Enhanced Diagnostic Accuracy for Septic Arthritis Through Multivariate Analysis of Serum and Synovial Biomarkers
Abstract
1. Introduction
2. Material and Methods
2.1. Study Design and Population
2.2. Laboratory Evaluation
2.3. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AUC | area under the curve |
| BMI | body mass index |
| CRP | C-reactive protein |
| IL-6 | interleukin 6 |
| PJI | periprosthetic joint injection |
| PMNs | polymorphonuclear leukocytes |
| PTX3 | pentraxin 3 |
| sTNF-R2 | soluble tumor necrosis factor receptor 2 |
| WBCs | white blood cells |
References
- Margaretten, M.E.; Kohlwes, J.; Moore, D.; Bent, S. Does this adult patient have septic arthritis? JAMA 2007, 297, 1478–1488. [Google Scholar] [CrossRef]
- Mathews, C.J.; Weston, V.C.; Jones, A.; Field, M.; Coakley, G. Bacterial septic arthritis in adults. Lancet 2010, 375, 846–855. [Google Scholar] [CrossRef]
- Elsissy, J.G.; Liu, J.N.; Wilton, P.J.; Nwachuku, I.; Gowd, A.K.; Amin, N.H. Bacterial Septic Arthritis of the Adult Native Knee Joint: A Review. JBJS Rev. 2020, 8, e0059. [Google Scholar] [CrossRef] [PubMed]
- Lin, A.P.; Nguyen, B.T.T.; Tran, S.Q.; Kuo, Y.J.; Huang, S.W.; Chen, Y.P. Does septic arthritis after anterior cruciate ligament reconstruction lead to poor outcomes? A systematic review and meta-analysis of observational studies. Knee Surg. Relat. Res. 2024, 36, 45. [Google Scholar] [CrossRef] [PubMed]
- Kaandorp, C.J.; Van Schaardenburg, D.; Krijnen, P.; Habbema, J.D.; van de Laar, M.A. Risk factors for septic arthritis in patients with joint disease. A prospective study. Arthritis Rheumatol. 1995, 38, 1819–1825. [Google Scholar] [CrossRef] [PubMed]
- He, M.; Arthur Vithran, D.T.; Pan, L.; Zeng, H.; Yang, G.; Lu, B.; Zhang, F. An update on recent progress of the epidemiology, etiology, diagnosis, and treatment of acute septic arthritis: A review. Front. Cell Infect. Microbiol. 2023, 13, 1193645. [Google Scholar] [CrossRef]
- Goldenberg, D.L. Septic arthritis. Lancet 1998, 351, 197–202. [Google Scholar] [CrossRef]
- Tarkowski, A. Infection and musculoskeletal conditions: Infectious arthritis. Best Pract. Res. Clin. Rheumatol. 2006, 20, 1029–1044. [Google Scholar] [CrossRef]
- Turner, E.H.G.; Lang, M.D.H.; Spiker, A.M. A narrative review of the last decade’s literature on the diagnostic accuracy of septic arthritis of the native joint. J. Exp. Orthop. 2021, 8, 3. [Google Scholar] [CrossRef]
- Carpenter, C.R.; Schuur, J.D.; Everett, W.W.; Pines, J.M. Evidence-based diagnostics: Adult septic arthritis. Acad. Emerg. Med. 2011, 18, 781–796. [Google Scholar] [CrossRef]
- Hassan, A.S.; Rao, A.; Manadan, A.M.; Block, J.A. Peripheral Bacterial Septic Arthritis: Review of Diagnosis and Management. J. Clin. Rheumatol. 2017, 23, 435–442. [Google Scholar] [CrossRef] [PubMed]
- Abdullah, S.; Young-Min, S.A.; Hudson, S.J.; Kelly, C.A.; Heycock, C.R.; Hamilton, J.D. Gross synovial fluid analysis in the differential diagnosis of joint effusion. J. Clin. Pathol. 2007, 60, 1144–1147. [Google Scholar] [CrossRef] [PubMed]
- Dechnik, A.; Kahane, C.G.; Nigrovic, L.E.; Lyons, T.W. Utility of Synovial Fluid Biomarkers for Culture-Positive Septic Arthritis in a Lyme Disease-Endemic Region. Pediatr. Emerg. Care 2024, 40, e82–e88. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, L.; Bell, J.; Kumar, A.; Heckman, M.G.; Lesser, E.; Whalen, J.; Shi, G.G.; Ledford, C.; Wilke, B. A Retrospective Review of Native Septic Arthritis in Patients: Can We Diagnose Based on Laboratory Values? Cureus 2020, 12, e8577. [Google Scholar] [CrossRef]
- Deirmengian, C.; Kardos, K.; Kilmartin, P.; Cameron, A.; Schiller, K.; Parvizi, J. Diagnosing periprosthetic joint infection: Has the era of the biomarker arrived? Clin. Orthop. Relat. Res. 2014, 472, 3254–3262. [Google Scholar] [CrossRef]
- Keemu, H.; Vaura, F.; Maksimow, A.; Maksimow, M.; Jokela, A.; Hollmen, M.; Makela, K. Novel Biomarkers for Diagnosing Periprosthetic Joint Infection from Synovial Fluid and Serum. JBJS Open Access 2021, 6, e20.00067. [Google Scholar] [CrossRef]
- Lenski, M.; Scherer, M.A. The significance of interleukin-6 and lactate in the synovial fluid for diagnosing native septic arthritis. Acta Orthop. Belg. 2014, 80, 18–25. [Google Scholar]
- Loppini, M.; Di Maio, M.; Avigni, R.; Leone, R.; Inforzato, A.; Grappiolo, G.; Mantovani, A.; Bottazzi, B. Long Pentraxin 3 as a New Biomarker for Diagnosis of Hip and Knee Periprosthetic Joint Infections. J. Clin. Med. 2023, 12, 1055. [Google Scholar] [CrossRef]
- Randau, T.M.; Friedrich, M.J.; Wimmer, M.D.; Reichert, B.; Kuberra, D.; Stoffel-Wagner, B.; Limmer, A.; Wirtz, D.C.; Gravius, S. Interleukin-6 in serum and in synovial fluid enhances the differentiation between periprosthetic joint infection and aseptic loosening. PLoS ONE 2014, 9, e89045. [Google Scholar] [CrossRef]
- Saleh, A.; Ramanathan, D.; Siqueira, M.B.P.; Klika, A.K.; Barsoum, W.K.; Rueda, C.A.H. The Diagnostic Utility of Synovial Fluid Markers in Periprosthetic Joint Infection: A Systematic Review and Meta-analysis. J. Am. Acad. Orthop. Surg. 2017, 25, 763–772. [Google Scholar] [CrossRef]
- Lee, B.H.; Na, Y.G.; Ham, S.H.; Jin, M.; Kim, Y.T.; Kim, K.O.; Sim, J.A. Tryptophanyl tRNA synthetase is an alternative synovial biomarker for diagnosis of septic arthritis in knee joint. Knee Surg. Relat. Res. 2024, 36, 27. [Google Scholar] [CrossRef] [PubMed]
- Delva, M.L.; Samuel, L.T.; Acuna, A.J.; Kamath, A.F. Presepsin as a diagnostic biomarker of peri-prosthetic joint infection: A review of the literature. Eur. J. Orthop. Surg. Traumatol. 2023, 33, 695–700. [Google Scholar] [CrossRef] [PubMed]
- Fisher, J.J.; Grace, T.; Castles, N.A.; Jones, E.A.; Delforce, S.J.; Peters, A.E.; Crombie, G.K.; Hoedt, E.C.; Warren, K.E.; Kahl, R.G.; et al. Methodology for Biological Sample Collection, Processing, and Storage in the Newcastle 1000 Pregnancy Cohort: Protocol for a Longitudinal, Prospective Population-Based Study in Australia. JMIR Res. Protoc. 2024, 13, e63562. [Google Scholar] [CrossRef]
- McHugh, M.L. Interrater reliability: The kappa statistic. Biochem. Med. 2012, 22, 276–282. [Google Scholar] [CrossRef]
- Lenski, M.; Scherer, M.A. Diagnostic potential of inflammatory markers in septic arthritis and periprosthetic joint infections: A clinical study with 719 patients. Infect. Dis. 2015, 47, 399–409. [Google Scholar] [CrossRef]
- Song, J.; Park, D.W.; Moon, S.; Cho, H.J.; Park, J.H.; Seok, H.; Choi, W.S. Diagnostic and prognostic value of interleukin-6, pentraxin 3, and procalcitonin levels among sepsis and septic shock patients: A prospective controlled study according to the Sepsis-3 definitions. BMC Infect. Dis. 2019, 19, 968. [Google Scholar] [CrossRef]
- Varady, N.H.; Schwab, P.E.; Kheir, M.M.; Dilley, J.E.; Bedair, H.; Chen, A.F. Synovial Fluid and Serum Neutrophil-to-Lymphocyte Ratio: Novel Biomarkers for the Diagnosis and Prognosis of Native Septic Arthritis in Adults. J. Bone Jt. Surg. Am. 2022, 104, 1516–1522. [Google Scholar] [CrossRef]
- Yoon, J.R.; Yang, S.H.; Shin, Y.S. Diagnostic accuracy of interleukin-6 and procalcitonin in patients with periprosthetic joint infection: A systematic review and meta-analysis. Int. Orthop. 2018, 42, 1213–1226. [Google Scholar] [CrossRef]
- Imagama, T.; Seki, K.; Seki, T.; Tokushige, A.; Matsuki, Y.; Yamazaki, K.; Nakashima, D.; Okazaki, T.; Hirata, K.; Yamamoto, M.; et al. Synovial fluid presepsin as a novel biomarker for the rapid differential diagnosis of native joint septic arthritis from crystal arthritis. Int. J. Infect. Dis. 2021, 102, 472–477. [Google Scholar] [CrossRef]
- Deeks, J.J.; Bossuyt, P.M.; Leeflang, M.M.; Takwoingi, Y. Cochrane Handbook for Systematic Reviews of Diagnostic Test Accuracy; John Wiley & Sons: Hoboken, NJ, USA, 2023. [Google Scholar]
- Festa, E.; Ascione, T.; Di Gennaro, D.; De Mauro, D.; Mariconda, M.; Balato, G. Synovial calprotectin in prosthetic joint infection. A systematic review and meta-analysis of the literature. Arch. Orthop. Trauma. Surg. 2024, 144, 5217–5227. [Google Scholar] [CrossRef]
- Deirmengian, C.; Kardos, K.; Kilmartin, P.; Cameron, A.; Schiller, K.; Booth, R.E., Jr.; Parvizi, J. The alpha-defensin test for periprosthetic joint infection outperforms the leukocyte esterase test strip. Clin. Orthop. Relat. Res. 2015, 473, 198–203. [Google Scholar] [CrossRef]
- Hantouly, A.T.; Salameh, M.; Toubasi, A.A.; Salman, L.A.; Alzobi, O.; Ahmed, A.F.; Hameed, S.; Zikria, B.; Ahmed, G. Synovial fluid calprotectin in diagnosing periprosthetic joint infection: A meta-analysis. Int. Orthop. 2022, 46, 971–981. [Google Scholar] [CrossRef]
- Cooper, K.B.; Siegel, E.R.; Stambough, J.B.; Bumpass, D.B.; Mears, S.C. The Alpha-Defensin Prosthetic Joint Infection Test Has Poor Validity for Native Knee Joint Infection. J. Arthroplast. 2021, 36, 2957–2961. [Google Scholar] [CrossRef] [PubMed]
- Baillet, A.; Trocme, C.; Romand, X.; Nguyen, C.M.V.; Courtier, A.; Toussaint, B.; Gaudin, P.; Epaulard, O. Calprotectin discriminates septic arthritis from pseudogout and rheumatoid arthritis. Rheumatology 2019, 58, 1644–1648. [Google Scholar] [CrossRef] [PubMed]
- Holzmeister, A.; Frazzetta, J.; Yuan, F.F.N.; Cherones, A.; Summers, H.; Cohen, J.; Lack, W.D. Evaluation for septic arthritis of the native adult knee is aided by multivariable assessment. Am. J. Emerg. Med. 2021, 46, 614–618. [Google Scholar] [CrossRef] [PubMed]
- Balato, G.; Dall’Anese, R.; Balboni, F.; Ascione, T.; Pezzati, P.; Bartolini, G.; Quercioli, M.; Baldini, A. Synovial fluid alpha-defensin in periprosthetic knee infection workup: Liquid chromatography-mass spectrometry detection of alpha-defensin in synovial fluid. Bone Jt. Surg. 2022, 104-B, 1047–1051. [Google Scholar] [CrossRef]
- Knapper, T.; Murphy, R.J.; Rocos, B.; Fagg, J.; Murray, N.; Whitehouse, M.R. Utility of bedside leucocyte esterase testing to rule out septic arthritis. Emerg. Med. J. 2021, 38, 707–710. [Google Scholar] [CrossRef]
- Litman, K. A rational approach to the diagnosis of arthritis. Am. Fam. Physician 1996, 53, 1295–1310. [Google Scholar]
- Kim, C.W.; Lee, C.R.; Park, D.H.; Kim, D.Y.; Kim, J.W. Clinical outcomes of two-stage revision for chronic periprosthetic joint infection of the knee: Culture-negative versus culture-positive. Knee Surg. Relat. Res. 2021, 33, 28. [Google Scholar] [CrossRef]
- Man, S.L.; Chau, W.W.; Chung, K.Y.; Ho, K.K.W. Hypoalbuminemia and obesity class II are reliable predictors of peri-prosthetic joint infection in patient undergoing elective total knee arthroplasty. Knee Surg. Relat. Res. 2020, 32, 21. [Google Scholar] [CrossRef]
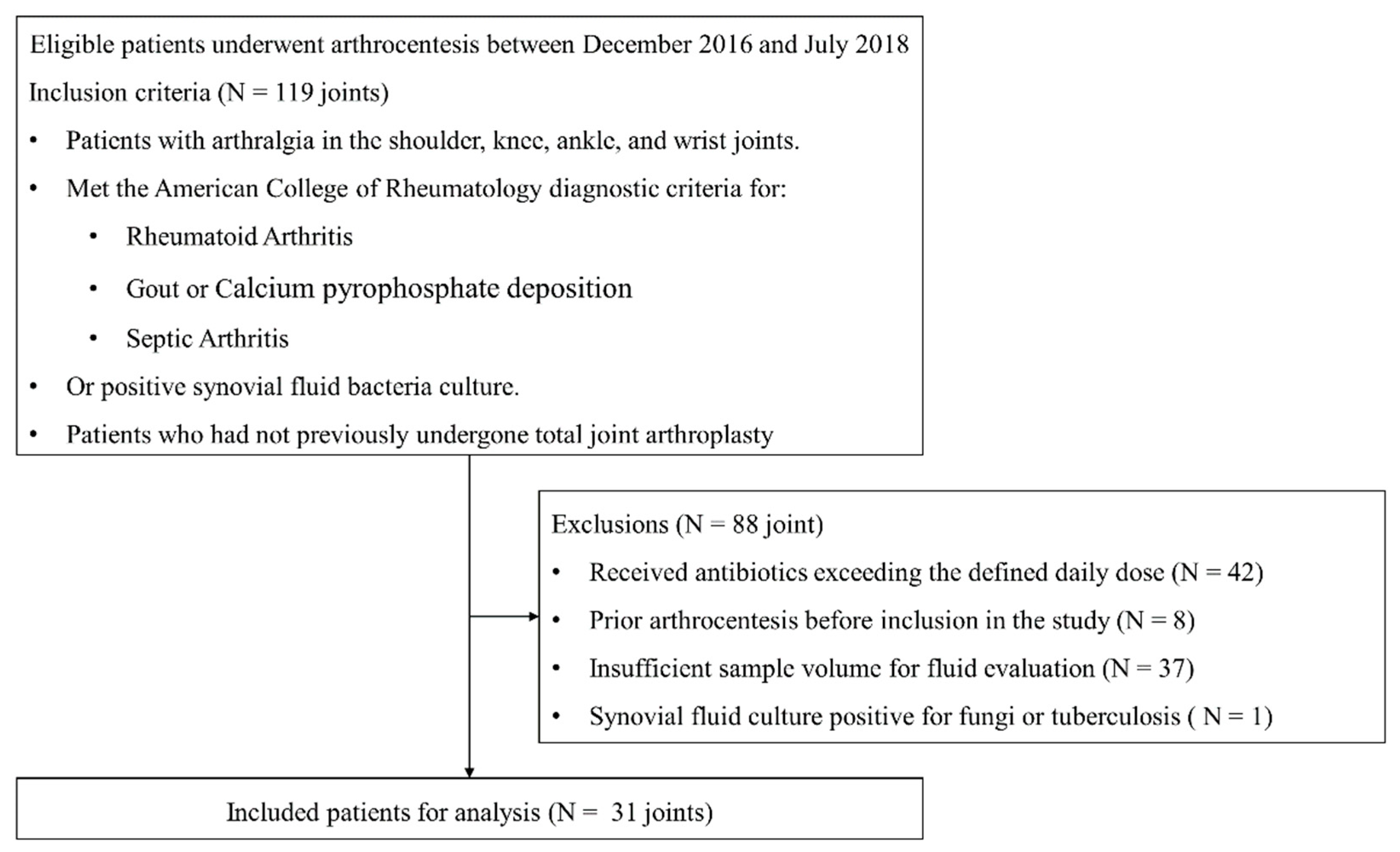

| Non-Septic Arthritis (N = 25) | Septic Arthritis (N = 6) | p-Value | |
|---|---|---|---|
| Age, years | 61.5 ± 14.6 | 45.8 ± 24.8 | 0.174 * |
| Sex (female/male), N, % | 13/25 (52.0) | 2/4 (50.0) | 0.654 † |
| Height, cm | 158.8 ± 10.9 | 168.4 ± 7.4 | 0.036 * |
| Weight, kg | 62.2 ± 11.3 | 70.6 ± 7.8 | 0.114 * |
| BMI, kg/m2 | 24.6 ± 3.6 | 24.9 ± 1.8 | 0.777 * |
| Affected joint | 0.420 ‡ | ||
| Knee, % | 22 (88.0) | 6 (100) | |
| Ankle, % | 1 (4.0) | 0 (0) | |
| Shoulder, % | 1 (4.0) | 0 (0) | |
| Wrist, % | 1 (4.0) | 0 (0) | |
| Laterality (right/left), N, % | 11/14 (78.6) | 5/6 (83.3%) | 0.172 † |
| Hypertension | 12 (48.0) | 3 (50.0) | 1.000 † |
| Diabetes mellitus | 7 (28.0) | 2 (33.3) | 1.000 † |
| Chronic kidney disease | 1 (4.0) | 0 (0) | 1.000 † |
| Cerebrovascular disease | 1 (4.0) | 0 (0) | 1.000 † |
| Rheumatologic disease | 2 (8.0) | 0 (0) | 1.000 † |
| Endocrinologic disease | 3 (12.0) | 0 (0) | 1.000 † |
| Hepatologic disease | 0 (0) | 1 (16.7) | 0.194 † |
| Non-Septic Arthritis (N = 25) | Septic Arthritis (N = 6) | p-Value * | |
|---|---|---|---|
| Blood | |||
| Hb, g/dL | 12.1 ± 2.3 | 11.8 ± 1.6 | 0.955 |
| WBC, cells/μL | 9478.4 ± 3412.0 | 12,260.0 ± 4910.5 | 0.279 |
| CRP, mg/dL | 7.5 ± 7.5 | 18.1 ± 13.8 | 0.022 |
| ESR, mm/hr | 45.0 ± 17.1 | 41.3 ± 9.7 | 0.395 |
| Albumin, g/dL | 3.7 ± 0.5 | 4.9 ± 2.8 | 0.066 |
| Uric acid, mg/dL | 5.6 ± 3.0 | 4.9 ± 2.8 | 0.770 |
| Joint fluid | |||
| SG | 1.027 ± 0.005 | 1.030 ± 0.005 | 0.148 |
| pH | 7.9 ± 0.4 | 7.9 ± 0.2 | 0.721 |
| WBC, cells/μL | 25,042.8 ± 26,784.2 | 72,028.6 ± 59,222.3 | 0.023 |
| Neutrophil, cells/μL | 22,488.2 ± 24,587.9 | 77,624.0 ± 64,225.6 | 0.031 |
| Lymphocyte, cells/μL | 3.3 ± 3.4 | 3.8 ± 3.6 | 0.314 |
| Eosinophils, cells/μL | 0.0 ± 0.2 | 0.0 ± 0.0 | 0.789 |
| Neutrophil, % | 69.9 ± 34.0 | 89.0 ± 7.3 | 0.327 |
| Lymphocyte, % | 12.5 ± 20.5 | 8.0 ± 12.4 | 0.865 |
| Eosinophil, % | 0.3 ± 1.1 | 0.0 ± 0.0 | 0.789 |
| Pentraxin 3, ng/mL | 53.6 ± 99.3 | 366.7 ± 618.0 | 0.017 |
| IL-6, ng/mL | 110.9 ± 146.0 | 190.7 ± 171.9 | 0.227 |
| Presepsin, pg/mL | 159.2 ± 516.6 | 1098.2 ± 1398.8 | 0.053 |
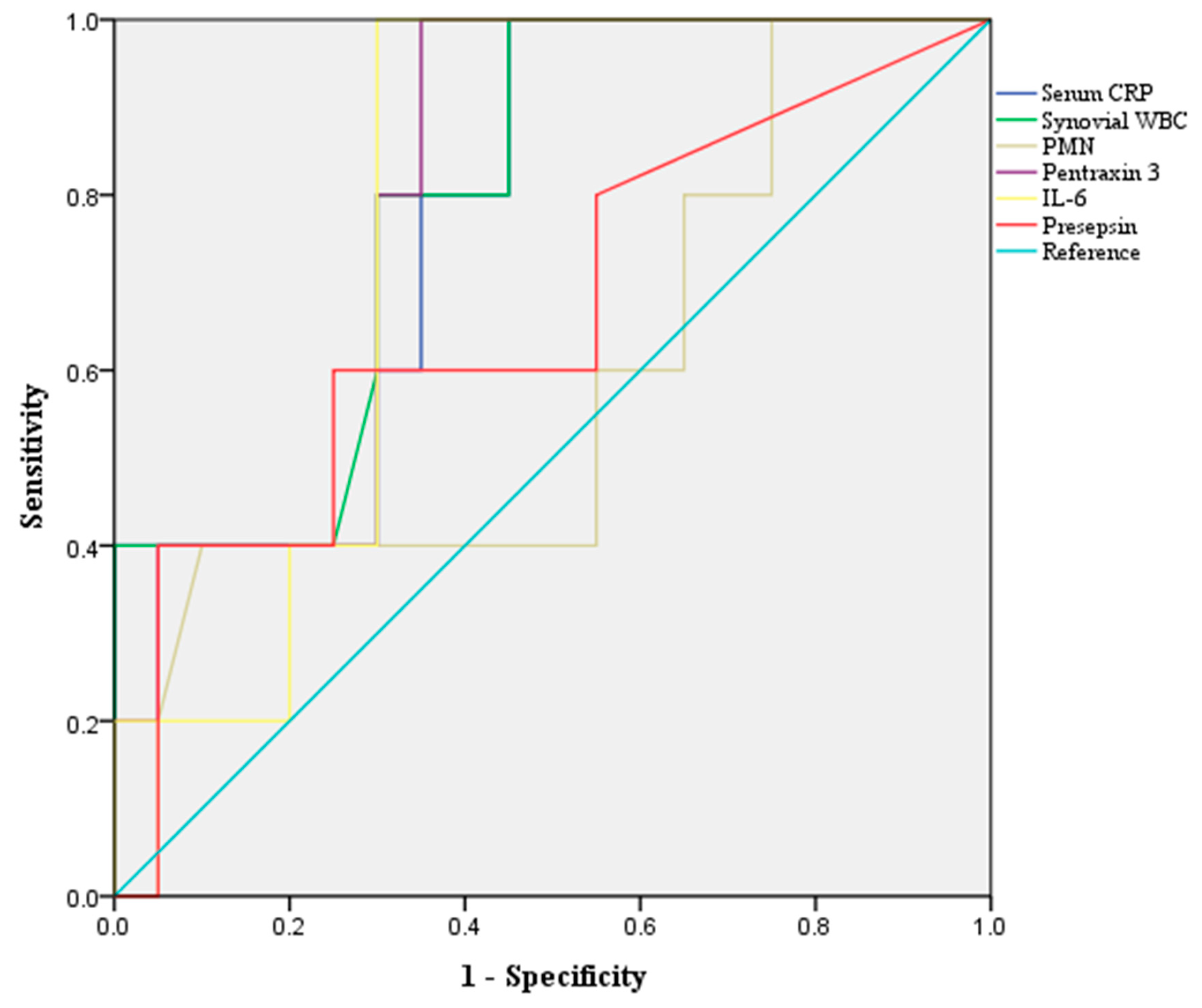
| AUC (95% CI) | p-Value | Yonden Index | Cut Off | Accuracy | Sensitivity | Specificity | PPV | NPV | |
|---|---|---|---|---|---|---|---|---|---|
| Serum CRP | 0.817 (0.631–1.000) | 0.021 | 0.550 | 5.2 mg/dL | 71.0 | 100 | 64.0 | 40.0 | 100 |
| Synovial WBC | 0.837 (0.678–0.995) | 0.012 | 0.593 | 34,200.0 cells/μL | 77.4 | 83.3 | 76.0 | 45.5 | 95.0 |
| PMN, % | 0.648 (0.388–0.908) | 0.303 | 0.320 | 94.5% | 80.6 | 33.3 | 92.0 | 50.0 | 85.2 |
| Pentraxin 3 | 0.813 (0.658–0.969) | 0.019 | 0.680 | 36.8 ng/mL | 74.2 | 100 | 68.0 | 42.9 | 100 |
| IL-6 | 0.667 (0.391–0.942) | 0.211 | 0.537 | 105.8 ng/mL | 74.2 | 83.3 | 72.0 | 41.7 | 94.7 |
| Presepsin | 0.760 (0.526–0.994) | 0.051 | 0.389 | 35.5 pg/mL | 77.4 | 66.7 | 80.0 | 44.4 | 90.9 |
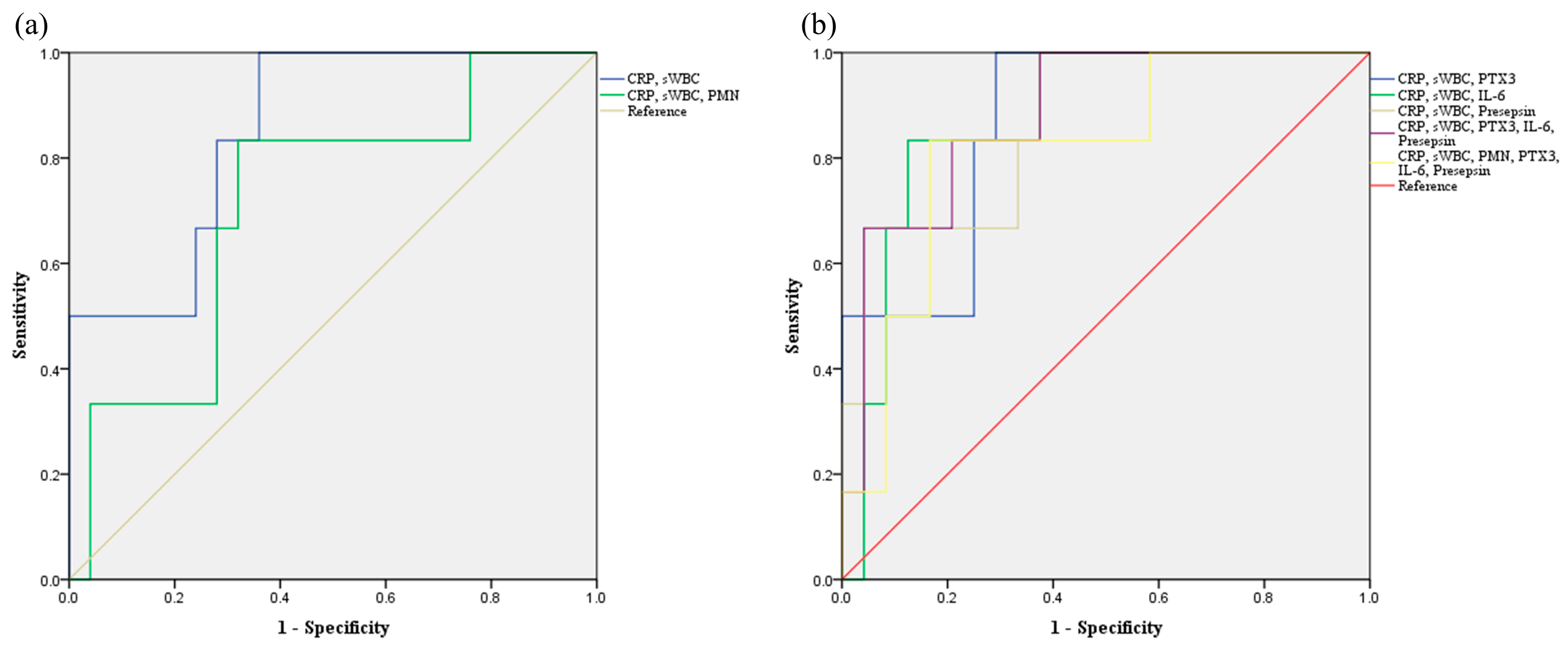
| AUC (95% CI) | p-Value | Yonden Index | Cut Off | Accuracy | Sensitivity | Specificity | PPV | NPV | |
|---|---|---|---|---|---|---|---|---|---|
| sWBC, PMN 1 | 0.540 (0.249–0.831) | 0.764 | 0.253 | −3.026 | 80.6 | 33.3 | 92.0 | 50.0 | 85.2 |
| CRP, sWBC 2 | 0.853 (0.702–1.000) | 0.008 | 0.640 | −4.208 | 74.2 | 100 | 68.0 | 42.9 | 100 |
| CRP, sWBC, PMN 3 | 0.713 (0.487–0.940) | 0.110 | 0.440 | −0.690 | 71.0 | 83.3 | 68.0 | 38.5 | 94.4 |
| CRP, sWBC, Pentraxin 3 4 | 0.873 (0.737–1.000) | 0.005 | 0.720 | −3.186 | 74.2 | 100 | 68.0 | 42.9 | 100 |
| CRP, sWBC, IL-6 5 | 0.880 (0.749–1.000) | 0.004 | 0.600 | −2.860 | 71.0 | 83.3 | 68.0 | 38.5 | 94.4 |
| CRP, sWBC, Presepsin 6 | 0.873 (0.727–1.000) | 0.005 | 0.600 | −4.196 | 87.1 | 50.0 | 96.0 | 75.0 | 88.9 |
| CRP, sWBC, Pentraxin 3, IL-6, Presepsin 7 | 0.887 (0.755–1.000) | 0.004 | 0.560 | −4.437 | 71.0 | 100 | 64.0 | 40.0 | 100 |
| CRP, sWBC, PMN, Pentraxin 3, IL-6, Presepsin 8 | 0.819 (0.636–1.000) | 0.017 | 0.667 | 2.031 | 83.3 | 83.3 | 83.3 | 55.6 | 95.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, H.J.; Jeon, J.H.; Song, J.; Seok, H.; Choi, H.K.; Choi, W.S.; Choi, S.; Nam, M.-H.; Suh, D.H.; Kim, J.G.; et al. Enhanced Diagnostic Accuracy for Septic Arthritis Through Multivariate Analysis of Serum and Synovial Biomarkers. J. Clin. Med. 2025, 14, 5415. https://doi.org/10.3390/jcm14155415
Park HJ, Jeon JH, Song J, Seok H, Choi HK, Choi WS, Choi S, Nam M-H, Suh DH, Kim JG, et al. Enhanced Diagnostic Accuracy for Septic Arthritis Through Multivariate Analysis of Serum and Synovial Biomarkers. Journal of Clinical Medicine. 2025; 14(15):5415. https://doi.org/10.3390/jcm14155415
Chicago/Turabian StylePark, Hyung Jun, Ji Hoon Jeon, Juhyun Song, Hyeri Seok, Hee Kyoung Choi, Won Suk Choi, Sungjae Choi, Myung-Hyun Nam, Dong Hun Suh, Jae Gyoon Kim, and et al. 2025. "Enhanced Diagnostic Accuracy for Septic Arthritis Through Multivariate Analysis of Serum and Synovial Biomarkers" Journal of Clinical Medicine 14, no. 15: 5415. https://doi.org/10.3390/jcm14155415
APA StylePark, H. J., Jeon, J. H., Song, J., Seok, H., Choi, H. K., Choi, W. S., Choi, S., Nam, M.-H., Suh, D. H., Kim, J. G., & Park, D. W. (2025). Enhanced Diagnostic Accuracy for Septic Arthritis Through Multivariate Analysis of Serum and Synovial Biomarkers. Journal of Clinical Medicine, 14(15), 5415. https://doi.org/10.3390/jcm14155415







