Analysis of Echocardiography and Risk Factors Related to Prognosis in Adult Patients with Isolated Congenitally Corrected Transposition of the Great Arteries
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Clinical Data and Echocardiographic Data
2.3. Tricuspid Valve Replacement/Repair
2.4. Follow-Up
2.5. Statistical Analysis
3. Results
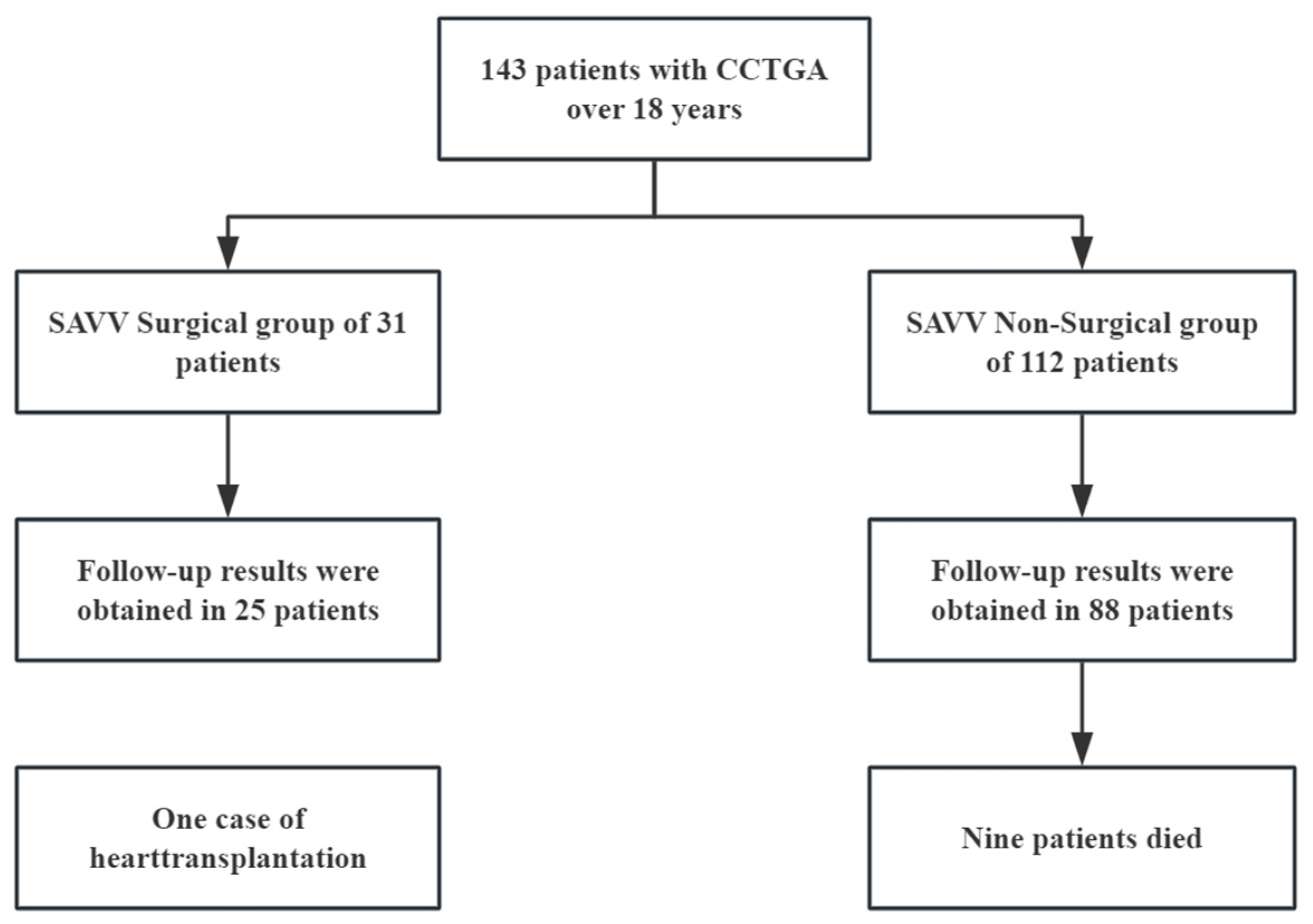
3.1. Study Population (n = 143)
3.2. Anatomical Characteristics of the Overall Population
3.3. Comparison of Initial Visit Echocardiographic Data Between Systemic Atrioventricular Valve Surgical and Non-Surgical Groups
3.4. Comparison of Follow-Up Results Between Surgical and Non-Surgical Groups of Systemic Atrioventricular Valve
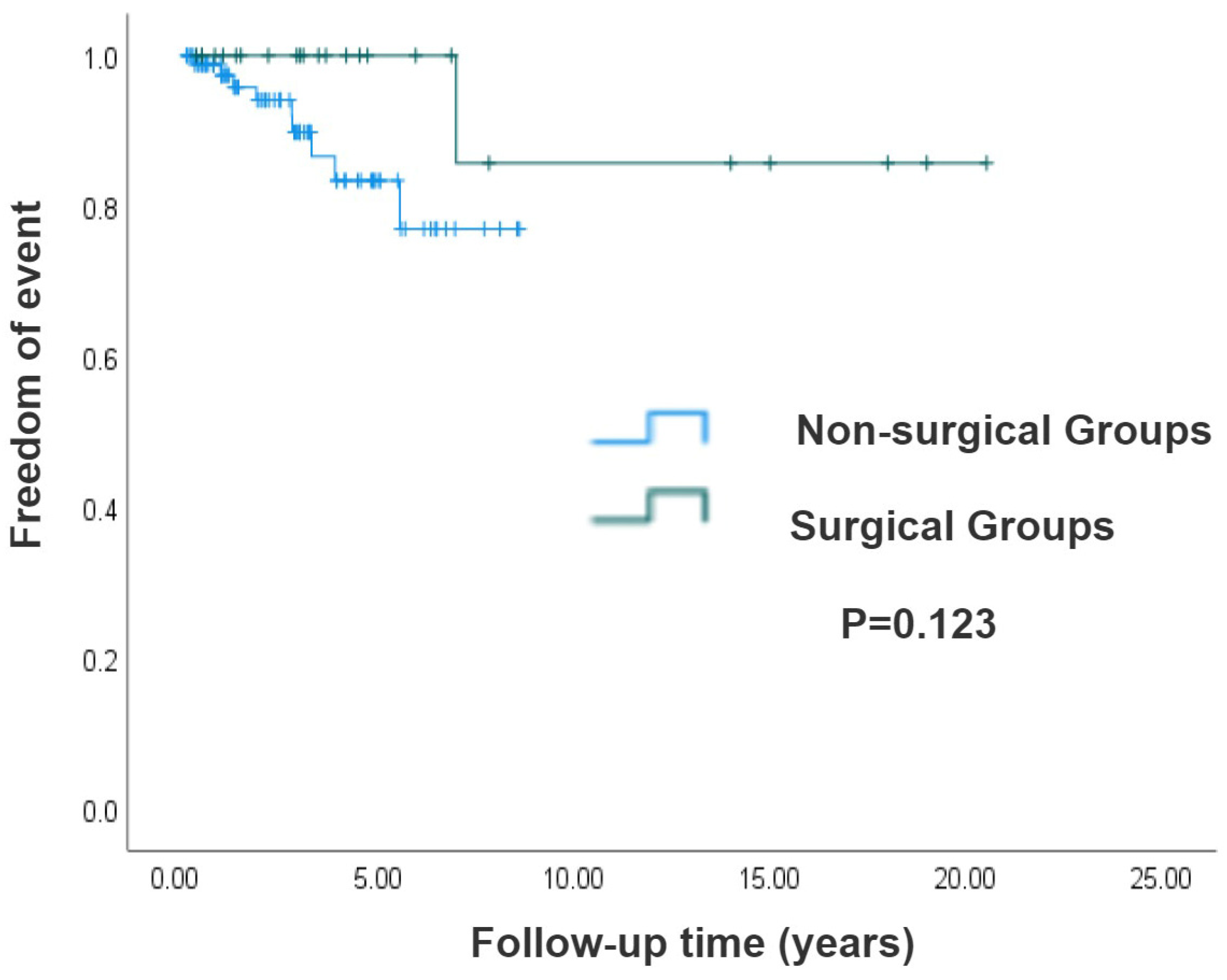
3.5. Survival Analysis of Surgical and Non-Surgical Groups of Systemic Atrioventricular Valve in CCTGA Patients
3.6. Risk Factors Analysis in Isolated CCTGA Patients
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wallis, G.A.; Debich-Spicer, D.; Anderson, R.H. Congenitally corrected transposition. Orphanet J. Rare Dis. 2011, 6, 22. [Google Scholar] [CrossRef] [PubMed]
- Hornung, T.S.; Calder, L. Congenitally corrected transposition of the great arteries. Heart 2010, 96, 1154–1161. [Google Scholar] [CrossRef]
- Presbitero, P.; Somerville, J.; Rabajoli, F.; Stone, S.; Conte, M.R. Corrected transposition of the great arteries without associated defects in adult patients: Clinical profile and follow up. Br. Heart J. 1995, 74, 57–59. [Google Scholar] [CrossRef]
- Beauchesne, L.M.; Warnes, C.A.; Connolly, H.M.; Ammash, N.M.; Tajik, A.J.; Danielson, G.K. Outcome of the unoperated adult who presents with congenitally corrected transposition of the great arteries. J. Am. Coll. Cardiol. 2002, 40, 285–290. [Google Scholar] [CrossRef]
- Abdelrehim, A.A.; Stephens, E.H.; Miranda, W.R.; Todd, A.L.; Connolly, H.M.; Egbe, A.C.; Burchill, L.J.; Ashikhmina, E.A.; Dearani, J.A. Systemic Atrioventricular Valve Surgery in Patients With Congenitally Corrected Transposition of the Great Vessels. J. Am. Coll. Cardiol. 2023, 82, 2197–2208. [Google Scholar] [CrossRef]
- Scherptong, R.W.; Vliegen, H.W.; Winter, M.M.; Holman, E.R.; Mulder, B.J.; van der Wall, E.E.; Hazekamp, M.G. Tricuspid valve surgery in adults with a dysfunctional systemic right ventricle: Repair or replace? Circulation 2009, 119, 1467–1472. [Google Scholar] [CrossRef]
- Westerman, G.R.; Lang, P.; Castaneda, A.R.; Norwood, W.I. Corrected transposition and repair of associated intracardiac defects. Circulation 1982, 66, 197–202. [Google Scholar]
- van Son, J.A.; Danielson, G.K.; Huhta, J.C.; Warnes, C.A.; Edwards, W.D.; Schaff, H.V.; Puga, F.J.; Ilstrup, D.M. Late results of systemic atrioventricular valve replacement in corrected transposition. J. Thorac. Cardiovasc. Surg. 1995, 109, 642–652, discussion 652–653. [Google Scholar] [CrossRef]
- Vahanian, A.; Beyersdorf, F.; Praz, F.; Milojevic, M.; Baldus, S.; Bauersachs, J.; Capodanno, D.; Conradi, L.; De Bonis, M.; De Paulis, R.; et al. Linee guida ESC/EACTS 2021 per il trattamento delle valvulopatie elaborate dalla Task Force per il trattamento delle valvulopatie della Società Europea di Cardiologia (ESC) e dell’Associazione Europea di Chirurgia Cardio-Toracica (EACTS). G. Ital. Di Cardiol. 2022, 23, e1–e75. [Google Scholar] [CrossRef]
- van Dissel, A.C.; Opotowsky, A.R.; Burchill, L.J.; Aboulhosn, J.; Grewal, J.; Lubert, A.M.; Antonova, P.; Shah, S.; Cotts, T.; Antonova, P.; et al. End-stage heart failure in congenitally corrected transposition of the great arteries: A multicentre study. Eur. Heart J. 2023, 44, 3278–3291. [Google Scholar] [CrossRef] [PubMed]
- Rutledge, J.M.; Nihill, M.R.; Fraser, C.D.; Smith, O.E.; McMahon, C.J.; Bezold, L.I. Outcome of 121 patients with congenitally corrected transposition of the great arteries. Pediatr. Cardiol. 2002, 23, 137–145. [Google Scholar] [CrossRef]
- Prieto, L.R.; Hordof, A.J.; Secic, M.; Rosenbaum, M.S.; Gersony, W.M. Progressive tricuspid valve disease in patients with congenitally corrected transposition of the great arteries. Circulation 1998, 98, 997–1005. [Google Scholar] [CrossRef]
- Connelly, M.S.; Liu, P.P.; Williams, W.G.; Webb, G.D.; Robertson, P.; McLaughlin, P.R. Congenitally corrected transposition of the great arteries in the adult: Functional status and complications. J. Am. Coll. Cardiol. 1996, 27, 1238–1243. [Google Scholar] [CrossRef] [PubMed]
- McCombe, A.; Touma, F.; Jackson, D.; Canniffe, C.; Choudhary, P.; Pressley, L.; Tanous, D.; Robinson, P.J.; Celermajer, D. Sudden cardiac death in adults with congenitally corrected transposition of the great arteries. Open Heart 2016, 3, e000407. [Google Scholar] [CrossRef] [PubMed]
- Baumgartner, H.; De Backer, J.; Babu-Narayan, S.V.; Budts, W.; Chessa, M.; Diller, G.P.; Lung, B.; Kluin, J.; Lang, I.M.; Meijboom, F.; et al. 2020 ESC Guidelines for the management of adult congenital heart disease. Eur. Heart J. 2021, 42, 563–645. [Google Scholar] [CrossRef]
- Lundstrom, U.; Bull, C.; Wyse, R.K.; Somerville, J. The natural and “unnatural” history of congenitally corrected transposition. Am. J. Cardiol. 1990, 65, 1222–1229. [Google Scholar] [CrossRef] [PubMed]
- Filippov, A.A.; Del Nido, P.J.; Vasilyev, N.V. Management of Systemic Right Ventricular Failure in Patients With Congenitally Corrected Transposition of the Great Arteries. Circulation 2016, 134, 1293–1302. [Google Scholar] [CrossRef]
- Graham, T.P., Jr.; Bernard, Y.D.; Mellen, B.G.; Celermajer, D.; Baumgartner, H.; Cetta, F.; Connolly, H.M.; Davidson, W.R.; Dellborg, M.; Foster, E.; et al. Long-term outcome in congenitally corrected transposition of the great arteries: A multi-institutional study. J. Am. Coll. Cardiol. 2000, 36, 255–261. [Google Scholar] [CrossRef]
- Adachi, O.; Masaki, N.; Kawatsu, S.; Yoshioka, I.; Masuda, S.; Fujiwara, H.; Akiyama, M.; Kumagai, K.; Kawamoto, S.; Saiki, Y. Long-term results after physiologic repair for congenitally corrected transposition of the great arteries. Gen. Thorac. Cardiovasc. Surg. 2016, 64, 715–721. [Google Scholar] [CrossRef]
- Said, S.M.; Dearani, J.A.; Burkhart, H.M.; Connolly, H.M.; Eidem, B.; Stensrud, P.E.; Schaff, H.V. Management of tricuspid regurgitation in congenital heart disease: Is survival better with valve repair? J. Thorac. Cardiovasc. Surg. 2014, 147, 412–417. [Google Scholar] [CrossRef]
- Lester, S.J.; McElhinney, D.B.; Viloria, E.; Reddy, G.P.; Ryan, E.; Tworetzky, W.; Schiller, N.B.; Foster, E. Effects of losartan in patients with a systemically functioning morphologic rights ventricle after atrial repair of transposition of the great arteries. Am. J. Cardiol. 2001, 88, 1314–1316. [Google Scholar] [CrossRef] [PubMed]
- Dobson, R.; Danton, M.; Nicola, W.; Hamish, W. The natural and unnatural history of the systemic right ventricle in adult survivers. J. Thorac. Cardiovasc. Surg. 2013, 145, 1493–1501, discussion 1501–1503. [Google Scholar] [CrossRef]
- Kral Kollars, C.A.; Gelehrter, S.; Bove, E.L.; Ensing, G. Effects of Morphologic Left Ventricular Pressure on Right Ventricular Geometry and Tricuspid Valve Regurgitation in Patients with Congenitally Corrected Transposition of the Great Arteries. Am. J. Cardiol. 2010, 105, 735–739. [Google Scholar] [CrossRef]
- Hraska, V.; Duncan, B.W.; Mayer, J.E., Jr.; Freed, M.; del Nido, P.J.; Jonas, R.A. Long-term outcome of surgically treated patients with corrected transposition of the great arteries. J. Thorac. Cardiovasc. Surg. 2005, 129, 182–191. [Google Scholar] [CrossRef]
- Mongeon, F.P.; Connolly, H.M.; Dearani, J.A.; Li, Z.; Warnes, C.A. Congenitally corrected transposition of the great arteries ventricular function at the time of systemic atrioventricular valve replacement predicts long-term ventricular function. J. Am. Coll. Cardiol. 2011, 57, 2008–2017. [Google Scholar] [CrossRef]
- Graham, T.P., Jr.; Parrish, M.D.; Boucek, R.J., Jr.; Boerth, R.C.; Breitweser, R.C.; Thompson, S.; Robertson, R.M.; Morgan, J.R.; Friesinger, G.C. Assessment of ventricular size and function in congenitally corrected transposition of the great arteries. Am. J. Cardiol. 1983, 51, 244–251. [Google Scholar] [CrossRef]
- Huhta, J. The natural history of congenitally corrected transposition of the great arteries. World J. Pediatr. Congenit. Heart Surg. 2011, 2, 59–63. [Google Scholar] [CrossRef] [PubMed]
- Graham, T.P., Jr.; Markham, L.; Parra, D.A.; Bichell, D. Congenitally corrected transposition of the great arteries: An update. Curr. Treat Options Cardiovasc. Med. 2007, 9, 407–413. [Google Scholar] [CrossRef]
- Barrios, P.A.; Zia, A.; Pettersson, G.; Najm, H.K.; Rajeswaran, J.; Bhimani, S.; Karamlou, T. Outcomes of treatment pathways in 240 patients with congenitally corrected transposition of great arteries. J. Thorac. Cardiovasc. Surg. 2021, 161, 1080–1093. [Google Scholar] [CrossRef] [PubMed]

| Characteristics | Values |
|---|---|
| Total | 143 |
| Age at initial visit (years) | 42.08 ± 13.83 |
| Sex, men | 74 (51.7) |
| Arrhythmia | 32 (22.4) |
| Pacemaker implanted | 5 (3.5) |
| Atrial fibrillation | 11 (7.7) |
| Supraventricular tachycardia | 6 (4.2) |
| High-degree atrioventricular block | 8 (5.6) |
| Premature ventricular complexes | 2 (1.4) |
| Medication | 45 (31.5) |
| ACEI/ARB | 6 (4.2) |
| Beta-blocker | 8 (5.6) |
| Digoxin | 16 (11.1) |
| Diuretic | 30 (20.1) |
| Anticoagulant | 10 (7.0) |
| Characteristics | Values |
|---|---|
| Segmental anatomy | |
| SLL | 121 (84.6) |
| IDD | 22 (15.4) |
| Cardiac malposition | |
| Dextrocardia | 15 (10.5) |
| Mesocardia | 7 (4.9) |
| Surgical Group | Non-Surgical Group | p Value | |
|---|---|---|---|
| Cases | 23 | 112 | |
| Age (years) | 41.78 ± 11.56 | 42.72 ± 14.08 | 0.765 |
| SAVV regurgitation grade | |||
| Mild | 0 | 39 (34.8) | |
| ≥Moderate | 23 (100) | 73 (65.2) | <0.001 |
| SVEF (%) | 52.26 ± 10.13 | 50.61 ± 11.91 | 0.494 |
| SVEF < 40% | 1 (4.3) | 24 (21.4) | |
| RV diastolic diameter (cm) | 61.65 ± 8.51 | 54.78 ± 10.76 | 0.008 |
| RV systolic diameter (cm) | 46.35 ± 10.69 | 40.22 ± 11.31 | 0.041 |
| LV diastolic diameter (cm) | 44.60 ± 10.52 | 46.00 ± 12.34 | 0.664 |
| LV systolic diameter (cm) | 38.00 ± 4.32 | 28.93 ± 8.93 | 0.056 |
| Pulmonary artery diameter (cm) | 29.13 ± 9.42 | 24.72 ± 6.44 | 0.007 |
| LA anterior–posterior diameter (cm) | 51.75 ± 13.50 | 41.10 ± 9.27 | <0.001 |
| LA superior–inferior diameter (cm) | 67.64 ± 20.83 | 58.95 ± 11.68 | 0.073 |
| LA left–right diameter (cm) | 58.24 ± 19.63 | 49.49 ± 10.42 | 0.061 |
| RA superior–inferior diameter (cm) | 47.82 ± 13.62 | 46.39 ± 9.35 | 0.614 |
| RA left–right diameter (cm) | 38.59 ± 13.63 | 38.52 ± 7.86 | 0.985 |
| Surgical Group | Non-Surgical Group | p Value | |
|---|---|---|---|
| Total follow-up time (years) | 3.62 ± 3.70 | ||
| Follow-up time (years) | 6.08 ± 6.19 | 2.92 ± 2.19 | 0.019 |
| Number of patients followed | 25 | 88 | |
| Mild regurgitation | 4 (16.0) | 20 (22.7) | 0.468 |
| ≥Moderate regurgitation | 4 (16.0) | 50 (56.8) | <0.001 |
| LA anterior–posterior diameter (cm) | 46.86 ± 8.34 | 40.93 ± 6.92 | 0.001 |
| RV end-diastolic diameter (cm) | 57.26 ± 11.25 | 54.59 ± 9.35 | 0.254 |
| RV end-systolic diameter (cm) | 40.93 ± 10.87 | 40.27 ± 10.28 | 0.832 |
| SVEF (%) | 47.40 ± 11.55 | 51.89 ± 9.91 | 0.060 |
| SVEF < 40% | 7 (28.0) | 11 (12.5) | 0.119 |
| Death/heart transplantation | 1 (4.0) | 9 (10.2) | 0.570 |
| Univariate Analysis | Hazard Ratio | 95% Confidence Interval | p Value |
|---|---|---|---|
| Age (years) | 1.068 | 1.019–1.120 | 0.006 |
| Women | 1.472 | 0.228–4.566 | 0.548 |
| Surgery | 0.217 | 0.049–3.413 | 0.155 |
| RV end-diastolic diameter (cm) | 1.110 | 1.019–1.154 | <0.001 |
| RV end-systolic diameter (cm) | 1.114 | 1.028–1.164 | <0.001 |
| LV end-diastolic diameter (cm) | 1.057 | 1.010–1.171 | 0.047 |
| LV end-systolic diameter (cm) | 1.122 | 1.016–1.234 | 0.021 |
| Pulmonary artery diameter (cm) | 1.046 | 0.993–1.107 | 0.088 |
| LA anterior–posterior diameter (cm) | 1.111 | 1.007–1.225 | 0.036 |
| LA superior–inferior diameter (cm) | 1.023 | 0.966–1.083 | 0.435 |
| LA left–right diameter (cm) | 1.055 | 1.000–1.113 | 0.050 |
| RA superior–inferior diameter (cm) | 1.033 | 0.986–1.083 | 0.169 |
| RA left–right diameter (cm) | 1.028 | 0.970–1.089 | 0.353 |
| SVEF (%) | 0.866 | 0.808–0.928 | <0.001 |
| Severe regurgitation | 0.017 | 0.000–7.874 | 0.194 |
| ≥Moderate regurgitation | 0.033 | 0.000–68.304 | 0.382 |
| Multivariate analysis | |||
| Age (years) | 1.103 | 1.021–1.191 | 0.013 |
| RV end-diastolic diameter (cm) | 1.116 | 1.018–1.224 | 0.020 |
| SVEF (%) | 0.909 | 0.851–0.971 | 0.005 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, L.; Wu, Y.; Xie, J.; Ruan, Y.; Hao, X.; Wang, H.; Zhang, Y.; Han, J.; He, Y.; Gu, X. Analysis of Echocardiography and Risk Factors Related to Prognosis in Adult Patients with Isolated Congenitally Corrected Transposition of the Great Arteries. J. Clin. Med. 2025, 14, 5313. https://doi.org/10.3390/jcm14155313
Zhang L, Wu Y, Xie J, Ruan Y, Hao X, Wang H, Zhang Y, Han J, He Y, Gu X. Analysis of Echocardiography and Risk Factors Related to Prognosis in Adult Patients with Isolated Congenitally Corrected Transposition of the Great Arteries. Journal of Clinical Medicine. 2025; 14(15):5313. https://doi.org/10.3390/jcm14155313
Chicago/Turabian StyleZhang, Lixin, Yuduo Wu, Jiaoyang Xie, Yanping Ruan, Xiaoyan Hao, Hairui Wang, Ye Zhang, Jiancheng Han, Yihua He, and Xiaoyan Gu. 2025. "Analysis of Echocardiography and Risk Factors Related to Prognosis in Adult Patients with Isolated Congenitally Corrected Transposition of the Great Arteries" Journal of Clinical Medicine 14, no. 15: 5313. https://doi.org/10.3390/jcm14155313
APA StyleZhang, L., Wu, Y., Xie, J., Ruan, Y., Hao, X., Wang, H., Zhang, Y., Han, J., He, Y., & Gu, X. (2025). Analysis of Echocardiography and Risk Factors Related to Prognosis in Adult Patients with Isolated Congenitally Corrected Transposition of the Great Arteries. Journal of Clinical Medicine, 14(15), 5313. https://doi.org/10.3390/jcm14155313








