Impact of Metabolic Syndrome on Renal and Cardiovascular Outcomes in Renal Transplant Recipients: A Single-Center Study in Japan
Abstract
1. Introduction
2. Subjects and Methods
2.1. Diagnostic Criteria for MetS
2.2. Patients
2.3. Measurements
2.4. Definition of Cardiovascular Disease at Baseline
2.5. Outcomes
2.6. Statistical Analysis
3. Results
3.1. Diagnosis of MetS Using Four Criteria
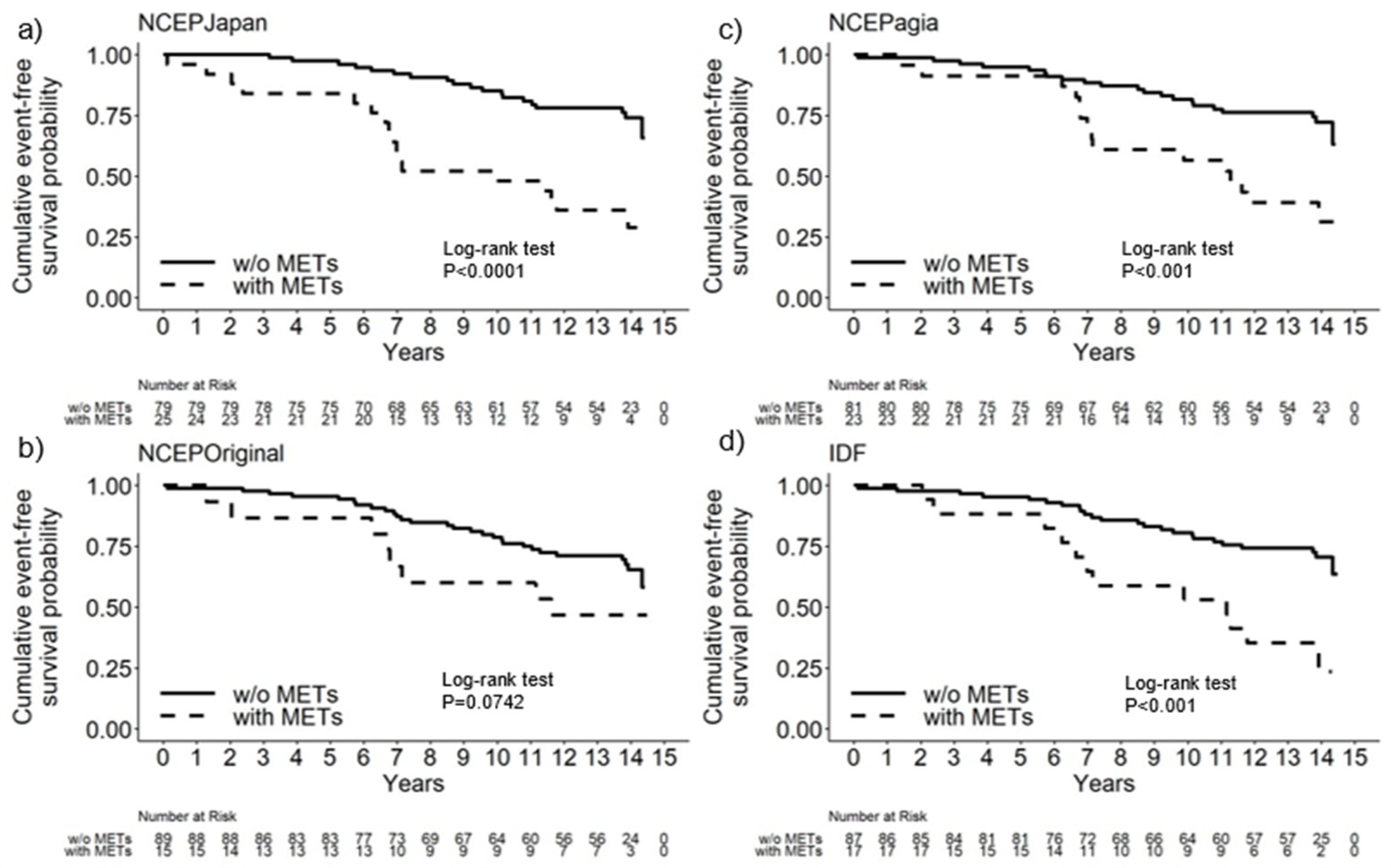
3.2. Cumulative Event-Free Survival Probability Using Each Set of Diagnostic Criteria for Mets
3.3. Impact of MetS on Composite Vascular Events Depends on Diagnostic Criteria
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Eckel, R.H.; Grundy, S.M.; Zimmet, P.Z. The metabolic syndrome. Lancet 2005, 365, 1415–1428. [Google Scholar] [CrossRef]
- Chen, J.; Muntner, P.; Hamm, L.L.; Jones, D.W.; Batuman, V.; Fonseca, V.; Whelton, P.K.; He, J. The metabolic syndrome and chronic kidney disease in U.S. adults. Ann. Intern. Med. 2004, 140, 167–174. [Google Scholar] [CrossRef]
- Ford, E.S.; Giles, W.H.; Mokdad, A.H. Increasing prevalence of the metabolic syndrome among u.s. Adults. Diabetes Care 2004, 27, 2444–2449. [Google Scholar] [CrossRef]
- Tanaka, H.; Shiohira, Y.; Uezu, Y.; Higa, A.; Iseki, K. Metabolic syndrome and chronic kidney disease in Okinawa, Japan. Kidney Int. 2006, 69, 369–374. [Google Scholar] [CrossRef]
- Kim, C.S.; Choi, J.S.; Bae, E.H.; Ma, S.K.; Ahn, Y.K.; Jeong, M.H.; Kim, Y.J.; Cho, M.C.; Kim, C.J.; Kim, S.W.; et al. Association of metabolic syndrome and renal insufficiency with clinical outcome in acute myocardial infarction. Metabolism 2013, 62, 669–676. [Google Scholar] [CrossRef]
- Chien, K.L.; Hsu, H.C.; Lee, Y.T.; Chen, M.F. Renal function and metabolic syndrome components on cardiovascular and all-cause mortality. Atherosclerosis 2008, 197, 860–867. [Google Scholar] [CrossRef]
- Lin, J.H.; Wu, H.C.; Huang, W.H.; Lu, C.L.; Cheng, M.H.; Wang, H.T.; Yen, T.H.; Wang, W.J. Association between management of metabolic syndrome and progression of early-stage chronic kidney disease: An observational cohort study. Ren. Fail. 2015, 37, 29–36. [Google Scholar] [CrossRef]
- Navaneethan, S.D.; Schold, J.D.; Kirwan, J.P.; Arrigain, S.; Jolly, S.E.; Poggio, E.D.; Beddhu, S.; Nally, J.V., Jr. Metabolic syndrome, ESRD, and death in CKD. Clin. J. Am. Soc. Nephrol. 2013, 8, 945–952. [Google Scholar] [CrossRef]
- Campistol, J.M.; Romero, R.; Paul, J.; Gutierrez-Dalmau, A. Epidemiology of arterial hypertension in renal transplant patients: Changes over the last decade. Nephrol. Dial. Transplant. 2004, 19 (Suppl. S3), iii62–iii66. [Google Scholar] [CrossRef]
- Dugonjic-Taletovic, M.; Tulumovic, D.; Aleckovic-Halilovic, M.; Pjanic, M.; Hajder, M.; Halilcevic-Terzic, A.; Loncar, D.; Jasarevic, A. Single-centre experience with the treatment of high-prevalence metabolic syndrome in kidney transplant patients in Bosnia and Herzegovina. Med. Glas. 2024, 21, 85–90. [Google Scholar] [CrossRef]
- Hecking, M.; Sharif, A.; Eller, K.; Jenssen, T. Management of post-transplant diabetes: Immunosuppression, early prevention, and novel antidiabetics. Transpl. Int. 2021, 34, 27–48. [Google Scholar] [CrossRef]
- Meier-Kriesche, H.U.; Schold, J.D.; Srinivas, T.R.; Reed, A.; Kaplan, B. Kidney transplantation halts cardiovascular disease progression in patients with end-stage renal disease. Am. J. Transplant. 2004, 4, 1662–1668. [Google Scholar] [CrossRef]
- Piotti, G.; Gandolfini, I.; Palmisano, A.; Maggiore, U. Metabolic risk profile in kidney transplant candidates and recipients. Nephrol. Dial. Transplant. 2019, 34, 388–400. [Google Scholar] [CrossRef]
- Ponticelli, C.; Arnaboldi, L.; Moroni, G.; Corsini, A. Treatment of dyslipidemia in kidney transplantation. Expert. Opin. Drug Saf. 2020, 19, 257–267. [Google Scholar] [CrossRef]
- Porrini, E.; Delgado, P.; Bigo, C.; Alvarez, A.; Cobo, M.; Checa, M.D.; Hortal, L.; Fernandez, A.; Garcia, J.J.; Velazquez, S.; et al. Impact of metabolic syndrome on graft function and survival after cadaveric renal transplantation. Am. J. Kidney Dis. 2006, 48, 134–142. [Google Scholar] [CrossRef]
- Sgambat, K.; Clauss, S.; Moudgil, A. Cardiovascular effects of metabolic syndrome after transplantation: Convergence of obesity and transplant-related factors. Clin. Kidney J. 2018, 11, 136–146. [Google Scholar] [CrossRef]
- Tantisattamo, E.; Ho, B.T.; Workeneh, B.T. Editorial: Metabolic Changes After Kidney Transplantation. Front. Med. 2021, 8, 709644. [Google Scholar] [CrossRef]
- Vincenti, F.; Friman, S.; Scheuermann, E.; Rostaing, L.; Jenssen, T.; Campistol, J.M.; Uchida, K.; Pescovitz, M.D.; Marchetti, P.; Tuncer, M.; et al. Results of an international, randomized trial comparing glucose metabolism disorders and outcome with cyclosporine versus tacrolimus. Am. J. Transplant. 2007, 7, 1506–1514. [Google Scholar] [CrossRef]
- Armstrong, K.A.; Campbell, S.B.; Hawley, C.M.; Nicol, D.L.; Johnson, D.W.; Isbel, N.M. Obesity is associated with worsening cardiovascular risk factor profiles and proteinuria progression in renal transplant recipients. Am. J. Transplant. 2005, 5, 2710–2718. [Google Scholar] [CrossRef]
- de Vries, A.P.; Bakker, S.J.; van Son, W.J.; van der Heide, J.J.; Ploeg, R.J.; The, H.T.; de Jong, P.E.; Gans, R.O. Metabolic syndrome is associated with impaired long-term renal allograft function; not all component criteria contribute equally. Am. J. Transplant. 2004, 4, 1675–1683. [Google Scholar] [CrossRef]
- Israni, A.K.; Snyder, J.J.; Skeans, M.A.; Kasiske, B.L.; Investigators, P. Clinical diagnosis of metabolic syndrome: Predicting new-onset diabetes, coronary heart disease, and allograft failure late after kidney transplant. Transpl. Int. 2012, 25, 748–757. [Google Scholar] [CrossRef]
- Lee, Y.H.; Song, S.H.; Song, S.H.; Shin, H.S.; Yang, J.; Kim, M.S.; Hwang, H.S.; Group, K.S. Clinical implications of changes in metabolic syndrome status after kidney transplantation: A nationwide prospective cohort study. Nephrol. Dial. Transplant. 2023, 38, 2743–2753. [Google Scholar] [CrossRef]
- Potluri, K.; Hou, S. Obesity in kidney transplant recipients and candidates. Am. J. Kidney Dis. 2010, 56, 143–156. [Google Scholar] [CrossRef]
- Prasad, G.V.; Huang, M.; Silver, S.A.; Al-Lawati, A.I.; Rapi, L.; Nash, M.M.; Zaltzman, J.S. Metabolic syndrome definitions and components in predicting major adverse cardiovascular events after kidney transplantation. Transpl. Int. 2015, 28, 79–88. [Google Scholar] [CrossRef]
- Kishikawa, H.; Nishimura, K.; Kato, T.; Kobayashi, Y.; Arichi, N.; Okuno, A.; Fujii, N.; Kyo, M.; Takahara, S.; Ichikawa, Y. Prevalence of the metabolic syndrome in kidney transplantation. Transplant. Proc. 2009, 41, 181–183. [Google Scholar] [CrossRef]
- Naganuma, T.; Uchida, J.; Kinoshita, Y.; Kuroki, Y.; Takemoto, Y.; Yoshimura, R.; Sugimura, K.; Nakatani, T. The prevalence of metabolic syndrome in Japanese renal transplant recipients. Nephrology 2007, 12, 413–417. [Google Scholar] [CrossRef]
- Alberti, K.G.; Zimmet, P.; Shaw, J.; IDF Epidemiology Task Force Consensus Group. The metabolic syndrome--a new worldwide definition. Lancet 2005, 366, 1059–1062. [Google Scholar] [CrossRef]
- Grundy, S.M.; Brewer, H.B., Jr.; Cleeman, J.I.; Smith, S.C., Jr.; Lenfant, C.; American Heart, A.; National Heart, L.; Blood, I. Definition of metabolic syndrome: Report of the National Heart, Lung, and Blood Institute/American Heart Association conference on scientific issues related to definition. Circulation 2004, 109, 433–438. [Google Scholar] [CrossRef]
- Friedewald, W.T.; Levy, R.I.; Fredrickson, D.S. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin. Chem. 1972, 18, 499–502. [Google Scholar] [CrossRef]
| Modified NCEP-ATPIII Criteria for Japanese | Original NCEP-ATPIII Criteria | Modified NCEP-ATPIII Criteria for Asians | IDF Criteria for Japanese | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Level | No | Yes | No | Yes | No | Yes | No | Yes | |
| N | 79 | 25 | 89 | 15 | 81 | 23 | 87 | 17 | |
| Underlying diseases % (freq) | Diabetic Nephropathy | 2.5 (2) | 16.0 (4) | 5.6 (5) | 6.7 (1) | 2.5 (2) | 17.4 (4) | 3.4 (3) | 17.6 (3) |
| Others | 97.5 (77) | 84.0 (21) | 94.4 (84) | 93.3 (14) | 97.5 (79) | 82.6 (19) | 96.6 (84) | 82.4 (14) | |
| Age (median [IQR]) | years | 41.00 [35.00, 53.00] | 54.00 [47.00, 56.00] | 45.00 [35.00, 54.00] | 52.00 [43.00, 55.50] | 43.00 [35.00, 53.00] | 55.00 [47.00, 56.00] | 43.00 [35.00, 54.00] | 54.00 [51.00, 56.00] |
| Sex % (freq) | female | 43.0 (34) | 12.0 (3) | 38.2 (34) | 20.0 (3) | 40.7 (33) | 17.4 (4) | 41.4 (36) | 5.9 (1) |
| male | 57.0 (45) | 88.0 (22) | 61.8 (55) | 80.0 (12) | 59.3 (48) | 82.6 (19) | 58.6 (51) | 94.1 (16) | |
| eGFR (median [IQR]) | mL/min/1.73 m2 | 49.23 [40.88, 57.37] | 46.41 [38.39, 51.05] | 49.23 [40.37, 57.10] | 43.88 [39.65, 54.33] | 48.55 [40.01, 56.57] | 49.60 [41.22, 55.84] | 48.55 [39.75, 57.37] | 49.60 [42.29, 53.21] |
| CVD history % (freq) | absence | 91.1 (72) | 76.0 (19) | 87.6 (78) | 86.7 (13) | 88.9 (72) | 82.6 (19) | 90.8 (79) | 70.6 (12) |
| presence | 8.9 (7) | 24.0 (6) | 12.4 (11) | 13.3 (2) | 11.1 (9) | 17.4 (4) | 9.2 (8) | 29.4 (5) | |
| CRP (median [IQR]) | mg/dL | 0.02 [0.01, 0.04] | 0.05 [0.04, 0.11] | 0.02 [0.01, 0.06] | 0.04 [0.03, 0.06] | 0.02 [0.01, 0.05] | 0.04 [0.04, 0.10] | 0.02 [0.01, 0.05] | 0.05 [0.03, 0.14] |
| Proteinuria % (freq) | absence | 88.6 (70) | 76.0 (19) | 86.5 (77) | 80.0 (12) | 87.7 (71) | 78.3 (18) | 87.4 (76) | 76.5 (13) |
| presence | 11.4 (9) | 24.0 (6) | 13.5 (12) | 20.0 (3) | 12.3 (10) | 21.7 (5) | 12.6 (11) | 23.5 (4) | |
| Hemodialysis duration (median [IQR]) | years | 2.00 [0.83, 6.50] | 3.00 [1.43, 4.35] | 2.08 [1.00, 5.80] | 3.67 [1.80, 9.34] | 2.03 [0.92, 6.25] | 3.00 [1.38, 6.07] | 2.26 [0.88, 6.33] | 3.11 [1.43, 4.35] |
| CNI % (freq) | cyclosporine | 57.0 (45) | 80.0 (20) | 59.6 (53) | 80.0 (12) | 55.6 (45) | 87.0 (20) | 58.6 (51) | 82.4 (14) |
| tacrolimus | 43.0 (34) | 20.0 (5) | 40.4 (36) | 20.0 (3) | 44.4 (36) | 13.0 (3) | 41.4 (36) | 17.6 (3) | |
| AR % (freq) | absence | 77.2 (61) | 84.0 (21) | 78.7 (70) | 80.0 (12) | 76.5 (62) | 87.0 (20) | 78.2 (68) | 82.4 (14) |
| presence | 22.8 (18) | 16.0 (4) | 21.3 (19) | 20.0 (3) | 23.5 (19) | 13.0 (3) | 21.8 (19) | 17.6 (3) | |
| Donor % (freq) | living donor | 83.5 (66) | 56.0 (14) | 83.1 (74) | 40.0 (6) | 84.0 (68) | 52.2 (12) | 81.6 (71) | 52.9 (9) |
| deceased donor | 16.5 (13) | 44.0 (11) | 16.9 (15) | 60.0 (9) | 16.0 (13) | 47.8 (11) | 18.4 (16) | 47.1 (8) | |
| LDL (median [IQR]) | mg/dL | 113.20 [91.50, 131.60] | 114.20 [95.00, 131.20] | 113.60 [92.80, 131.80] | 112.60 [92.80, 120.00] | 111.20 [91.20, 131.40] | 116.20 [99.70, 131.60] | 113.60 [91.70, 130.50] | 112.60 [95.00, 132.00] |
| Post-transplant duration (median [IQR]) | years | 3.98 [2.33, 9.73] | 6.31 [2.21, 9.97] | 4.14 [2.29, 9.59] | 5.11 [2.24, 12.46] | 4.14 [2.38, 9.3] | 5.11 [2.14, 10.92] | 4.14 [2.33, 9.9] | 5.11 [2.06, 9.63] |
| Uric acid level (median [IQR]) | mg/dL | 6.7 [5.6, 7.9] | 7.1 [6.7, 8.0] | 6.8 [5.6, 7.9] | 7.1 [6.8, 8.1] | 6.7 [5.5, 7.8] | 7.2 [6.8, 8.1] | 7.0 [5.6, 8.0] | 7.1 [6.7, 7.9] |
| Calcium level (median [IQR]) | mg/dL | 9.7 [9.50, 10.00] | 9.8 [9.50, 10.30] | 9.7 [9.50, 10.00] | 9.9 [9.75, 10.35] | 9.7 [9.50, 10.00] | 9.9 [9.35, 10.30] | 9.7 [9.50, 10.00] | 9.9 [9.50, 10.30] |
| Phosphorus level (median [IQR]) | mg/dL | 3.1 [2.7, 3.4] | 3.1 [2.7, 3.6] | 3.1 [2.7, 3.4] | 3.1 [2.6, 3.5] | 3.1 [2.7, 3.4] | 3.0 [2.7, 3.5] | 3.1 [2.7, 3.4] | 3.2 [2.7, 3.5] |
| Use of antiplatelet drugs % (freq) | No | 77.2 (61) | 68.0 (17) | 76.4 (68) | 66.7 (10) | 77.8 (63) | 82.6 (19) | 74.7 (65) | 76.5 (13) |
| Yes | 22.8 (18) | 32.0 (8) | 23.6 (21) | 33.3 (5) | 22.2 (18) | 17.4 (4) | 25.3 (22) | 23.5 (4) | |
| Scale | HR | 95% Confidence Interval | p | p for Interaction | |
|---|---|---|---|---|---|
| Lower | Upper | ||||
| NCEP-ATPIIIJapan | 2.78 | 1.15 | 6.75 | 0.024 | 0.371 |
| NCEP-ATPIIIOriginal | 2.65 | 1.04 | 6.8 | 0.042 | |
| NCEP-ATPIIIagia | 2.37 | 0.93 | 6.01 | 0.07 | |
| IDF | 1.91 | 0.77 | 4.75 | 0.164 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Naganuma, T.; Iwai, T.; Kabata, D.; Machida, Y.; Takemoto, Y.; Uchida, J. Impact of Metabolic Syndrome on Renal and Cardiovascular Outcomes in Renal Transplant Recipients: A Single-Center Study in Japan. J. Clin. Med. 2025, 14, 5303. https://doi.org/10.3390/jcm14155303
Naganuma T, Iwai T, Kabata D, Machida Y, Takemoto Y, Uchida J. Impact of Metabolic Syndrome on Renal and Cardiovascular Outcomes in Renal Transplant Recipients: A Single-Center Study in Japan. Journal of Clinical Medicine. 2025; 14(15):5303. https://doi.org/10.3390/jcm14155303
Chicago/Turabian StyleNaganuma, Toshihide, Tomoaki Iwai, Daijiro Kabata, Yuichi Machida, Yoshiaki Takemoto, and Junji Uchida. 2025. "Impact of Metabolic Syndrome on Renal and Cardiovascular Outcomes in Renal Transplant Recipients: A Single-Center Study in Japan" Journal of Clinical Medicine 14, no. 15: 5303. https://doi.org/10.3390/jcm14155303
APA StyleNaganuma, T., Iwai, T., Kabata, D., Machida, Y., Takemoto, Y., & Uchida, J. (2025). Impact of Metabolic Syndrome on Renal and Cardiovascular Outcomes in Renal Transplant Recipients: A Single-Center Study in Japan. Journal of Clinical Medicine, 14(15), 5303. https://doi.org/10.3390/jcm14155303





