HMGB1 as a Key Modulator in Nasal Inflammatory Disorders: A Narrative Review
Abstract
1. Introduction
1.1. HMGB1 Overview
1.2. HMGB1 in the Extracellular Space
1.3. HMGB1 Modifications and Functions
1.4. CRS Overview
1.5. Allergic Rhinitis Overview
2. Materials and Methods
2.1. Research and Screening of Literature
2.2. Inclusion and Exclusion Criteria
3. Results
3.1. HMGB1 and CRS
3.2. HMGB1 and AR
3.3. HMGB1 and Both CRS and AR
3.4. HMGB1 and Non-Specific Nasal Inflammatory Diseases
4. Discussion
4.1. Discrepant Results
4.2. Therapeutic Possibilities
4.2.1. Preclinical Evidence for HMGB1 Inhibition
4.2.2. Challenges in Clinical Translation
4.2.3. Key Considerations for Clinical Trial Design
- The identification of reliable biomarkers, such as (potentially) HMGB1 levels in nasal lavage or tissue samples;
- Standardized clinical outcome measures, including changes in SNOT-22 scores, polyp size, or levels of inflammatory cytokines; and
- Clear inclusion criteria based on clinical phenotype and inflammatory profile.
4.2.4. Future Perspectives
4.3. Challenges Due to Limited Sample Sizes
4.4. Spatiotemporal Dynamics and Mechanistic Implications of HMGB1 Action
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AP-1 | Activator Protein 1 |
| AR | Allergic Rhinitis |
| BAFF | B-cell Activating Factor |
| CRP | C-Reactive Protein |
| CRS | Chronic Rhinosinusitis |
| CRSwNP | Chronic Rhinosinusitis with Nasal Polyps |
| CRSsNP | Chronic Rhinosinusitis without Nasal Polyps |
| CXCL1 | C-X-C Motif Chemokine Ligand 1 |
| ECM | Extracellular Matrix |
| ECRSwNP | Eosinophilic Chronic Rhinosinusitis with Nasal Polyps |
| EMT | Epithelial–Mesenchymal Transition |
| ERK | Extracellular Signal-Regulated Kinase |
| GA | Glycyrrhetinic Acid |
| GSDMD | Gasdermin D |
| HIF-1α | Hypoxia-Inducible Factor 1 Alpha |
| HMGB1 | High Mobility Group Box 1 |
| HPGD | 15-Hydroxyprostaglandin Dehydrogenase |
| HNEC | Human Nasal Epithelial Cells |
| ICAM-1 | Intercellular Adhesion Molecule 1 |
| IFN-γ | Interferon Gamma |
| IgE | Immunoglobulin E |
| IL-1α | Interleukin 1 Alpha |
| IL-1β | Interleukin 1 Beta |
| IL-4 | Interleukin 4 |
| IL-5 | Interleukin 5 |
| IL-6 | Interleukin 6 |
| IL-8 | Interleukin 8 |
| IL-10 | Interleukin 10 |
| IL-13 | Interleukin 13 |
| IL-17A | Interleukin 17A |
| JNK | c-Jun N-terminal Kinase |
| LPS | Lipopolysaccharides |
| MAPK | Mitogen-Activated Protein Kinase |
| MCP-1 | Monocyte Chemoattractant Protein 1 |
| MEK | Mitogen-Activated Protein Kinase |
| MLKL | Mixed Lineage Kinase Domain-Like Protein |
| MUC5AC | Mucin 5AC |
| NAC | N-Acetyl Cysteine |
| NF-κB | Nuclear Factor Kappa-Light-Chain-Enhancer of Activated B Cells |
| NECRSwNP | Non-Eosinophilic Chronic Rhinosinusitis with Nasal Polyps |
| NETs | Neutrophil Extracellular Traps |
| NLS | Nuclear Localization Signals |
| NLRP3 | NOD-, LRR-, and Pyrin Domain-Containing Protein 3 |
| PAI-1 | Plasminogen Activator Inhibitor-1 |
| PGE2 | Prostaglandin E2 |
| PPAR-γ | Peroxisome Proliferator-Activated Receptor Gamma |
| PRRs | Pattern Recognition Receptors |
| RAGE | Receptor for Advanced Glycation End Products |
| RIPK3 | Receptor-Interacting Protein Kinase 3 |
| ROG | Rosiglitazone |
| ROS | Reactive Oxygen Species |
| RNS | Reactive Nitrogen Species |
| SIRT6 | Sirtuin 6 |
| SNAI1 | Snail Family Transcriptional Repressor 1 |
| SNOT-20 | Sino-Nasal Outcome Test-20 |
| TGF-β | Transforming Growth Factor Beta |
| Th1 | T-helper 1 Cells |
| Th2 | T-helper 2 Cells |
| Th17 | T-helper 17 Cells |
| TLR | Toll-Like Receptor |
| TLR2 | Toll-Like Receptor 2 |
| TLR4 | Toll-Like Receptor 4 |
| TLR9 | Toll-Like Receptor 9 |
| TNF-α | Tumor Necrosis Factor Alpha |
| VCAM-1 | Vascular Cell Adhesion Molecule 1 |
| VEGF | Vascular Endothelial Growth Factor |
| Wnt | Wnt Signaling Pathway |
| ZO-1 | Zona Occludens-1 |
References
- Dumitriu, I.E.; Baruah, P.; Manfredi, A.A.; Bianchi, M.E.; Rovere-Querini, P. HMGB1: An immune odyssey. Discov. Med. 2005, 5, 388–392. [Google Scholar] [PubMed]
- Andersson, U.; Erlandsson-Harris, H.; Yang, H.; Tracey, K.J. HMGB1 as a DNA-binding cytokine. J. Leukoc. Biol. 2002, 72, 1084–1091. [Google Scholar] [CrossRef] [PubMed]
- Lange, S.S.; Vasquez, K.M. HMGB1: The jack-of-all-trades protein is a master DNA repair mechanic. Mol. Carcinog. 2009, 48, 571–580. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Nightingale, K.; Dimitrov, S.; Reeves, R.; Wolffe, A.P. Evidence for a shared structural role for HMG1 and linker histones B4 and H1 in organizing chromatin. EMBO J. 1996, 15, 548–561. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- van Beijnum, J.R.; Buurman, W.A.; Griffioen, A.W. Convergence and amplification of toll-like receptor (TLR) and receptor for advanced glycation end products (RAGE) signaling pathways via high mobility group B1 (HMGB1). Angiogenesis 2008, 11, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Xu, L.; Yang, T.; Wang, F. High-mobility group box-1 and its role in angiogenesis. J. Leukoc. Biol. 2014, 95, 563–574. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Kang, R.; Tang, D. The mechanism of HMGB1 secretion and release. Exp. Mol. Med. 2022, 54, 91–102. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yang, H.; Wang, H.; Andersson, U. Targeting Inflammation Driven by HMGB1. Front. Immunol. 2020, 11, 484. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Andersson, U.; Yang, H.; Harris, H. High-mobility group box 1 protein (HMGB1) operates as an alarmin outside as well as inside cells. Semin. Immunol. 2018, 38, 40–48. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Wang, H.; Czura, C.J.; Tracey, K.J. The cytokine activity of HMGB1. J. Leukoc. Biol. 2005, 78, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Ward, M.F.; Sama, A.E.; Wang, H. Extracellular HMGB1 as a proinflammatory cytokine. J. Interf. Cytokine Res. 2004, 24, 329–333. [Google Scholar] [CrossRef] [PubMed]
- Erlandsson Harris, H.; Andersson, U. Mini-review: The nuclear protein HMGB1 as a proinflammatory mediator. Eur. J. Immunol. 2004, 34, 1503–1512. [Google Scholar] [CrossRef] [PubMed]
- Sun, N.K.; Chao, C.C. The cytokine activity of HMGB1—Extracellular escape of the nuclear protein. Chang Gung Med. J. 2005, 28, 673–682. [Google Scholar] [PubMed]
- Bianchi, M.E.; Manfredi, A.A. High-mobility group box 1 (HMGB1) protein at the crossroads between innate and adaptive immunity. Immunol. Rev. 2007, 220, 35–46. [Google Scholar] [CrossRef] [PubMed]
- Ulloa, L.; Messmer, D. High-mobility group box 1 (HMGB1) protein: Friend and foe. Cytokine Growth Factor Rev. 2006, 17, 189–201. [Google Scholar] [CrossRef] [PubMed]
- Kang, R.; Chen, R.; Zhang, Q.; Hou, W.; Wu, S.; Cao, L.; Huang, J.; Yu, Y.; Fan, X.-G.; Yan, Z.; et al. HMGB1 in health and disease. Mol. Asp. Med. 2014, 40, 1–116. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chen, D.; Bellussi, L.M.; Passali, D.; Chen, L. LPS may enhance expression and release of HMGB1 in human nasal epithelial cells in vitro. Acta Otorhinolaryngol. Ital. 2013, 33, 398–404. [Google Scholar] [PubMed]
- Chen, D.; Mao, M.; Bellussi, L.M.; Passali, D.; Chen, L. Increase of high mobility group box chromosomal protein 1 in eosinophilic chronic rhinosinusitis with nasal polyps. Int. Forum Allergy Amp. Rhinol. 2014, 4, 453–462. [Google Scholar] [CrossRef]
- Ferrara, M.; Chialli, G.; Ferreira, L.M.; Ruggieri, E.; Careccia, G.; Preti, A.; Piccirillo, R.; Bianchi, M.E.; Sitia, G.; Venereau, E. Oxidation of HMGB1 Is a Dynamically Regulated Process in Physiological and Pathological Conditions. Front. Immunol. 2020, 11, 1122. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bianchi, M.E.; Crippa, M.P.; Manfredi, A.A.; Mezzapelle, R.; Rovere Querini, P.; Venereau, E. High-mobility group box 1 protein orchestrates responses to tissue damage via inflammation, innate and adaptive immunity, and tissue repair. Immunol. Rev. 2017, 280, 74–82. [Google Scholar] [CrossRef] [PubMed]
- Singh, H.; Agrawal, D.K. Therapeutic Potential of Targeting the HMGB1/RAGE Axis in Inflammatory Diseases. Molecules 2022, 27, 7311. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Paudel, Y.N.; Angelopoulou, E.; Piperi, C.; Balasubramaniam, V.R.; Othman, I.; Shaikh, M.F. Enlightening the role of high mobility group box 1 (HMGB1) in inflammation: Updates on receptor signalling. Eur. J. Pharmacol. 2019, 858, 172487. [Google Scholar] [CrossRef] [PubMed]
- Rauvala, H.; Rouhiainen, A. RAGE as a receptor of HMGB1 (Amphoterin): Roles in health and disease. Curr. Mol. Med. 2007, 7, 725–734. [Google Scholar] [CrossRef] [PubMed]
- Rouhiainen, A.; Kuja-Panula, J.; Tumova, S.; Rauvala, H. RAGE-mediated cell signaling. Methods Mol. Biol. 2013, 963, 239–263. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, Z.A.; Armour, C.L.; Phipps, S.; Sukkar, M.B. RAGE and TLRs: Relatives, friends or neighbours? Mol. Immunol. 2013, 56, 739–744. [Google Scholar] [CrossRef] [PubMed]
- Fiuza, C.; Bustin, M.; Talwar, S.; Tropea, M.; Gerstenberger, E.; Shelhamer, J.H.; Suffredini, A.F. Inflammation-promoting activity of HMGB1 on human microvascular endothelial cells. Blood 2003, 101, 2652–2660. [Google Scholar] [CrossRef] [PubMed]
- Treutiger, C.J.; Mullins, G.E.; Johansson, A.M.; Rouhiainen, A.; Rauvala, H.M.E.; Erlandsson-Harris, H.; Andersson, U.; Yang, H.; Tracey, K.J.; Andersson, J.; et al. High mobility group 1 B-box mediates activation of human endothelium. J. Intern. Med. 2003, 254, 375–385. [Google Scholar] [CrossRef] [PubMed]
- Bernard, N.J. HMGB1+ platelet microparticles damage the endothelium. Nat. Rev. Rheumatol. 2018, 14, 499. [Google Scholar] [CrossRef] [PubMed]
- Leng, Y.; Chen, R.; Chen, R.; He, S.; Shi, X.; Zhou, X.; Zhang, Z.; Chen, A.F. HMGB1 mediates homocysteine-induced endothelial cells pyroptosis via cathepsin V-dependent pathway. Biochem. Biophys. Res. Commun. 2020, 532, 640–646. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Wang, H.; Chavan, S.S.; Andersson, U. High Mobility Group Box Protein 1 (HMGB1): The Prototypical Endogenous Danger Molecule. Mol. Med. 2015, 21 (Suppl. S1), S6–S12. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lee, S.A.; Kwak, M.S.; Kim, S.; Shin, J.S. The role of high mobility group box 1 in innate immunity. Yonsei Med. J. 2014, 55, 1165–1176. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yanai, H.; Ban, T.; Taniguchi, T. High-mobility group box family of proteins: Ligand and sensor for innate immunity. Trends Immunol. 2012, 33, 633–640. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Tracey, K.J. Targeting HMGB1 in inflammation. Biochim. Biophys. Acta (BBA)-Gene Regul. Mech. 2010, 1799, 149–156. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Liu, J.; Song, K.; Lin, B.; Chen, Z.; Zuo, Z.; Fang, Y.; He, Q.; Yao, X.; Liu, Z.; Huang, Q.; et al. HMGB1 promotes neutrophil PD-L1 expression through TLR2 and mediates T cell apoptosis leading to immunosuppression in sepsis. Int. Immunopharmacol. 2024, 133, 112130. [Google Scholar] [CrossRef] [PubMed]
- Lou, H.; Zhang, N.; Bachert, C.; Zhang, L. Highlights of eosinophilic chronic rhinosinusitis with nasal polyps in definition, prognosis, and advancement. Int. Forum Allergy Rhinol. 2018, 8, 1218–1225. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cao, P.P.; Wang, Z.C.; Schleimer, R.P.; Liu, Z. Pathophysiologic mechanisms of chronic rhinosinusitis and their roles in emerging disease endotypes. Ann. Allergy Asthma Immunol. 2019, 122, 33–40. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Vanderhaegen, T.; Gengler, I.; Dendooven, A.; Chenivesse, C.; Lefèvre, G.; Mortuaire, G. Eosinophils in the Field of Nasal Polyposis: Towards a Better Understanding of Biologic Therapies. Clin. Rev. Allergy Immunol. 2022, 62, 90–102. [Google Scholar] [CrossRef] [PubMed]
- Tsuda, T.; Suzuki, M.; Kato, Y.; Kidoguchi, M.; Kumai, T.; Fujieda, S.; Sakashita, M. The current findings in eosinophilic chronic rhinosinusitis. Auris Nasus Larynx 2024, 51, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Bachert, C.; Akdis, C.A. Phenotypes and Emerging Endotypes of Chronic Rhinosinusitis. J. Allergy Clin. Immunol. Pract. 2016, 4, 621–628. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Pan, L.; Liu, Z. Neutrophils as a Protagonist and Target in Chronic Rhinosinusitis. Clin. Exp. Otorhinolaryngol. 2019, 12, 337–347. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Siddiqui, Z.A.; Walker, A.; Pirwani, M.M.; Tahiri, M.; Syed, I. Allergic rhinitis: Diagnosis and management. Br. J. Hosp. Med. 2022, 83, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Ponda, P.; Carr, T.; Rank, M.A.; Bousquet, J. Nonallergic Rhinitis, Allergic Rhinitis, and Immunotherapy: Advances in the Last Decade. J. Allergy Clin. Immunol. Pract. 2023, 11, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Yu, Y.; Zheng, Z.; Cheng, Q. High mobility group box-1: A potential therapeutic target for allergic rhinitis. Eur. J. Med. Res. 2023, 28, 430. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bellussi, L.M.; Iosif, C.; Sarafoleanu, C.; Jianu, E.; Duda, R.; Panaitescu, E.; Passali, F.M.; Passali, D. Are HMGB1 protein expression and secretion markers of upper airways inflammatory diseases? J. Biol. Regul. Homeost. Agents 2013, 27, 791–804. [Google Scholar] [PubMed]
- Pesold, V.V.; Wendler, O.; Morgenthaler, L.; Gröhn, F.; Mueller, S.K. Analysis of CRSsNP Proteome Using a Highly Multiplexed Approach in Nasal Mucus. Am. J. Rhinol. Allergy 2023, 37, 348–359. [Google Scholar] [CrossRef] [PubMed]
- Bellussi, L.M.; Cocca, S.; Chen, L.; Passali, F.M.; Sarafoleanu, C.; Passali, D. Rhinosinusal Inflammation and High Mobility Group Box 1 Protein: A New Target for Therapy. ORL J. Otorhinolaryngol. Relat. Spec. 2016, 78, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Dyer, K.D.; Rosenberg, H.F. Physiologic concentrations of HMGB1 have no impact on cytokine-mediated eosinophil survival or chemotaxis in response to Eotaxin-2 (CCL24). PLoS ONE 2015, 10, e0118887. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Xie, Y.; Li, M.; Chen, K.; Zhu, H.; Tang, M.; Zhou, C.; Zheng, Y.; Wen, J.; Han, M.; Zhang, J.; et al. Necroptosis Underlies Neutrophilic Inflammation Associated with the Chronic Rhinosinusitis with Nasal Polyps (CRSwNP). J. Inflamm. Res. 2021, 14, 3969–3983. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ciprandi, G.; Bellussi, L.M.; Passali, G.C.; Damiani, V.; Passali, D. HMGB1 in nasal inflammatory diseases: A reappraisal 30 years after its discovery. Expert Rev. Clin. Immunol. 2020, 16, 457–463. [Google Scholar] [CrossRef] [PubMed]
- Bellussi, L.M.; Cocca, S.; Passali, G.C.; Passali, D. HMGB1 in the Pathogenesis of Nasal Inflammatory Diseases and its Inhibition as New Therapeutic Approach: A Review from the Literature. Int. Arch. Otorhinolaryngol. 2017, 21, 390–398. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Dzaman, K.; Szczepanski, M.J.; Molinska-Glura, M.; Krzeski, A.; Zagor, M. Expression of the receptor for advanced glycation end products, a target for high mobility group box 1 protein, and its role in chronic recalcitrant rhinosinusitis with nasal polyps. Arch. Immunol. Ther. Exp. 2015, 63, 223–230. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Dzaman, K.; Zagor, M.; Molinska-Glura, M.; Krzeski, A. High motility group box 1 (HMGB1) protein and its receptor for advanced glycation end products (RAGE) expression in chronic rhinosinusitis without nasal polyps. Folia Histochem. Cytobiol. 2015, 53, 70–78. [Google Scholar] [CrossRef] [PubMed]
- Min, H.J.; Kim, S.J.; Kim, T.H.; Chung, H.J.; Yoon, J.H.; Kim, C.H. Level of secreted HMGB1 correlates with severity of inflammation in chronic rhinosinusitis. Laryngoscope 2015, 125, E225–E230. [Google Scholar] [CrossRef] [PubMed]
- Taziki, M.H.; Azarhoush, R.; Taziki, M.M.; Naghavi-Alhosseini, M.; Javid, N.; Davoodi, H. Correlation Between HMGB1 and TLR4 Expression in Sinonasal Mucosa in Patients With Chronic Rhinosinusitis. Ear Nose Throat J. 2019, 98, 599–605. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Cho, J.H.; Park, J.H.; Cho, J.S.; Lee, H.M. High Mobility Group Box Chromosomal Protein-1 Induces Myofibroblast Differentiation and Extracellular Matrix Production via RAGE, p38, JNK and AP-1 Signaling Pathways in Nasal Fibroblasts. Am. J. Rhinol. Allergy 2021, 35, 774–780. [Google Scholar] [CrossRef] [PubMed]
- Hao, W.; Zhu, Y.; Guo, Y.; Wang, H. miR-1287-5p upregulation inhibits the EMT and pro-inflammatory cytokines in LPS-induced human nasal epithelial cells (HNECs). Transpl. Immunol. 2021, 68, 101429, Erratum in Transpl. Immunol. 2022, 71, 101496. [Google Scholar] [CrossRef] [PubMed]
- Choi, T.; Ryu, S.; Bae, J.S.; Yoo, S.H.; Mo, J.H. Epithelial-Mesenchymal Transition in Chronic Rhinosinusitis. J. Rhinol. 2024, 31, 67–77. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yang, P.; Chen, S.; Zhong, G.; Kong, W.; Wang, Y. Agonist of PPAR-γ Reduced Epithelial-Mesenchymal Transition in Eosinophilic Chronic Rhinosinusitis with Nasal Polyps via Inhibition of High Mobility Group Box1. Int. J. Med. Sci. 2019, 16, 1631–1641. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Shimizu, S.; Kouzaki, H.; Kato, T.; Tojima, I.; Shimizu, T. HMGB1-TLR4 signaling contributes to the secretion of interleukin 6 and interleukin 8 by nasal epithelial cells. Am. J. Rhinol. Allergy 2016, 30, 167–172. [Google Scholar] [CrossRef] [PubMed]
- Min, H.J.; Kim, K.S. Expression Pattern of HMGB1 Differs Between Eosinophilic Chronic Rhinosinusitis with Nasal Polyp and Non-Eosinophilic Chronic Rhinosinusitis With Nasal Polyp: A Preliminary Study. Am. J. Rhinol. Allergy 2020, 35, 474–481. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Chen, J.; Chen, J.; Wang, Y. Altered expression of 15-hydroxyprostaglandin dehydrogenase in chronic rhinosinusitis with nasal polyps. Lin Chuang Er Bi Yan Hou Tou Jing Wai Ke Za Zhi (J. Clin. Otorhinolaryngol. Head Neck Surg.) 2023, 37, 891–896. (In Chinese) [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Xu, J.; Lee, J.-W.; Park, S.-K.; Lee, S.-B.; Yoon, Y.-H.; Yeon, S.-H.; Rha, K.-S.; Choi, J.-A.; Song, C.-H.; Kim, Y.M. Toll-like receptor 9 ligands increase type I interferon induced B-cell activating factor expression in chronic rhinosinusitis with nasal polyposis. Clin. Immunol. 2018, 197, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Cavone, L.; Cuppari, C.; Manti, S.; Grasso, L.; Arrigo, T.; Calamai, L.; Salpietro, C.; Chiarugi, A. Increase in the Level of Proinflammatory Cytokine HMGB1 in Nasal Fluids of Patients with Rhinitis and its Sequestration by Glycyrrhizin Induces Eosinophil Cell Death. Clin. Exp. Otorhinolaryngol. 2015, 8, 123–128. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Passali, D.; Cappello, C.; Passali, G.C.; Cingi, C.; Sarafoleanu, C.; Bellussi, L.M. Nasal Muco-ciliary transport time alteration: Efficacy of 18 B Glycyrrhetinic acid. Multidiscip. Respir. Med. 2017, 12, 29. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chen, D.; Bellussi, L.M.; Cocca, S.; Wang, J.; Passali, G.C.; Hao, X.; Chen, L.; Passali, D. Glycyrrhetinic acid suppressed hmgb1 release by up-regulation of Sirt6 in nasal inflammation. J. Biol. Regul. Homeost. Agents 2017, 31, 269–277. [Google Scholar] [PubMed]
- Cho, H.J.; Kim, C.H. Oxygen matters: Hypoxia as a pathogenic mechanism in rhinosinusitis. BMB Rep. 2018, 51, 59–64. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhong, N.; Luo, Q.; Huang, X.; Yu, J.; Ye, J.; Zhang, J. High Mobility Group Box-1 Protein and Interleukin 33 Expression in Allergic Rhinitis. ORL J. Otorhinolaryngol. Relat. Spec. 2022, 84, 315–323. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Cong, J.; Yang, B.; Sun, Y. Association analysis of high-mobility group box-1 protein 1 (HMGB1)/toll-like receptor (TLR) 4 with nasal interleukins in allergic rhinitis patients. Cytokine 2020, 126, 154880. [Google Scholar] [CrossRef] [PubMed]
- Xing, X.; Wang, H. Correlation of serum HMGB1 and HMGB2 levels with clinical symptoms in allergic rhinitis children. Medicine 2023, 102, e34921. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhu, Y.M.; Wu, F.; Zhou, J.Y. Analysis the effect of miR-141-3p/HMGB1 in LPS-induced mucus production and the apoptosis in nasal epithelial cells. Kaohsiung J. Med. Sci. 2020, 36, 622–629. [Google Scholar] [CrossRef] [PubMed]
- Ohwada, K.; Konno, T.; Kohno, T.; Nakano, M.; Ohkuni, T.; Miyata, R.; Kakuki, T.; Kondoh, M.; Takano, K.; Kojima, T. Effects of HMGB1 on Tricellular Tight Junctions via TGF-β Signaling in Human Nasal Epithelial Cells. Int. J. Mol. Sci. 2021, 22, 8390. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zheng, J.; Wei, X.; Zhan, J.B.; Jiang, H.Y. High mobility group box1 contributes to hypoxia-induced barrier dysfunction of nasal epithelial cells. Lin Chuang Er Bi Yan Hou Tou Jing Wai Ke Za Zhi (J. Clin. Otorhinolaryngol. Head Neck Surg.) 2017, 31, 1178–1181. (In Chinese) [Google Scholar] [CrossRef] [PubMed]
- Min, H.J.; Kim, J.H.; Yoo, J.E.; Oh, J.H.; Kim, K.S.; Yoon, J.H.; Kim, C.H. ROS-dependent HMGB1 secretion upregulates IL-8 in upper airway epithelial cells under hypoxic condition. Mucosal Immunol. 2017, 10, 685–694. [Google Scholar] [CrossRef] [PubMed]
- Bianchi, M.E. HMGB1 loves company. J. Leukoc. Biol. 2009, 86, 573–576. [Google Scholar] [CrossRef] [PubMed]
- Hreggvidsdottir, H.S.; Östberg, T.; Wähämaa, H.; Schierbeck, H.; Aveberger, A.-C.; Klevenvall, L.; Palmblad, K.; Ottosson, L.; Andersson, U.; Harris, H.E. The alarmin HMGB1 acts in synergy with endogenous and exogenous danger signals to promote inflammation. J. Leukoc. Biol. 2009, 86, 655–662. [Google Scholar] [CrossRef] [PubMed]
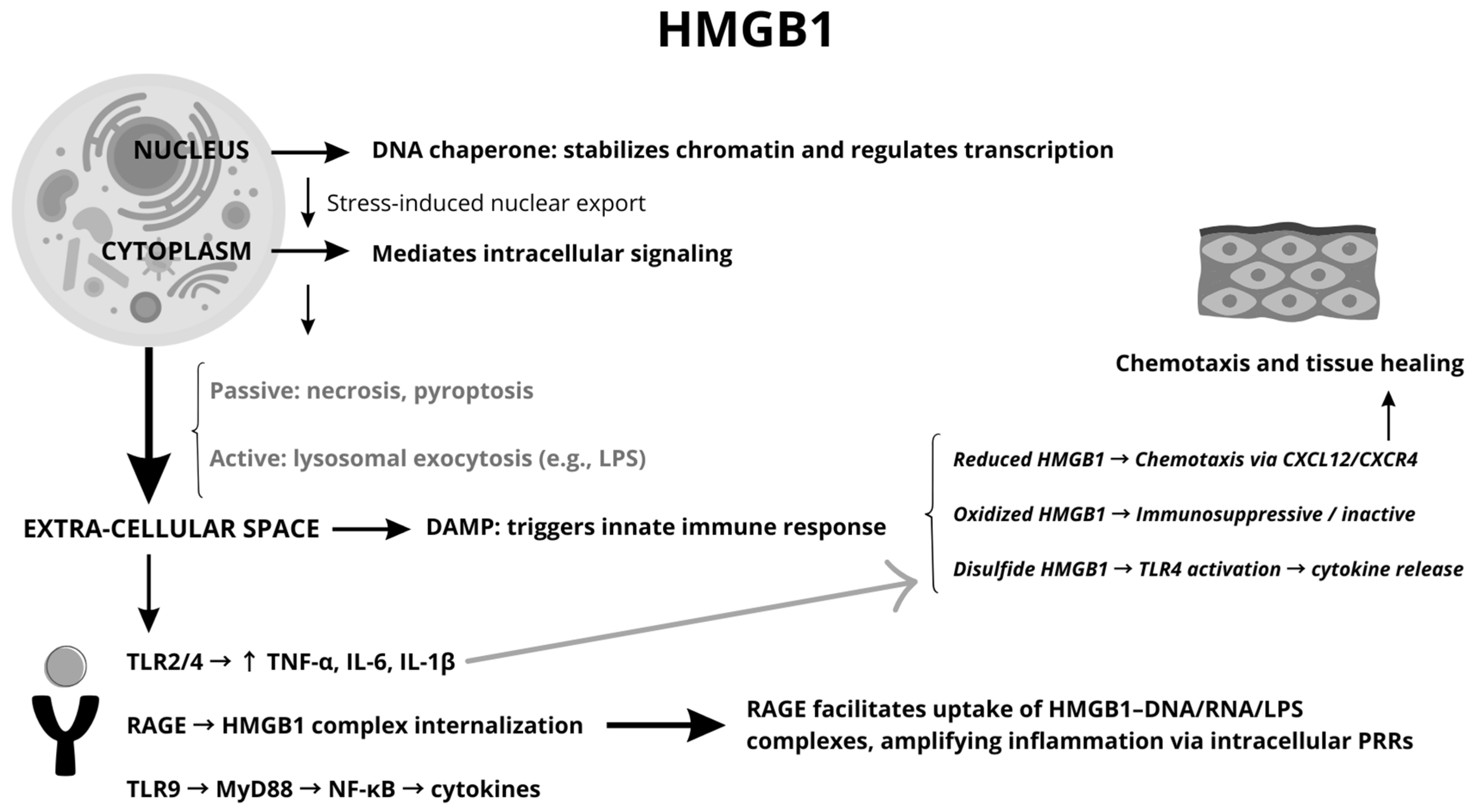

| Feature | Description | Observations |
|---|---|---|
| Discovery | Identified in 1973 as a non-histone protein extracted from calf thymus chromatin; named ‘High Mobility Group’ for its high mobility in gel electrophoresis. | Significant for its rapid migration in electrophoresis and role in chromatin biology. |
| Localization | Nucleus: Functions as a DNA chaperone, maintains chromosomal structure, and regulates transcription. Cytoplasm: Interacts with cellular proteins. Extracellular: Acts as a damage-associated molecular pattern (DAMP) | Functions depend on its subcellular location; extracellular HMGB1 serves as an inflammatory modulator or mediator based on its interactions with the environment |
| Release Mechanisms | Passive: Released during cell death (e.g., necrosis, pyroptosis) as membrane integrity is lost. Active: Secreted via non-conventional pathways (e.g., lysosomal exocytosis) in response to stress signals like LPS. | Non-classical secretion pathways bypass the ER–Golgi system, ensuring release under stress conditions. |
| Post-Translational Modifications | Acetylation, phosphorylation, methylation, and oxidation regulate its translocation from the nucleus to the cytoplasm and extracellular space. | Post-translational modifications determine cellular localization and inflammatory activity of HMGB1. |
| Redox States | Reduced: Promotes chemotaxis by forming complexes with CXCL12. Disulfide: Activates TLR4-mediated inflammation. Overoxidized: Loses pro-inflammatory activity, acting as an immune suppressor. | The redox state is pivotal in defining HMGB1’s role in chemotaxis, cytokine activation, or immune suppression. |
| Cytokines whose release is induced by HMGB1 [6,10] | Tumor Necrosis Factor (TNF) Interleukin-1 (IL-1) Interleukin-6 (IL-6) Interleukin-8 (IL-8) Macrophage Inflammatory Protein-1 (MIP-1) Monocyte Chemoattractant Protein-1 (MCP-1) | TNF: Drives inflammation, immune cell recruitment, and apoptosis. IL-1: Triggers fever, acute inflammation, and leukocyte recruitment. IL-6: Promotes acute-phase protein production and adaptive immunity. IL-8: Attracts neutrophils to inflammation sites. MIP-1: Recruits macrophages and other immune cells. MCP-1: Recruits monocytes, macrophages, and T-cells to sites of inflammation. |
| Receptors | TLR 2 and 4: Drives production of pro-inflammatory cytokines. RAGE: Facilitates endocytosis of HMGB1 and its complexes, amplifying inflammation by delivering cargo to intracellular receptors. TLR9: Triggers cytokine release through MyD88- and NF-κB-dependent pathways. | RAGE’s role in lysosomal disruption links extracellular signals to intracellular inflammatory pathways. |
| Therapeutic Target | Strategies include neutralizing antibodies, TLR4/RAGE inhibitors, and drugs targeting HMGB1 release or interactions. Delayed HMGB1 release offers a therapeutic window in inflammatory diseases. | Promising results in preclinical models for sepsis, chronic inflammation, and autoimmune conditions. |
| Study | Receptor Activity | Type of Study | N of Subjects | Correlation with Severity | Activated Cytokines | Key Findings |
|---|---|---|---|---|---|---|
| Chen S et al., 2023 [61] | RAGE-MEK pathway; upregulation of HPGD | Experimental; in vitro and ex vivo analysis | Control group: 9; NE CRSwNP: 18; ECRSwNP: 13; | Elevated HPGD correlates with CRSwNP severity | PGE2 reduction; no cytokines directly reported | HMGB1 upregulates HPGD via RAGE-MEK; potential therapeutic target. |
| Pesold VV et al., 2023 [45] | HMGB1 significantly upregulated | Proteomic study using clinical samples | Western blot: CRSsNP (n = 25), Controls (n = 23); | N/A | MIP-1β, FOXP3 | HMGB1 identified as non-invasive biomarker for CRSsNP endotyping. |
| Bellussi LM et al., 2016 [46] | RAGE activity highlighted; inhibited by glycyrrhetic acid | Review and experimental study; | 10 biopsies from CRSsNP patients, 31 from CRSwNP patients, and 3 healthy nasal mucosa samples | HMGB1 increased in severe nasal obstruction | TNF-α, IL-5, IL-8 | Glycyrrhetic acid inhibits HMGB1; therapeutic potential for severe cases. |
| Min HJ et al., 2015 [53] | N/A | Cross-sectional study analyzing nasal lavage samples. | 38 CRS patients; Total 63 nasal lavage samples | HMGB1 levels correlate with inflammation severity | IL-8 only significant correlation | HMGB1 correlates strongly with inflammation severity in CRS. |
| Chen D et al., 2017 [65] | SIRT6 downregulated; modulates HMGB1 | Experimental study (in vitro and ex vivo analysis) | N/A | N/A | N/A | SIRT6 depletion triggers HMGB1 release; glycyrrhetinic acid mitigates by enhancing Sirt6 expression; |
| Min HJ & Kim KS, 2021 [60] | Higher HMGB1 in ECRSwNP compared to NECRSwNP | Comparative experimental study | 26 nasal polyp samples from patients with ECRSwNP and NECRSwNP | HMGB1 elevated in severe ECRSwNP | N/A | HMGB1 distinguishes ECRSwNP from NECRSwNP; potential biomarker. |
| Lee SH et al., 2021 [55] | RAGE-mediated signaling; ECM remodeling | Experimental study (in vitro analysis) using human nasal fibroblast cultures | 8 patients | HMGB1 promotes tissue remodeling severity | α-SMA, fibronectin, collagen | HMGB1 drives fibroblast differentiation and ECM production via RAGE. |
| Xu J et al., 2018 [62] | TLR9 activation increases BAFF via HMGB1 | Experimental study (ex vivo and in vitro analysis) | 17 patients with CRSwNP; 10 patients with CRSsNP; 18 control subjects | HMGB1 correlated with BAFF levels in NP tissues | Type-I IFN, BAFF | TLR9 activation via HMGB1 amplifies BAFF and inflammation; HMGB1 plays a role in TLR9-mediated immune activation. |
| Dzaman K et al., 2015 [51] | RAGE overexpressed; linked to inflammation | Comparative observational study | 25 patients with recalcitrant CRSwNP; 26 control subjects | RAGE expression correlates with disease severity | N/A | RAGE linked to severe recalcitrant CRSwNP; therapeutic target potential. |
| Hao W et al., 2021 [56] | miR-1287-5p inhibits HMGB1 and EMT | In vitro experimental study using HNEC cultures | N/A | N/A | IL-6, IL-8, TNF-α | miR-1287-5p inhibits HMGB1 and EMT; Inhibition of HMGB1 using Glycyrrhizin suppressed inflammatory cytokines and EMT in nasal epithelial cells. |
| Xie Y et al., 2021 [48] | HMGB1 as DAMP via necroptosis; RAGE/TLR4 | Comparative experimental study | 48 control subjects; 34 patients with ECRSwNP; 35 patients with NECRSwNP | HMGB1 release correlates with neutrophilic inflammation | IL-1a; IL-6; IL-8, CXCL1, TNF-α, IFN-γ | HMGB1 levels are significantly elevated in CRSwNP tissues; Necroptosis triggers HMGB1 release; promotes neutrophilic inflammation. |
| Choi T et al., 2024 [57] | HMGB1 role in EMT via TGF-β, Wnt | Review study | N/A | HMGB1 drives EMT in severe CRS cases | TNF-α, TGF-β | HMGB1 drives EMT in CRS, promoting tissue remodeling. |
| Taziki MH et al., 2019 [54] | TLR4 correlation with HMGB1 | Basic science study using qRT-PCR | 26 CRS patients; 26 control subjects | HMGB1-TLR4 correlation with inflammation | N/A | HMGB1-TLR4 synergistically amplifies inflammation in CRSwNP. |
| Yang P et al., 2019 [58] | PPAR-γ inhibition of HMGB1-driven EMT | Experimental study (ex vivo and in vitro analysis) | 18 ECRSwNP tissue samples; 12 control nasal mucosa samples | HMGB1-driven EMT correlates with ECRSwNP severity | N-cadherin, vimentin | PPAR-γ agonists inhibit HMGB1-driven EMT in ECRSwNP. |
| Dzaman K et al., 2015 [52] | RAGE overexpression in recalcitrant CRSsNP | Comparative observational study | 37 CRSsNP patients; 26 control subjects | RAGE overexpression correlates with severe inflammation | N/A | RAGE expression correlates with disease severity in CRSsNP. HMGB1 may not be a key differentiating factor in CRSsNP pathogenesis. |
| Cho HJ & Kim CH, 2018 [66] | Hypoxia induces HMGB1 via ROS | Review and experimental study | In vitro studies were conducted HNECs | Hypoxia-induced HMGB1 linked to inflammation severity | MUC5AC, VEGF, IL-8 | Hypoxia induces HMGB1 release, amplifying CRS inflammation; HMGB1 secretion under hypoxia is ROS-dependent, as it was blocked by the ROS scavenger NAC |
| Authors | Key Findings | Implications |
|---|---|---|
| Zhong N et al. [67] | HMGB1 and IL-33 expression were significantly higher in AR nasal mucosa; linked to allergen-induced inflammation. | Suggests HMGB1 and IL-33 as key players in AR inflammation; potential therapeutic targets. |
| Zhu X et al. [68] | HMGB1/TLR4 pathway correlates positively with pro-inflammatory ILs (IL-4, IL-5, IL-13, IL-17A) and negatively with IL-10 in AR. | Highlights HMGB1/TLR4 pathway’s role in AR pathogenesis and its potential for immunotherapy. |
| Zhu YM et al. [70] | miR-141-3p negatively regulates HMGB1 expression, reducing mucus production and apoptosis in LPS-treated cells. | Demonstrates a regulatory axis (miR-141-3p/HMGB1) as a possible therapeutic target for AR. |
| Xing X et al. [69] | Serum HMGB1 and HMGB2 levels correlate with inflammatory markers and clinical severity; potential biomarkers for AR. | Supports HMGB1 and HMGB2 as diagnostic and prognostic biomarkers in AR management. |
| Wu S et al. [43] | HMGB1 contributes to immune dysregulation and inflammation in AR; inhibitors show therapeutic promise in AR models. | Suggests targeting HMGB1 with inhibitors as a novel therapeutic strategy for AR. |
| Pathway | Key Cytokines | Relevance to Disease | Role of HMGB1 |
|---|---|---|---|
| Th1 | IFN-γ, TNF-α | Associated with non-eosinophilic CRS; neutrophilic inflammation | Amplifies cytokine release and neutrophil recruitment through TLR4/RAGE pathways |
| Th2 | IL-4, IL-5, IL-13 | Key driver in CRSwNP and AR; eosinophilic inflammation, IgE production | Promotes Th2 polarization, enhances eosinophil recruitment, and IgE production |
| Th17 | IL-17A, IL-22 | Linked to epithelial barrier dysfunction and severe inflammation | Drives epithelial barrier dysfunction and amplifies IL-17A-mediated inflammation |
| Common Findings | Relevant Studies |
|---|---|
| HMGB1 is consistently upregulated in CRS and AR patients compared to controls. | Pesold VV et al., 2023 [45]; Min HJ et al., 2015 [53]; Bellussi LM et al., 2016 [46]; |
| HMGB1 plays a role in EMT, contributing to tissue remodeling. | Lee SH et al., 2021 [55]; Choi T et al., 2024 [57]; Hao W et al., 2021 [56]; Yang P et al., 2019 [58]; |
| In eosinophilic CRS (ECRSwNP), HMGB1 levels are significantly higher than in non-eosinophilic forms. | Min HJ & Kim KS, 2021 [60]; Xie Y et al., 2021 [48] |
| Glycyrrhizin and glycyrrhetinic acid reduce HMGB1-related inflammation and show therapeutic potential. | Bellussi LM et al., 2016 [46]; Hao W et al., 2021 [56]; Chen D et al., 2017 [65]; |
| HMGB1-TLR4 and HMGB1-TLR9 signaling pathways are linked to increased cytokine production. | Taziki MH et al., 2019 [54]; Xu J et al., 2018 [62] |
| Hypoxia enhances HMGB1 release, further exacerbating inflammation. | Cho HJ & Kim CH, 2018 [66] |
| miRNAs (e.g., miR-1287-5p and miR-141-3p) regulate HMGB1 expression and inflammatory responses. | Hao W et al., 2021 [56]; Zhu YM et al., 2020 [70] |
| HMGB1 may serve as a potential biomarker for differentiating CRS subtypes. | Min HJ & Kim KS, 2021 [60]; Dzaman K et al., 2015 [52] |
| HMGB1-driven inflammation correlates with disease severity | Pesold VV et al., 2023 [45]; Xing X et al., 2023 [69]; Yang P et al., 2019 [58]; Dzaman K et al., 2015 [51]; Cho HJ & Kim CH, 2018 [66]; |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Passali, D.; Bellussi, L.M.; Santantonio, M.; Passali, G.C. HMGB1 as a Key Modulator in Nasal Inflammatory Disorders: A Narrative Review. J. Clin. Med. 2025, 14, 5392. https://doi.org/10.3390/jcm14155392
Passali D, Bellussi LM, Santantonio M, Passali GC. HMGB1 as a Key Modulator in Nasal Inflammatory Disorders: A Narrative Review. Journal of Clinical Medicine. 2025; 14(15):5392. https://doi.org/10.3390/jcm14155392
Chicago/Turabian StylePassali, Desiderio, Luisa Maria Bellussi, Mariaconsiglia Santantonio, and Giulio Cesare Passali. 2025. "HMGB1 as a Key Modulator in Nasal Inflammatory Disorders: A Narrative Review" Journal of Clinical Medicine 14, no. 15: 5392. https://doi.org/10.3390/jcm14155392
APA StylePassali, D., Bellussi, L. M., Santantonio, M., & Passali, G. C. (2025). HMGB1 as a Key Modulator in Nasal Inflammatory Disorders: A Narrative Review. Journal of Clinical Medicine, 14(15), 5392. https://doi.org/10.3390/jcm14155392







