; Garg, P.; Pavanello, S.; et al. Techniques for Tracking Biological Aging of the Cardiovascular System: JACC Family Series. JACC Cardiovasc. Imaging 2024, 17, 533–551. [Google Scholar] [CrossRef]
- Karakasis, P.; Theofilis, P.; Patoulias, D.; Vlachakis, P.K.; Pamporis, K.; Sagris, M.; Ktenopoulos, N.; Kassimis, G.; Antoniadis, A.P.; Fragakis, N. Sodium-Glucose Cotransporter 2 Inhibitors in Aortic Stenosis: Toward a Comprehensive Cardiometabolic Approach. Int. J. Mol. Sci. 2025, 26, 4494. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mazzone, A.; Esposito, A.; Foffa, I.; Berti, S. Calcific Aortic Valve Stenosis: A Focal Disease in Older and Complex Patients—What Could Be the Best Time for an Appropriate Interventional Treatment? J. Clin. Med. 2025, 14, 5560. https://doi.org/10.3390/jcm14155560
Mazzone A, Esposito A, Foffa I, Berti S. Calcific Aortic Valve Stenosis: A Focal Disease in Older and Complex Patients—What Could Be the Best Time for an Appropriate Interventional Treatment? Journal of Clinical Medicine. 2025; 14(15):5560. https://doi.org/10.3390/jcm14155560
Chicago/Turabian StyleMazzone, Annamaria, Augusto Esposito, Ilenia Foffa, and Sergio Berti. 2025. "Calcific Aortic Valve Stenosis: A Focal Disease in Older and Complex Patients—What Could Be the Best Time for an Appropriate Interventional Treatment?" Journal of Clinical Medicine 14, no. 15: 5560. https://doi.org/10.3390/jcm14155560
APA StyleMazzone, A., Esposito, A., Foffa, I., & Berti, S. (2025). Calcific Aortic Valve Stenosis: A Focal Disease in Older and Complex Patients—What Could Be the Best Time for an Appropriate Interventional Treatment? Journal of Clinical Medicine, 14(15), 5560. https://doi.org/10.3390/jcm14155560





