Decline in Serum Lysophosphatidylcholine Species in Patients with Severe Inflammatory Bowel Disease
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Measurement of Serum LPC Species
2.3. Statistical Analysis
3. Results
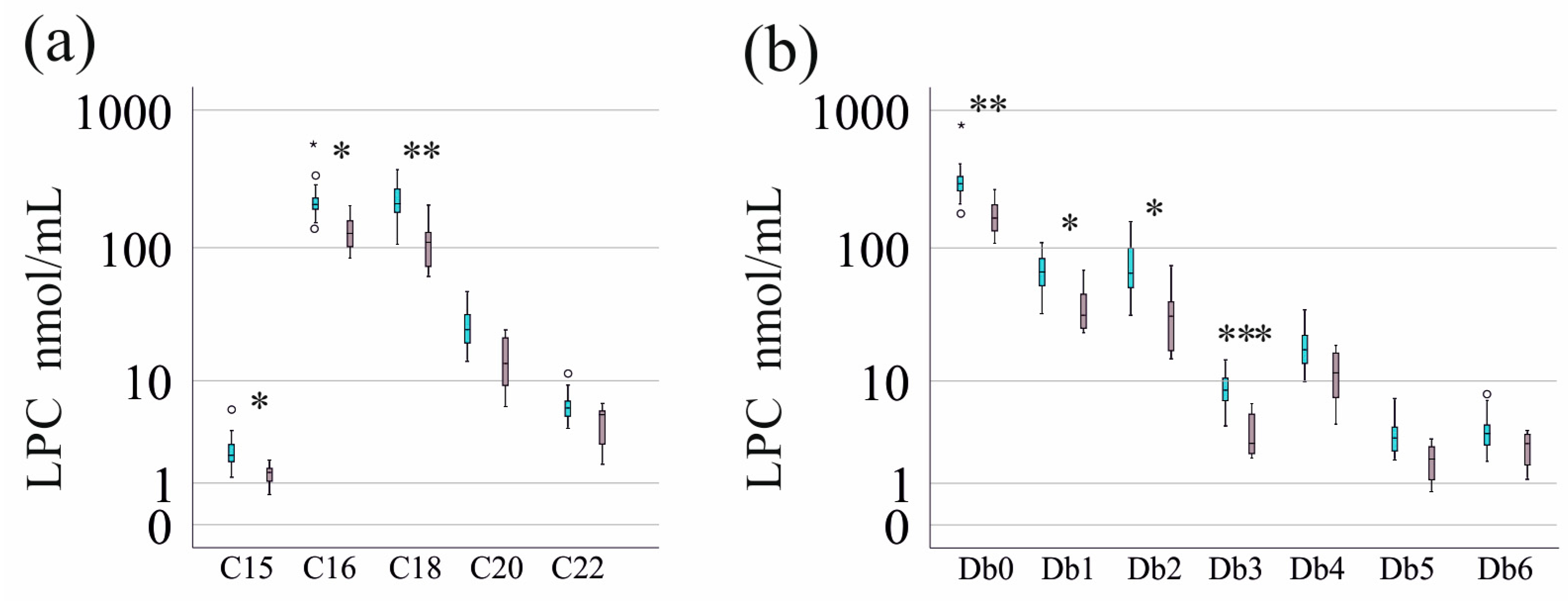
3.1. Study Cohort
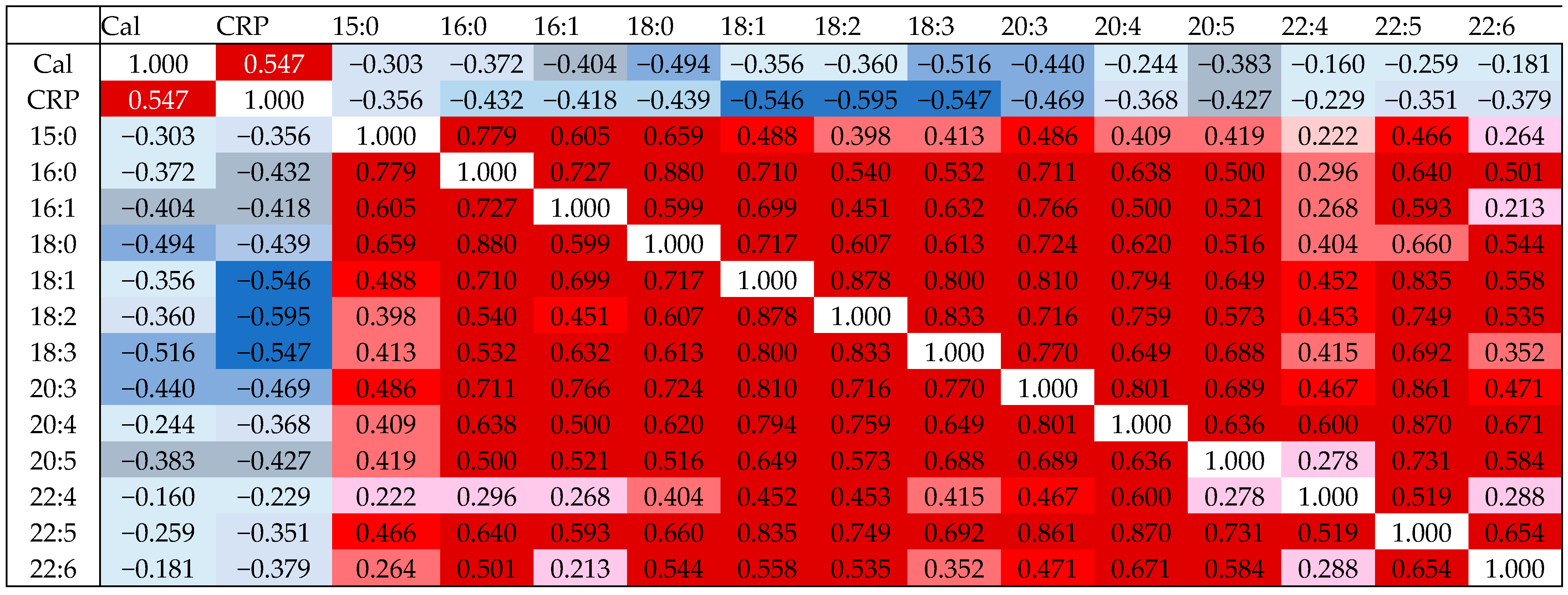
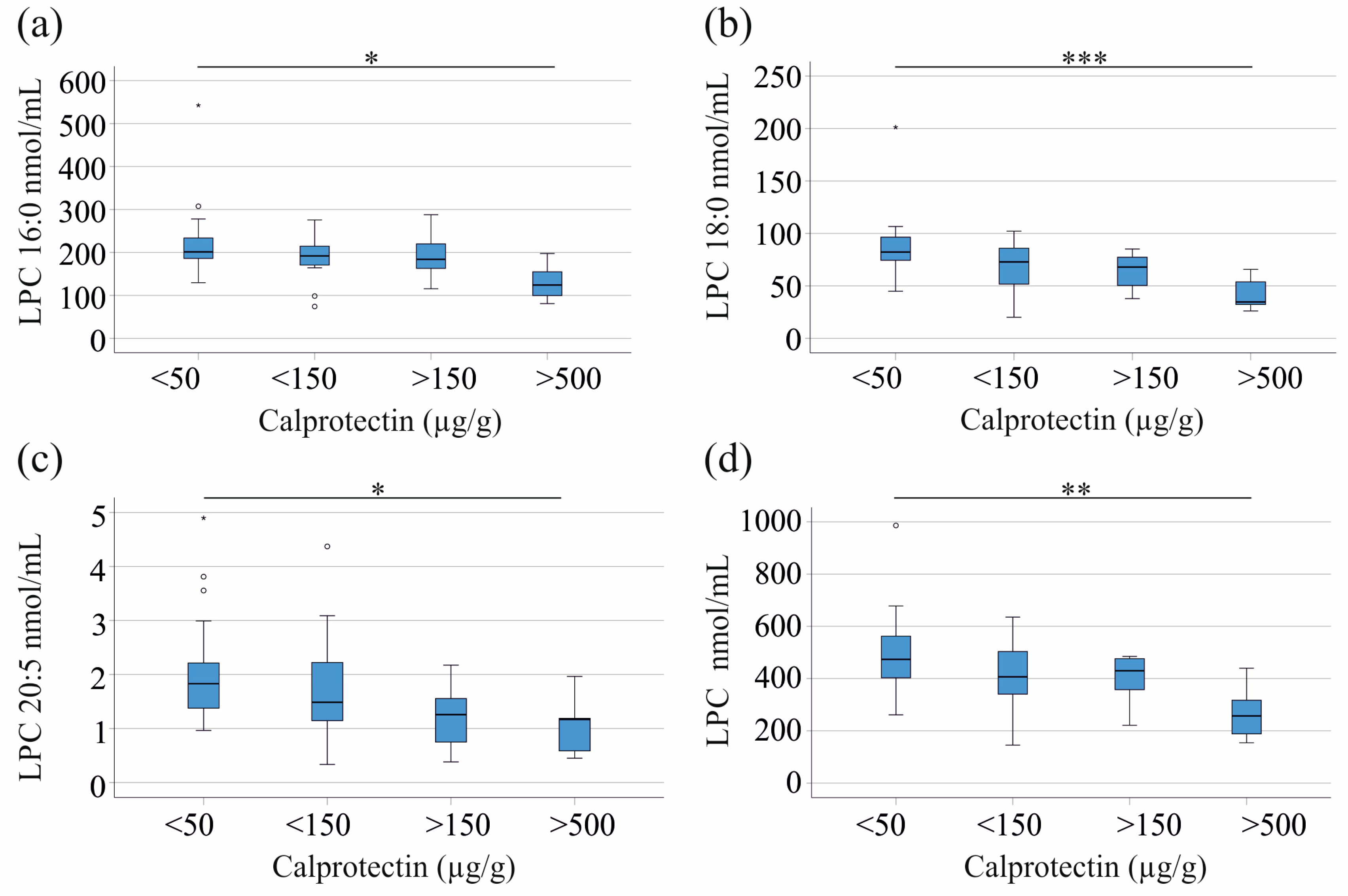
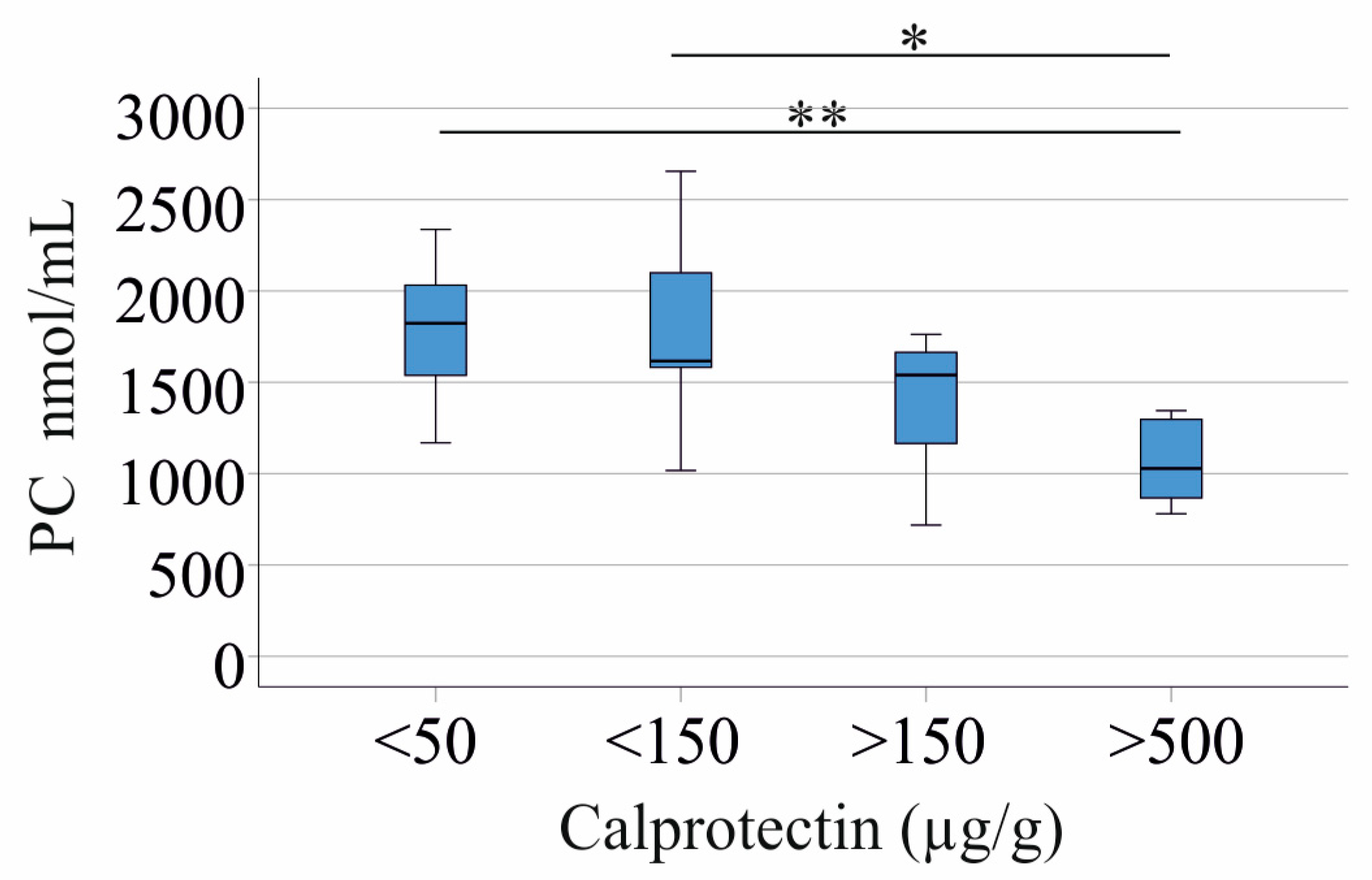
3.2. Correlation of LPC Species with CRP and Fecal Calprotectin
3.3. Correlation of LPC Species with Measures of Liver Function
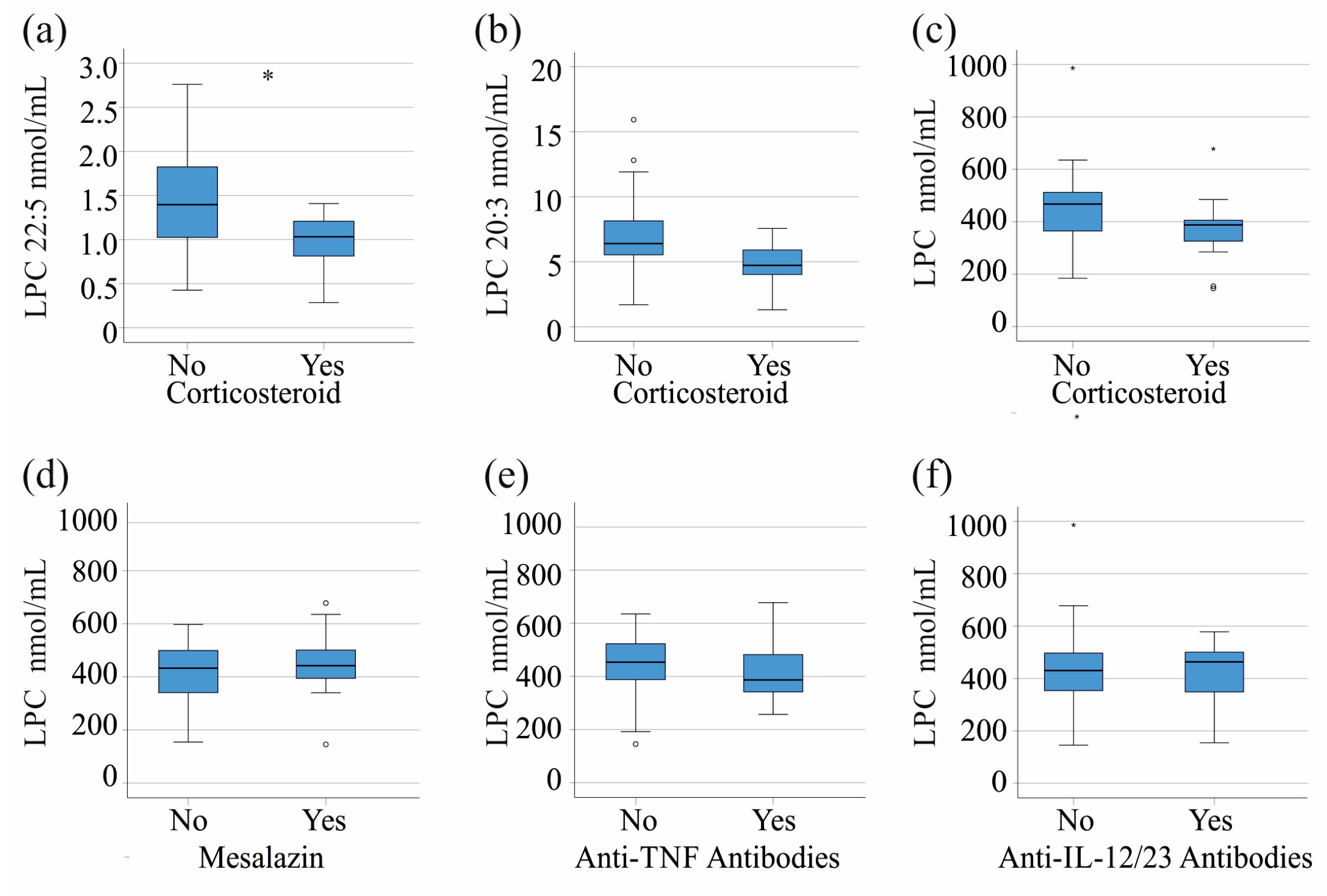
3.4. LPC Species Levels in Relation to Current Treatment
3.5. LPC Species Levels in Relation to Serum Cholesterol, Triglyceride, and Phosphatidylcholine Levels
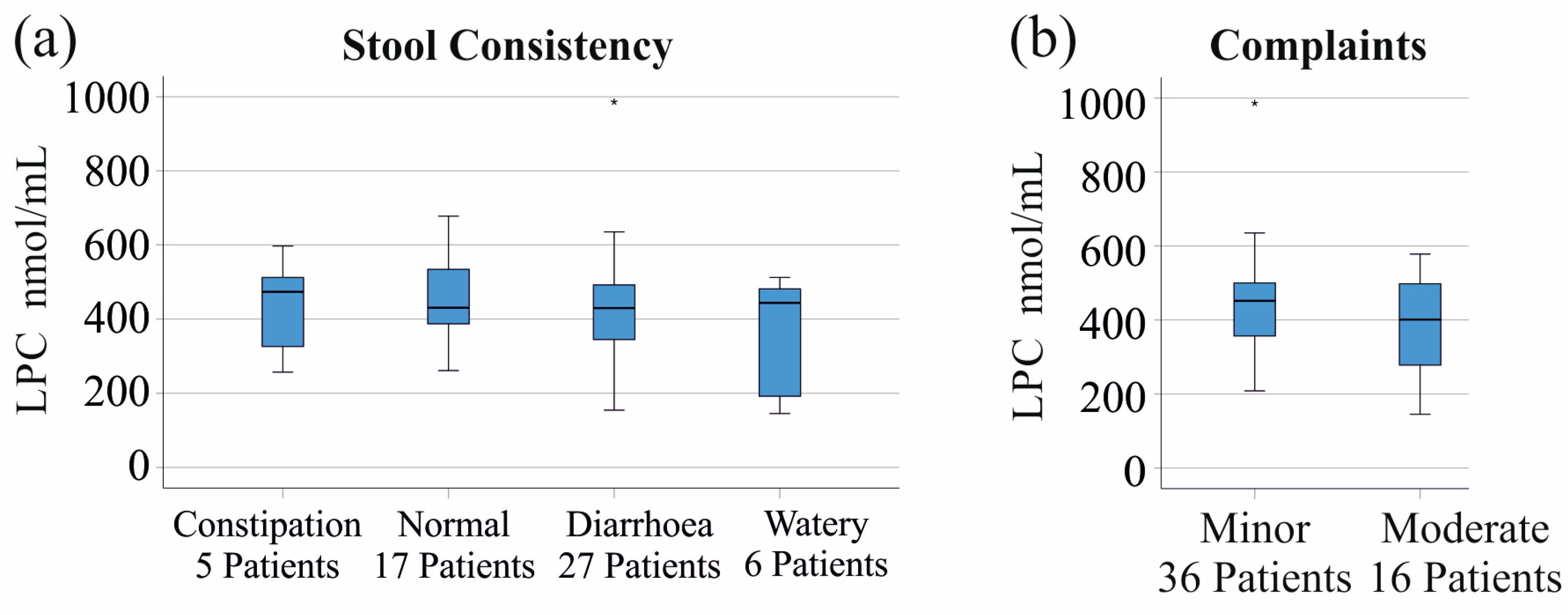
3.6. LPC Species Levels in Relation to Stool Consistency and Gastrointestinal Symptoms
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BMI | Body mass index |
| CD | Crohn’s disease |
| CRP | C-reactive protein |
| IBD | Inflammatory bowel disease |
| LPC | Lysophosphatidylcholine |
| PC | Phosphatidylcholine |
| UC | Ulcerative colitis |
References
- Feingold, K.R.; Grunfeld, C. The Effect of Inflammation and Infection on Lipids and Lipoproteins. In Endotext; Feingold, K.R., Anawalt, B., Blackman, M.R., Boyce, A., Chrousos, G., Corpas, E., de Herder, W.W., Dhatariya, K., Dungan, K., Hofland, J., et al., Eds.; Endotext: South Dartmouth, MA, USA, 2000. [Google Scholar]
- Wiesner, P.; Leidl, K.; Boettcher, A.; Schmitz, G.; Liebisch, G. Lipid profiling of FPLC-separated lipoprotein fractions by electrospray ionization tandem mass spectrometry. J. Lipid Res. 2009, 50, 574–585. [Google Scholar] [CrossRef]
- Pakiet, A.; Kobiela, J.; Stepnowski, P.; Sledzinski, T.; Mika, A. Changes in lipids composition and metabolism in colorectal cancer: A review. Lipids Health Dis. 2019, 18, 29. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.G.; Yoon, Y.C.; Yoon, J.; Lee, S.J.; Oh, Y.K.; Kwon, S.W. Systematic Review of Recent Lipidomics Approaches Toward Inflammatory Bowel Disease. Biomol. Ther. 2021, 29, 582–595. [Google Scholar] [CrossRef] [PubMed]
- Bauset, C.; Gisbert-Ferrandiz, L.; Cosin-Roger, J. Metabolomics as a Promising Resource Identifying Potential Biomarkers for Inflammatory Bowel Disease. J. Clin. Med. 2021, 10, 622. [Google Scholar] [CrossRef] [PubMed]
- Rogler, G.; Singh, A.; Kavanaugh, A.; Rubin, D.T. Extraintestinal Manifestations of Inflammatory Bowel Disease: Current Concepts, Treatment, and Implications for Disease Management. Gastroenterology 2021, 161, 1118–1132. [Google Scholar] [CrossRef]
- Agouridis, A.P.; Elisaf, M.; Milionis, H.J. An overview of lipid abnormalities in patients with inflammatory bowel disease. Ann. Gastroenterol. 2011, 24, 181–187. [Google Scholar]
- Alhouayek, M.; Ameraoui, H.; Muccioli, G.G. Bioactive lipids in inflammatory bowel diseases—From pathophysiological alterations to therapeutic opportunities. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2021, 1866, 158854. [Google Scholar] [CrossRef]
- Chen, H.; Li, W.; Hu, J.; Xu, F.; Lu, Y.; Zhu, L.; Shen, H. Association of serum lipids with inflammatory bowel disease: A systematic review and meta-analysis. Front. Med. 2023, 10, 1198988. [Google Scholar] [CrossRef]
- Sappati Biyyani, R.S.; Putka, B.S.; Mullen, K.D. Dyslipidemia and lipoprotein profiles in patients with inflammatory bowel disease. J. Clin. Lipidol. 2010, 4, 478–482. [Google Scholar] [CrossRef]
- Yan, D.; Ye, S.; He, Y.; Wang, S.; Xiao, Y.; Xiang, X.; Deng, M.; Luo, W.; Chen, X.; Wang, X. Fatty acids and lipid mediators in inflammatory bowel disease: From mechanism to treatment. Front. Immunol. 2023, 14, 1286667. [Google Scholar] [CrossRef]
- de Souza, H.S.; Fiocchi, C. Immunopathogenesis of IBD: Current state of the art. Nat. Rev. Gastroenterol. Hepatol. 2016, 13, 13–27. [Google Scholar] [CrossRef] [PubMed]
- Gajendran, M.; Loganathan, P.; Catinella, A.P.; Hashash, J.G. A comprehensive review and update on Crohn’s disease. Dis. Mon. 2018, 64, 20–57. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Feng, R.; Ben-Horin, S.; Zhuang, X.; Tian, Z.; Li, X.; Ma, R.; Mao, R.; Qiu, Y.; Chen, M. Systematic review with meta-analysis: Environmental and dietary differences of inflammatory bowel disease in Eastern and Western populations. Aliment. Pharmacol. Ther. 2022, 55, 266–276. [Google Scholar] [CrossRef]
- Tshikudi, D.M.; Bernstein, C.N.; Mishra, S.; Ghia, J.E.; Armstrong, H.K. Influence of biological sex in inflammatory bowel diseases. Nat. Rev. Gastroenterol. Hepatol. 2025, 22, 415–437. [Google Scholar] [CrossRef]
- Chang, M.C.; Lee, J.J.; Chen, Y.J.; Lin, S.I.; Lin, L.D.; Jein-Wen Liou, E.; Huang, W.L.; Chan, C.P.; Huang, C.C.; Jeng, J.H. Lysophosphatidylcholine induces cytotoxicity/apoptosis and IL-8 production of human endothelial cells: Related mechanisms. Oncotarget 2017, 8, 106177–106189. [Google Scholar] [CrossRef]
- Liu, P.; Zhu, W.; Chen, C.; Yan, B.; Zhu, L.; Chen, X.; Peng, C. The mechanisms of lysophosphatidylcholine in the development of diseases. Life Sci. 2020, 247, 117443. [Google Scholar] [CrossRef]
- Nakano, T.; Raines, E.W.; Abraham, J.A.; Klagsbrun, M.; Ross, R. Lysophosphatidylcholine upregulates the level of heparin-binding epidermal growth factor-like growth factor mRNA in human monocytes. Proc. Natl. Acad. Sci. USA 1994, 91, 1069–1073. [Google Scholar] [CrossRef]
- Drobnik, W.; Liebisch, G.; Audebert, F.X.; Frohlich, D.; Gluck, T.; Vogel, P.; Rothe, G.; Schmitz, G. Plasma ceramide and lysophosphatidylcholine inversely correlate with mortality in sepsis patients. J Lipid Res. 2003, 44, 754–761. [Google Scholar] [CrossRef]
- Murgia, A.; Hinz, C.; Liggi, S.; Denes, J.; Hall, Z.; West, J.; Santoru, M.L.; Piras, C.; Manis, C.; Usai, P.; et al. Italian cohort of patients affected by inflammatory bowel disease is characterised by variation in glycerophospholipid, free fatty acids and amino acid levels. Metabolomics 2018, 14, 140. [Google Scholar] [CrossRef]
- Guan, S.; Jia, B.; Chao, K.; Zhu, X.; Tang, J.; Li, M.; Wu, L.; Xing, L.; Liu, K.; Zhang, L.; et al. UPLC-QTOF-MS-Based Plasma Lipidomic Profiling Reveals Biomarkers for Inflammatory Bowel Disease Diagnosis. J. Proteome Res. 2020, 19, 600–609. [Google Scholar] [CrossRef]
- Tefas, C.; Ciobanu, L.; Tantau, M.; Moraru, C.; Socaciu, C. The potential of metabolic and lipid profiling in inflammatory bowel diseases: A pilot study. Bosn. J. Basic Med. Sci. 2020, 20, 262–270. [Google Scholar] [CrossRef]
- Daniluk, U.; Daniluk, J.; Kucharski, R.; Kowalczyk, T.; Pietrowska, K.; Samczuk, P.; Filimoniuk, A.; Kretowski, A.; Lebensztejn, D.; Ciborowski, M. Untargeted Metabolomics and Inflammatory Markers Profiling in Children With Crohn’s Disease and Ulcerative Colitis-A Preliminary Study. Inflamm. Bowel Dis. 2019, 25, 1120–1128. [Google Scholar] [CrossRef] [PubMed]
- Jonas, A. Lecithin cholesterol acyltransferase. Biochim. Biophys. Acta 2000, 1529, 245–256. [Google Scholar] [CrossRef] [PubMed]
- Woodruff, T.M.; Arumugam, T.V.; Shiels, I.A.; Newman, M.L.; Ross, P.A.; Reid, R.C.; Fairlie, D.P.; Taylor, S.M. A potent and selective inhibitor of group IIa secretory phospholipase A2 protects rats from TNBS-induced colitis. Int. Immunopharmacol. 2005, 5, 883–892. [Google Scholar] [CrossRef] [PubMed]
- Pruzanski, W.; Stefanski, E.; Vadas, P.; Ramamurthy, N.S. Inhibition of extracellular release of proinflammatory secretory phospholipase A2 (sPLA2) by sulfasalazine: A novel mechanism of anti-inflammatory activity. Biochem. Pharmacol. 1997, 53, 1901–1907. [Google Scholar] [CrossRef]
- Barrett, K.; Saxena, S.; Pollok, R. Using corticosteroids appropriately in inflammatory bowel disease: A guide for primary care. Br. J. Gen. Pract. 2018, 68, 497–498. [Google Scholar] [CrossRef]
- Nakano, T.; Ohara, O.; Teraoka, H.; Arita, H. Glucocorticoids suppress group II phospholipase A2 production by blocking mRNA synthesis and post-transcriptional expression. J. Biol. Chem. 1990, 265, 12745–12748. [Google Scholar] [CrossRef]
- Allgayer, H.; Stenson, W.F. A comparison of effects of sulfasalazine and its metabolites on the metabolism of endogenous vs. exogenous arachidonic acid. Immunopharmacology 1988, 15, 39–46. [Google Scholar] [CrossRef]
- van Dullemen, H.M.; Wolbink, G.J.; Wever, P.C.; van der Poll, T.; Hack, C.E.; Tytgat, G.N.; van Deventer, S.J. Reduction of circulating secretory phospholipase A2 levels by anti-tumor necrosis factor chimeric monoclonal antibody in patients with severe Crohn’s disease. Relation between tumor necrosis factor and secretory phospholipase A2 in healthy humans and in active Crohn’s disease. Scand. J. Gastroenterol. 1998, 33, 1094–1098. [Google Scholar] [CrossRef]
- Hrabovsky, V.; Zadak, Z.; Blaha, V.; Hyspler, R.; Karlik, T.; Martinek, A.; Mendlova, A. Cholesterol metabolism in active Crohn’s disease. Wien. Klin. Wochenschr. 2009, 121, 270–275. [Google Scholar] [CrossRef]
- Vermeire, S.; Van Assche, G.; Rutgeerts, P. Laboratory markers in IBD: Useful, magic, or unnecessary toys? Gut 2006, 55, 426–431. [Google Scholar] [CrossRef]
- Romanato, G.; Scarpa, M.; Angriman, I.; Faggian, D.; Ruffolo, C.; Marin, R.; Zambon, S.; Basato, S.; Zanoni, S.; Filosa, T.; et al. Plasma lipids and inflammation in active inflammatory bowel diseases. Aliment. Pharmacol. Ther. 2009, 29, 298–307. [Google Scholar] [CrossRef]
- Sakurai, T.; Saruta, M. Positioning and Usefulness of Biomarkers in Inflammatory Bowel Disease. Digestion 2023, 104, 30–41. [Google Scholar] [CrossRef]
- Siddiqui, I.; Majid, H.; Abid, S. Update on clinical and research application of fecal biomarkers for gastrointestinal diseases. World J. Gastrointest. Pharmacol. Ther. 2017, 8, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Sands, B.E. Biomarkers of Inflammation in Inflammatory Bowel Disease. Gastroenterology 2015, 149, 1275–1285.e2. [Google Scholar] [CrossRef] [PubMed]
- Sturm, A.; Maaser, C.; Calabrese, E.; Annese, V.; Fiorino, G.; Kucharzik, T.; Vavricka, S.R.; Verstockt, B.; van Rheenen, P.; Tolan, D.; et al. ECCO-ESGAR Guideline for Diagnostic Assessment in IBD Part 2: IBD scores and general principles and technical aspects. J. Crohns Colitis 2019, 13, 273–284. [Google Scholar] [CrossRef]
- Liebisch, G.; Schmitz, G. Quantification of lysophosphatidylcholine species by high-throughput electrospray ionization tandem mass spectrometry (ESI-MS/MS). Methods Mol. Biol. 2009, 580, 29–37. [Google Scholar] [CrossRef]
- Bligh, E.G.; Dyer, W.J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef]
- Liebisch, G.; Lieser, B.; Rathenberg, J.; Drobnik, W.; Schmitz, G. High-throughput quantification of phosphatidylcholine and sphingomyelin by electrospray ionization tandem mass spectrometry coupled with isotope correction algorithm. Biochim. Biophys. Acta 2004, 1686, 108–117. [Google Scholar] [CrossRef]
- Horing, M.; Ejsing, C.S.; Hermansson, M.; Liebisch, G. Quantification of Cholesterol and Cholesteryl Ester by Direct Flow Injection High-Resolution Fourier Transform Mass Spectrometry Utilizing Species-Specific Response Factors. Anal. Chem. 2019, 91, 3459–3466. [Google Scholar] [CrossRef]
- Weigand, K.; Peschel, G.; Grimm, J.; Horing, M.; Krautbauer, S.; Liebisch, G.; Muller, M.; Buechler, C. Serum Phosphatidylcholine Species 32:0 as a Biomarker for Liver Cirrhosis Pre- and Post-Hepatitis C Virus Clearance. Int. J. Mol. Sci. 2024, 25, 8161. [Google Scholar] [CrossRef]
- Horing, M.; Ejsing, C.S.; Krautbauer, S.; Ertl, V.M.; Burkhardt, R.; Liebisch, G. Accurate quantification of lipid species affected by isobaric overlap in Fourier-Transform mass spectrometry. J. Lipid Res. 2021, 62, 100050. [Google Scholar] [CrossRef] [PubMed]
- Nahm, F.S. Nonparametric statistical tests for the continuous data: The basic concept and the practical use. Korean J. Anesthesiol. 2016, 69, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Yin, P.; Zhao, X.; Xing, W.; Hu, C.; Zhou, L.; Xu, G. Serum lipid profiling of patients with chronic hepatitis B, cirrhosis, and hepatocellular carcinoma by ultra fast LC/IT-TOF MS. Electrophoresis 2013, 34, 2848–2856. [Google Scholar] [CrossRef] [PubMed]
- Ripolles Piquer, B.; Nazih, H.; Bourreille, A.; Segain, J.P.; Huvelin, J.M.; Galmiche, J.P.; Bard, J.M. Altered lipid, apolipoprotein, and lipoprotein profiles in inflammatory bowel disease: Consequences on the cholesterol efflux capacity of serum using Fu5AH cell system. Metabolism 2006, 55, 980–988. [Google Scholar] [CrossRef]
- Law, S.H.; Chan, M.L.; Marathe, G.K.; Parveen, F.; Chen, C.H.; Ke, L.Y. An Updated Review of Lysophosphatidylcholine Metabolism in Human Diseases. Int. J. Mol. Sci. 2019, 20, 1149. [Google Scholar] [CrossRef]
- Bazarganipour, S.; Hausmann, J.; Oertel, S.; El-Hindi, K.; Brachtendorf, S.; Blumenstein, I.; Kubesch, A.; Sprinzl, K.; Birod, K.; Hahnefeld, L.; et al. The Lipid Status in Patients with Ulcerative Colitis: Sphingolipids are Disease-Dependent Regulated. J. Clin. Med. 2019, 8, 971. [Google Scholar] [CrossRef]
- Kontush, A.; Lhomme, M.; Chapman, M.J. Unraveling the complexities of the HDL lipidome. J. Lipid Res. 2013, 54, 2950–2963. [Google Scholar] [CrossRef]
- Cameron, K.; Nguyen, A.L.; Gibson, D.J.; Ward, M.G.; Sparrow, M.P.; Gibson, P.R. Review Article: Albumin and Its Role in Inflammatory Bowel Disease: The Old, the New, and the Future. J. Gastroenterol. Hepatol. 2025, 40, 808–820. [Google Scholar] [CrossRef]
- Salguero, S.; Rojo, D.; Berenguer, J.; Gonzalez-Garcia, J.; Fernandez-Rodriguez, A.; Brochado-Kith, O.; Diez, C.; Hontanon, V.; Virseda-Berdices, A.; Martinez, J.; et al. Plasma metabolomic fingerprint of advanced cirrhosis stages among HIV/HCV-coinfected and HCV-monoinfected patients. Liver Int. 2020, 40, 2215–2227. [Google Scholar] [CrossRef]
- Sleutjes, J.A.M.; Roeters van Lennep, J.E.; Boersma, E.; Menchen, L.A.; Laudes, M.; Farkas, K.; Molnar, T.; Kennedy, N.A.; Pierik, M.J.; van der Woude, C.J.; et al. Systematic review with meta-analysis: Effect of inflammatory bowel disease therapy on lipid levels. Aliment. Pharmacol. Ther. 2021, 54, 999–1012. [Google Scholar] [CrossRef] [PubMed]
- Minami, T.; Tojo, H.; Shinomura, Y.; Tarui, S.; Okamoto, M. Raised serum activity of phospholipase A2 immunochemically related to group II enzyme in inflammatory bowel disease: Its correlation with disease activity of Crohn’s disease and ulcerative colitis. Gut 1992, 33, 914–921. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.J.; Chen, L.; Sun, R.; Yu, T.; Jiang, S.Y.; Chen, H. Characterization of serum polyunsaturated fatty acid profile in patients with inflammatory bowel disease. Ther. Adv. Chronic Dis. 2023, 14. [Google Scholar] [CrossRef] [PubMed]
- Piotrowska, M.; Binienda, A.; Fichna, J. The role of fatty acids in Crohn’s disease pathophysiology—An overview. Mol. Cell. Endocrinol. 2021, 538, 111448. [Google Scholar] [CrossRef]
- Tokumura, A.; Majima, E.; Kariya, Y.; Tominaga, K.; Kogure, K.; Yasuda, K.; Fukuzawa, K. Identification of human plasma lysophospholipase D, a lysophosphatidic acid-producing enzyme, as autotaxin, a multifunctional phosphodiesterase. J. Biol. Chem. 2002, 277, 39436–39442. [Google Scholar] [CrossRef]
- Hozumi, H.; Hokari, R.; Kurihara, C.; Narimatsu, K.; Sato, H.; Sato, S.; Ueda, T.; Higashiyama, M.; Okada, Y.; Watanabe, C.; et al. Involvement of autotaxin/lysophospholipase D expression in intestinal vessels in aggravation of intestinal damage through lymphocyte migration. Lab. Investig. 2013, 93, 508–519. [Google Scholar] [CrossRef][Green Version]
- Fu, J.; Cuppen, B.V.; Welsing, P.M.; van Wietmarschen, H.; Harms, A.C.; Berger, R.; Koval, S.; Fritsch-Stork, R.D.; Bijlsma, J.W.; Hankemeier, T.; et al. Differences between serum polar lipid profiles of male and female rheumatoid arthritis patients in response to glucocorticoid treatment. Inflammopharmacology 2016, 24, 397–402. [Google Scholar] [CrossRef]
| Statistical tests | Mann–Whitney U-test (two-group comparisons) Kruskal–Wallis test (multiple group comparisons) Spearman correlation (associations between variables) |
| Graphs | Boxplots (median, minimum, and maximum values) |
| Boxplots highlight outliers with circles and asterisks | |
| Normality testing | Shapiro–Wilk tests (p < 0.05 for all but LPC 16:1, 18:1, 18:2, 20:4, 22:4, and 22:5) and non-parametric tests were used for all analyses [44] |
| Correction for multiple comparisons | p-values were adjusted by multiplying by 13 (total number of LPC species analyzed) |
| Significance threshold | p < 0.05 |
| Data in Tables | Median, minimum, and maximum values |
| Software used | IBM SPSS Statistics 26.0 (IBM Corp., Armonk, NY, USA, released 2019) |
| Characteristics | IBD | Normal Values |
|---|---|---|
| Number (females/males) | 57 (26/31) | |
| Age (years) | 41.2 (19.1–69.9) | |
| Body mass index (kg/m2) | 24.1 (15.5–44.3) | |
| C-reactive protein (mg/L) | 2 (0–144) | <5 |
| Fecal calprotectin (µg/g) | 58 (0–1616) | <80 |
| Aspartate aminotransferase (U/L) | 25 (10–35) | Females: 0–35 Males: 0–50 |
| Alanine aminotransferase (U/L) | 18 (7–63) | Females: 0–35 Males: 0–50 |
| Gamma-glutamyl transferase (U/L) | 23 (8–100) | Females: <40 Males: <60 |
| Alkaline phosphatase (U/L) | 65 (38–142) | Females: 35–104 Males: 40–129 |
| Bilirubin (mg/dL) | 0.4 (0.1–1.9) | Females: 0–0.9 Males: 0–1.4 |
| LPC | Cholesterol | Triglycerides | Phosphatidylcholine |
|---|---|---|---|
| 15:0 | 0.525 *** | 0.427 * | 0.542 *** |
| 16:0 | 0.687 *** | 0.570 *** | 0.612 *** |
| 16:1 | 0.459 ** | 0.450 ** | 0.526 *** |
| 18:0 | 0.754 *** | 0.488 ** | 0.659 *** |
| 18:1 | 0.437 ** | 0.236 | 0.430 * |
| 18:2 | 0.345 | 0.108 | 0.385 * |
| 18:3 | 0.388 * | 0.198 | 0.476 ** |
| 20:3 | 0.454 ** | 0.501 *** | 0.449 ** |
| 20:4 | 0.342 | 0.318 | 0.243 |
| 20:5 | 0.318 | 0.234 | 0.335 |
| 22:4 | 0.300 | 0.119 | 0.200 |
| 22:5 | 0.348 | 0.402 * | 0.315 |
| 22:6 | 0.477 ** | 0.226 | 0.411 * |
| Total LPC Level | 0.601 *** | 0.402 * | 0.549 *** |
| CRP | −0.421 * | −0.116 | −0.437 * |
| Calprotectin | −0.468 ** | −0.259 | −0.484 ** |
| LPC | Control | IBD | ||||
|---|---|---|---|---|---|---|
| Median | Minimum | Maximum | Median | Minimum | Maximum | |
| 15:0 | 1.74 | 1.06 | 3.97 | 2.13 | 0.65 | 5.76 |
| 16:0 | 144.60 | 102.63 | 248.97 | 195.99 | 74.13 | 542.58 |
| 16:1 | 4.36 | 2.59 | 11.22 | 5.22 | 1.40 | 10.22 |
| 18:0 | 52.91 | 19.37 | 105.06 | 76.61 | 20.09 | 201.21 |
| 18:1 | 42.85 | 19.41 | 61.57 | 55.11 | 19.54 | 101.51 |
| 18:2 | 58.98 | 28.41 | 78.54 | 60.65 | 14.85 | 154.88 |
| 18:3 | 1.33 | 0.67 | 3.46 | 1.32 | 0.29 | 3.05 |
| 20:3 | 4.68 | 3.10 | 8.99 | 6.09 | 1.31 | 15.93 |
| 20:4 | 11.50 | 6.36 | 20.23 | 15.64 | 4.02 | 33.53 |
| 20:5 | 1.19 | 0.47 | 5.92 | 1.54 | 0.33 | 4.90 |
| 22:4 | 0.78 | 0.31 | 1.38 | 1.01 | 0.32 | 1.63 |
| 22:5 | 1.04 | 0.34 | 1.70 | 1.26 | 0.28 | 2.76 |
| 22:6 | 2.37 | 1.39 | 5.93 | 3.30 | 1.13 | 10.35 |
| Total LPC | 344.32 | 193.78 | 500.44 | 430.45 | 145.25 | 986.22 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tews, H.C.; Elger, T.; Huss, M.; Loibl, J.; Kandulski, A.; Müller, M.; Höring, M.; Liebisch, G.; Buechler, C. Decline in Serum Lysophosphatidylcholine Species in Patients with Severe Inflammatory Bowel Disease. J. Clin. Med. 2025, 14, 5485. https://doi.org/10.3390/jcm14155485
Tews HC, Elger T, Huss M, Loibl J, Kandulski A, Müller M, Höring M, Liebisch G, Buechler C. Decline in Serum Lysophosphatidylcholine Species in Patients with Severe Inflammatory Bowel Disease. Journal of Clinical Medicine. 2025; 14(15):5485. https://doi.org/10.3390/jcm14155485
Chicago/Turabian StyleTews, Hauke Christian, Tanja Elger, Muriel Huss, Johanna Loibl, Arne Kandulski, Martina Müller, Marcus Höring, Gerhard Liebisch, and Christa Buechler. 2025. "Decline in Serum Lysophosphatidylcholine Species in Patients with Severe Inflammatory Bowel Disease" Journal of Clinical Medicine 14, no. 15: 5485. https://doi.org/10.3390/jcm14155485
APA StyleTews, H. C., Elger, T., Huss, M., Loibl, J., Kandulski, A., Müller, M., Höring, M., Liebisch, G., & Buechler, C. (2025). Decline in Serum Lysophosphatidylcholine Species in Patients with Severe Inflammatory Bowel Disease. Journal of Clinical Medicine, 14(15), 5485. https://doi.org/10.3390/jcm14155485






