Leadless Pacemaker Implantation During Extraction in Patients with Active Infection: A Comprehensive Analysis of Safety, Patient Benefits and Costs
Abstract
1. Introduction
2. Methods
2.1. Extraction Procedure
2.2. LP Implantation Procedure
2.3. SP Implantation Procedure
2.4. Study Endpoints
2.5. Statistical Analysis
2.6. Cost Analysis
3. Results
3.1. Baseline Patient Characteristics
3.2. Extraction Procedure
3.3. Pacemaker Implantation
3.4. Primary and Secondary Endpoints
3.5. Cost Analysis of Both Approaches
4. Discussion
4.1. Study Limitations
4.2. Future Directions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chang, D.; Gabriels, J.K.; Soo Kim, B.; Ismail, H.; Willner, J.; Beldner, S.J.; John, R.M.; Epstein, L.M. Concomitant leadless pacemaker implantation and lead extraction during an active infection. J. Cardiovasc. Electrophysiol. 2020, 31, 860–867. [Google Scholar] [CrossRef]
- Reynolds, D.; Duray, G.Z.; Omar, R.; Soejima, K.; Neuzil, P.; Zhang, S.; Narasimhan, C.; Steinwender, C.; Brugada, J.; Lloyd, M.; et al. A leadless intracardiac transcatheter pacing system. N. Engl. J. Med. 2016, 374, 533–541. [Google Scholar] [CrossRef]
- Reddy, V.Y.; Exner, D.V.; Cantillon, D.J.; Doshi, R.; Bunch, T.J.; Tomassoni, G.F.; Friedman, P.A.; Estes, N.A., III; Ip, J.; Niazi, I.; et al. Percutaneous implantation of an entirely intracardiac leadless pacemaker. N. Engl. J. Med. 2015, 373, 1125–1135. [Google Scholar] [CrossRef] [PubMed]
- Kypta, A.; Blessberger, H.; Kammler, J.; Lambert, T.; Lichtenauer, M.; Brandstaetter, W.; Gabriel, M.; Steinwender, C. Leadless cardiac pacemaker implantation after lead extraction in patients with severe device infection. J. Cardiovasc. Electrophysiol. 2016, 27, 1067–1071. [Google Scholar] [CrossRef] [PubMed]
- Roberts, P.R.; Clementy, N.; Al Samadi, F.; Garweg, C.; Martinez-Sande, J.L.; Iacopino, S.; Johansen, J.B.; Prat, X.V.; Kowal, R.C.; Klug, D.; et al. A leadless pacemaker in the real-world setting: The Micra Transcatheter Pacing System Post-Approval Registry. Heart Rhythm 2017, 14, 1375–1379. [Google Scholar] [CrossRef]
- Beccarino, N.J.; Choi, E.Y.; Liu, B.; Kim, B.S.; Pagan, E.; Saleh, M.; Gabriels, J.K.; Epstein, L.M. Concomitant leadless pacing in pacemaker-dependent patients undergoing transvenous lead extraction for active infection: Mid-term follow-up. Heart Rhythm 2023, 20, 853–860. [Google Scholar] [CrossRef] [PubMed]
- Beurskens, N.E.G.; Tjong, F.V.Y.; Dasselaar, K.J.; Kuijt, W.J.; Wilde, A.A.M.; Knops, R.E. Leadless pacemaker implantation after explantation of infected conventional pacemaker systems: A viable solution? Heart Rhythm 2019, 16, 66–71. [Google Scholar] [CrossRef] [PubMed]
- Maille, B.; Behar, N.; Jacon, P.; Hourdain, J.; Franceschi, F.; Koutbi, L.; Tovmassian, L.; Bierme, C.; Seder, E.; Klein, V.; et al. Two-in-one procedure for transvenous lead extraction and leadless pacemaker reimplantation in pacemaker-dependent patients with device infection: Streamlined patient flow. Europace 2024, 2, 26. [Google Scholar] [CrossRef] [PubMed]
- Younis, A.; Glikson, M.; Meitus, A.; Arwas, N.; Natanzon, S.S.; Lotan, D.; Luria, D.; Beinart, R.; Nof, E. Transvenous lead extraction with laser reduces need for femoral approach during the procedure. PLoS ONE 2019, 14, e0215589. [Google Scholar] [CrossRef] [PubMed]
- La Fazia, V.M.; Lepone, A.; Pierucci, N.; Gianni, C.; Barletta, V.; Mohanty, S.; Rocca, D.G.D.; Valle, C.L.; Torlapati, P.G.; Al-Ahmad, M.; et al. Low prevalence of new-onset severe tricuspid regurgitation following leadless pacemaker implantation in a large series of consecutive patients. Heart Rhythm 2024, 21, 2603–2604. [Google Scholar] [CrossRef] [PubMed]
- El-Chami, M.F.; Garweg, C.; Clementy, N.; Al-Samadi, F.; Iacopino, S.; Martinez-Sande, J.L.; Roberts, P.R.; Tondo, C.; Johansen, J.B.; Vinolas-Prat, X.; et al. Leadless pacemakers at 5-year follow-up: The Micra transcatheter pacing system post-approval registry. Eur. Heart J. 2024, 45, 1241–1251. [Google Scholar] [CrossRef]
- Crossley, G.H.; Piccini, J.P.; Longacre, C.; Higuera, L.; Stromberg, K.; El-Chami, M.F. Leadless versus transvenous single-chamber ventricular pacemakers: 3 year follow-up of the Micra CED study. J. Cardiovasc. Electrophysiol. 2023, 34, 1015–1023. [Google Scholar] [CrossRef]
- El-Chami, M.F.; Soejima, K.; Piccini, J.P.; Reynolds, D.; Ritter, P.; Okabe, T.; Friedman, P.A.; Cha, Y.M.; Stromberg, K.; Holbrook, R.; et al. Incidence and outcomes of systemic infections in patients with leadless pacemakers: Data from the Micra IDE study. Pacing Clin. Electrophysiol. 2019, 42, 1105–1110. [Google Scholar] [CrossRef] [PubMed]
- El-Chami, M.F.; Bonner, M.; Holbrook, R.; Stromberg, K.; Mayotte, J.; Molan, A.; Sohail, M.R.; Epstein, L.M. Leadless pacemakers reduce risk of device-related infection: Review of the potential mechanisms. Heart Rhythm 2020, 17, 1393–1397. [Google Scholar] [CrossRef] [PubMed]
| Entire Cohort (n = 87) | Temporary Transvenous Pacemaker (n = 42) | Leadless Pacemaker (n = 45) | p-Value | |
|---|---|---|---|---|
| Demographics | ||||
| Age, years, mean (SD) | 74 (13) | 72 (14) | 76 (12) | 0.073 |
| Female gender, n (%) | 19 (21.8) | 8 (19.0) | 11 (24.4) | 0.543 |
| Diabetes mellitus, n (%) | 39 (45.3) | 21 (50) | 18 (40.9) | 0.397 |
| Hypertension, n (%) | 59 (68.6) | 29 (69) | 30 (68.2) | 0.931 |
| CVA/TIA, n (%) | 23 (26.7) | 12 (28.6) | 11 (25.0) | 0.283 |
| Chronic atrial fibrillation, n (%) | 36 (41.4) | 20 (47.6) | 16 (35.6) | 0.254 |
| Chronic kidney disease, n (%) | 22 (25.6) | 9 (21.4) | 13 (29.5) | 0.388 |
| LVEF, mean (SD) | 49 (12) | 45 (13) | 52 (9) | 0.018 |
| Cardiomyopathy, n (%) | 0.015 | |||
| Ischemic | 11 (13.8) | 6 (16.7) | 5 (11.4) | |
| Dilated | 4 (5.0) | 4 (11.1) | 0 (0.0) | |
| Hypertrophic | 3 (3.8) | 3 (8.3) | 0 (0.0) | |
| COPD, n (%) | 13 (15.3) | 7 (17.1) | 6 (13.6) | 0.660 |
| Severe pulmonary hypertension, n (%) | 15 (19.2) | 7 (20.6) | 8 (18.2) | 0.789 |
| Valve replacement, n (%) | 25 (40.3) | 9 (21.4) | 16 (35.6) | 0.396 |
| Number of electrodes (Median) | 2.3 (2) | 2.5 (2.5) | 2.1 (2) | 0.011 |
| Device type, n (%) | 0.013 | |||
| CRTD | 18 (20.9) | 14 (33.3) | 4 (9.1) | |
| CRTP | 10 (11.6) | 5 (11.9) | 5 (11.4) | |
| Single chamber | 10 (11.6) | 7 (16.7) | 3 (6.8) | |
| Dual chamber | 47 (54.7) | 16 (38.1) | 31 (70.4) | |
| Pacing indication, n (%) | 0.453 | |||
| SSS | 7 (8.04) | 3 (7.14) | 4 (8.9) | |
| High degree AV block | 53 (60.91) | 29 (69.04) | 24 (53.3) | |
| Atrial fibrillation with AV block | 18 (20.68) | 6 (14.28) | 12 (26.7) | |
| Indication for extraction, n (%) | 0.139 | |||
| Pocket infection | 33 (37.9) | 19 (45.2) | 14 (31.1) | |
| Systemic infection | 23 (26.4) | 8 (19.0) | 15 (33.3) | |
| Combined pocket + systemic | 28 (32.2) | 15 (35.7) | 13 (28.9) | |
| Type of infection, n (%) | 0.113 | |||
| Streptococcus | 4 (5.9) | 2 (5.1) | 2 (4.6) | |
| MSSA | 10 (14.7) | 7 (17.9) | 3 (6.8) | |
| Staphylococcus (other) | 29 (42.6) | 12 (31.2) | 17 (39.1) | |
| Enterococcus | 10 (14.7) | 2 (5.1) | 8 (18.2) |
| Temporary Transvenous Pacemaker Patients (n = 42) | Leadless Pacemaker Patients (n = 45) | p-Value | |
|---|---|---|---|
| Simple Traction (%) | 10 (23.8) | 18 (40) | 0.106 |
| Laser (%) | 24 (57.1) | 3 (6.7) | <0.001 |
| TightRail (%) | 11 (26.2) | 9 (20) | 0.493 |
| Sub C TightRail (%) | 0 (0) | 20 (44.4) | <0.001 |
| Femoral Work Station (%) | 3 (7.1) | 1 (2.2) | 0.273 |
| Temporary Transvenous Pacemaker Patients (n = 32) | Leadless Pacemaker Patients (n = 42) | p-Value | |
|---|---|---|---|
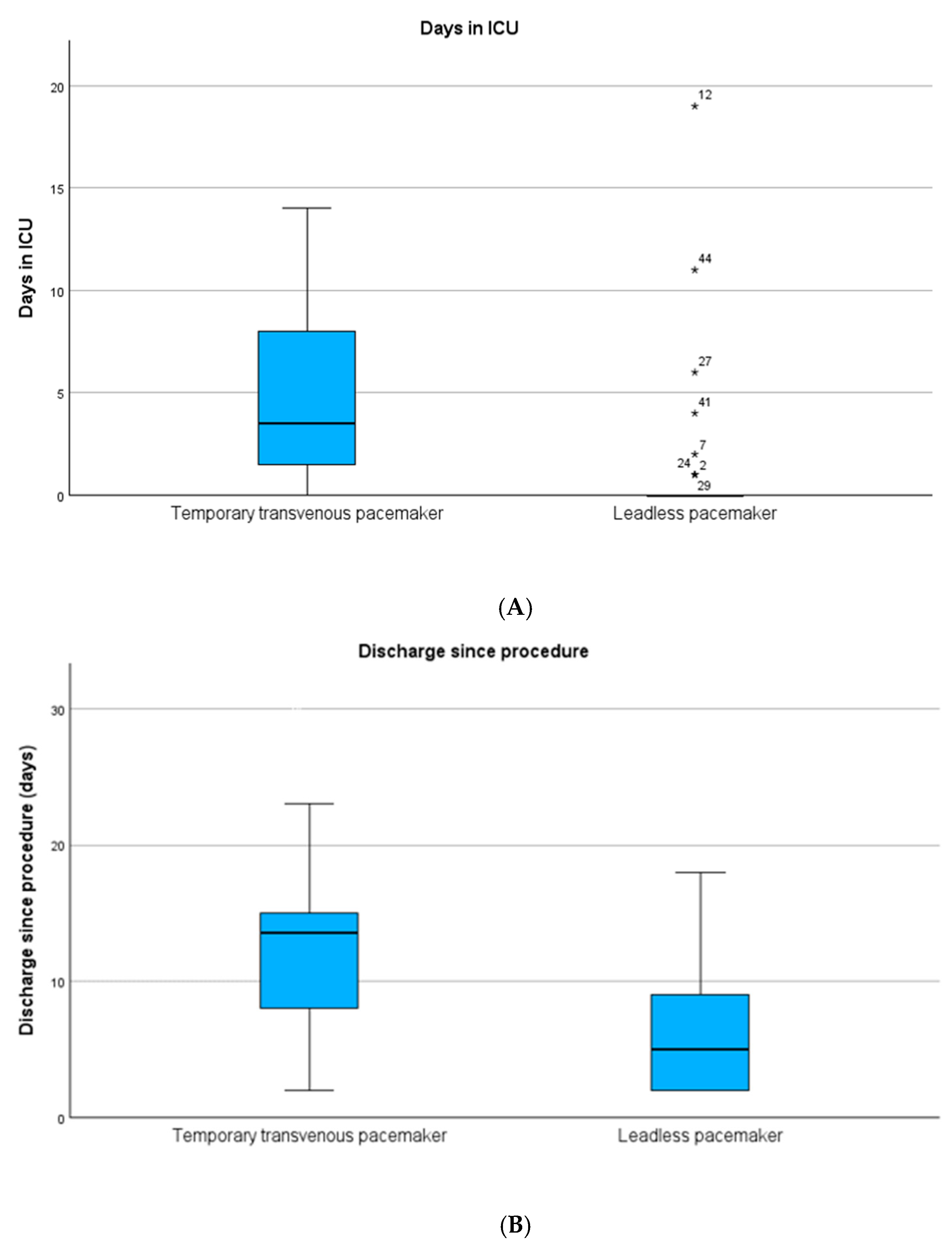
| Days in ICU, mean (SD) | 7 (12) | 1 (3) | <0.001 |
| Days in hospital, mean (SD) | 17 (17) | 11 (24) | <0.001 |
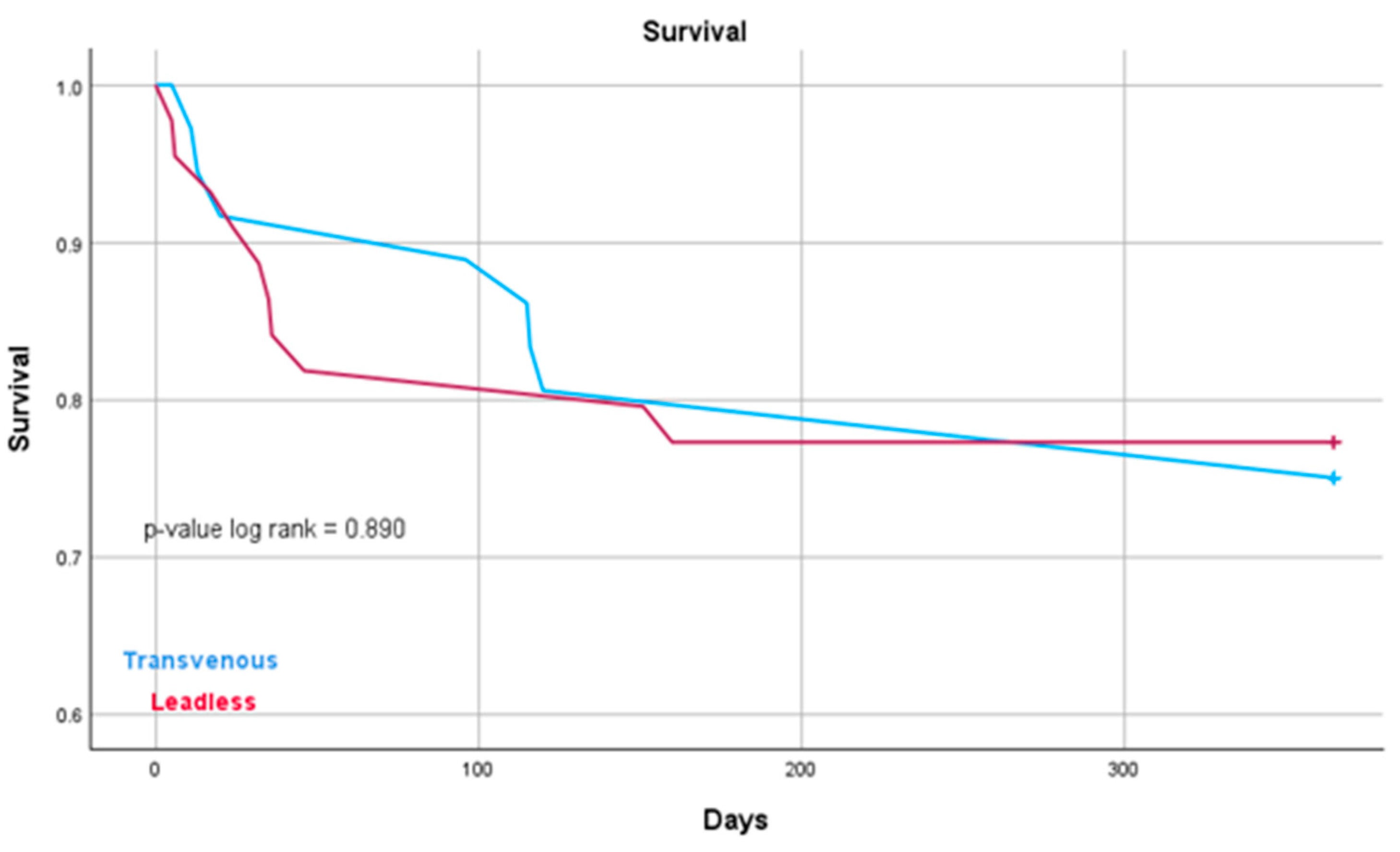
| 1-year mortality, n (%) | 9 (23.7) | 10 (22.7) | 0.890 |
| Temporary Transvenous Pacemaker Approach (USD) (n = 32) | Leadless Pacemaker Approach (USD) (n = 41) | p-Value | |
|---|---|---|---|
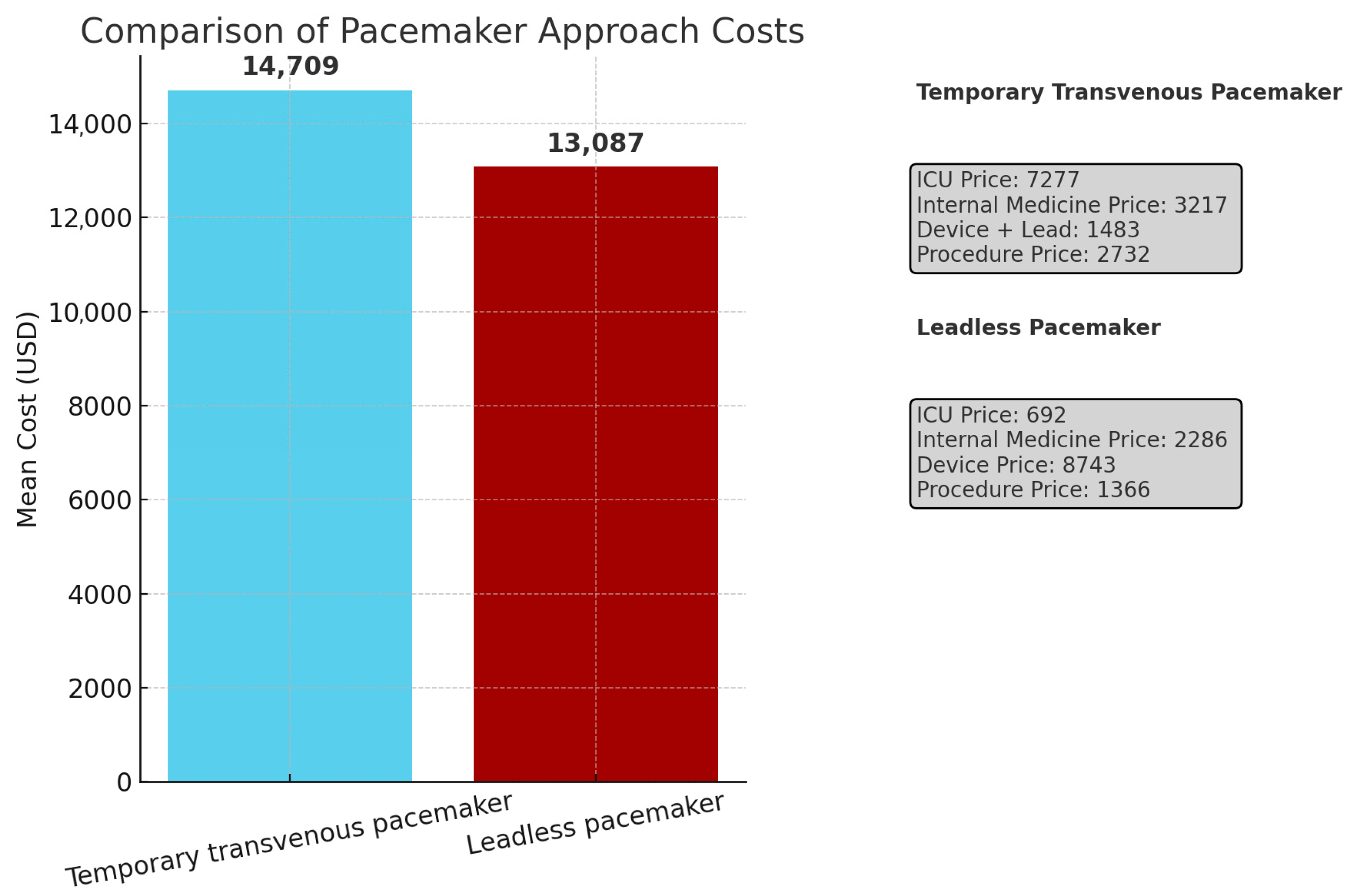
| ICU, mean (SD) | 7277 (13,457) | 692 (2210) | <0.001 |
| Internal Department, mean (SD) | 3217 (2789) | 2286 (3378) | 0.031 |
| Device + Temporary Screw in Lead | 1483 | 8743 | |
| Procedure | 2732 | 1366 | |
| Total, mean | 14,709 | 13,087 | 0.321 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Solomon, A.; Tzuberi, M.; Berkovitch, A.; Hoch, E.; Beinart, R.; Nof, E. Leadless Pacemaker Implantation During Extraction in Patients with Active Infection: A Comprehensive Analysis of Safety, Patient Benefits and Costs. J. Clin. Med. 2025, 14, 5450. https://doi.org/10.3390/jcm14155450
Solomon A, Tzuberi M, Berkovitch A, Hoch E, Beinart R, Nof E. Leadless Pacemaker Implantation During Extraction in Patients with Active Infection: A Comprehensive Analysis of Safety, Patient Benefits and Costs. Journal of Clinical Medicine. 2025; 14(15):5450. https://doi.org/10.3390/jcm14155450
Chicago/Turabian StyleSolomon, Aviv, Maor Tzuberi, Anat Berkovitch, Eran Hoch, Roy Beinart, and Eyal Nof. 2025. "Leadless Pacemaker Implantation During Extraction in Patients with Active Infection: A Comprehensive Analysis of Safety, Patient Benefits and Costs" Journal of Clinical Medicine 14, no. 15: 5450. https://doi.org/10.3390/jcm14155450
APA StyleSolomon, A., Tzuberi, M., Berkovitch, A., Hoch, E., Beinart, R., & Nof, E. (2025). Leadless Pacemaker Implantation During Extraction in Patients with Active Infection: A Comprehensive Analysis of Safety, Patient Benefits and Costs. Journal of Clinical Medicine, 14(15), 5450. https://doi.org/10.3390/jcm14155450