Planning and Problem-Solving Impairments in Fibromyalgia: The Predictive Role of Updating, Inhibition, and Mental Flexibility
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Procedure
2.2.1. Neuropsychological Assessment
2.2.2. Clinical Assessment
2.3. Statistical Analysis
3. Results
3.1. Group Differences in Clinical Measures
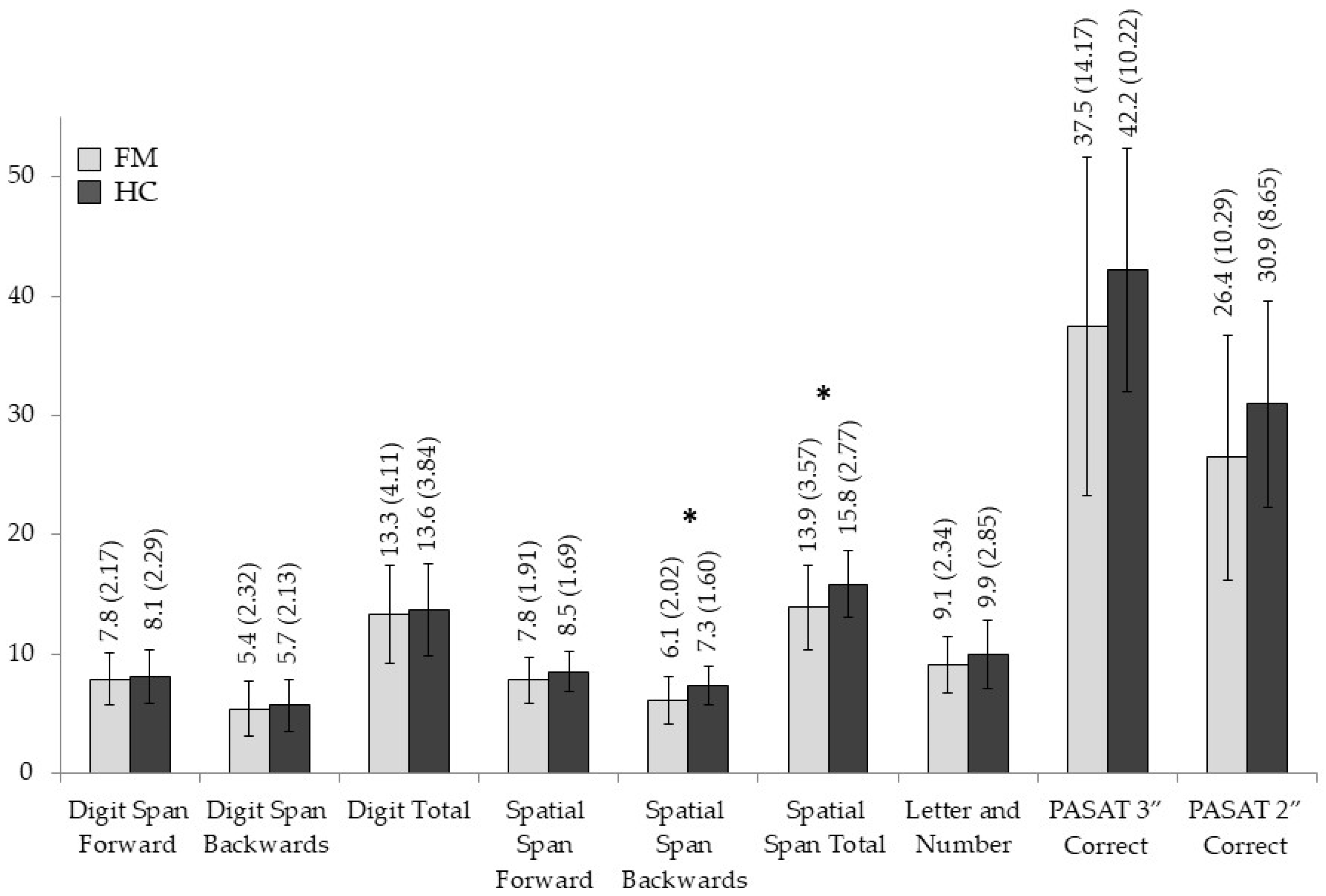
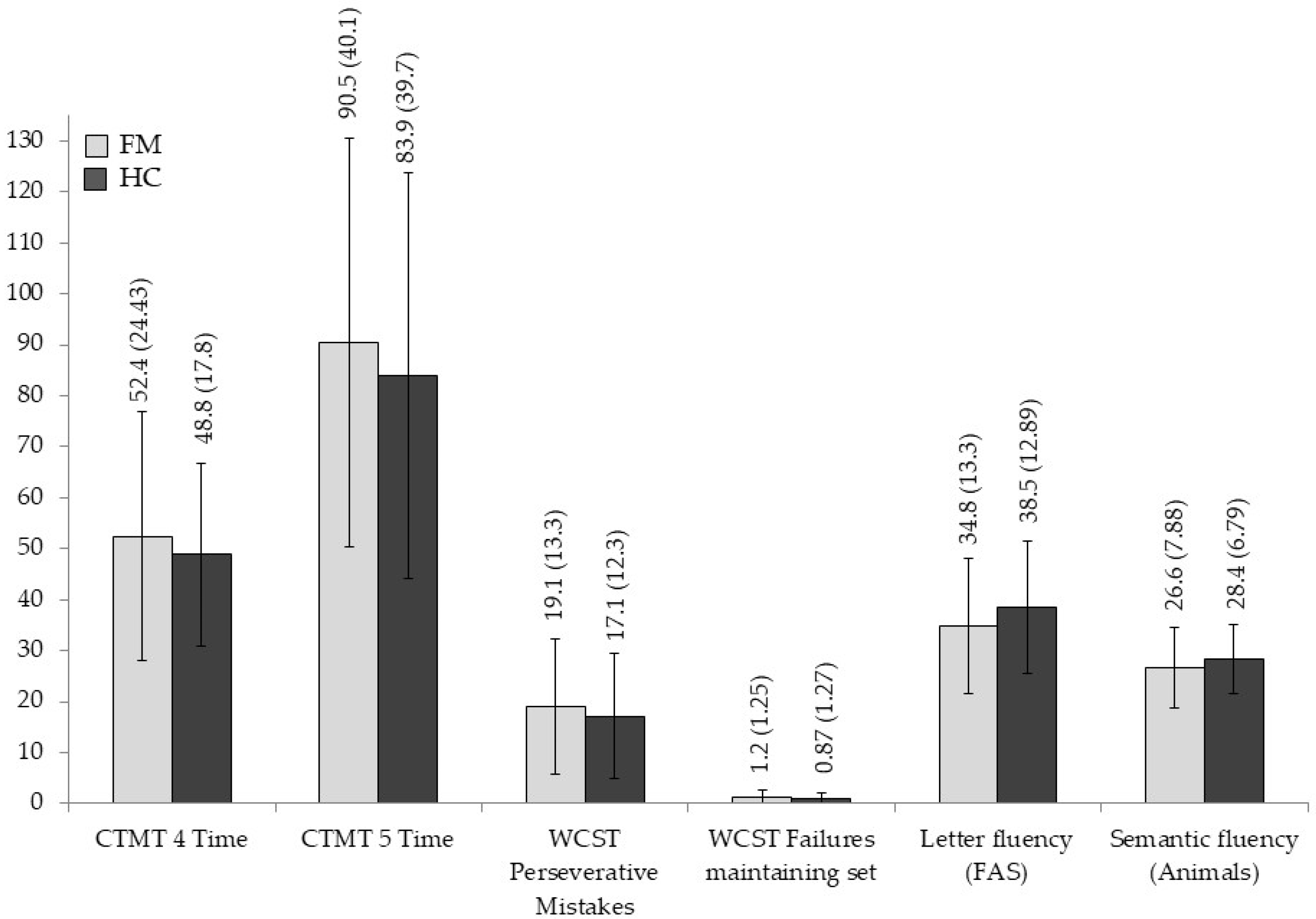
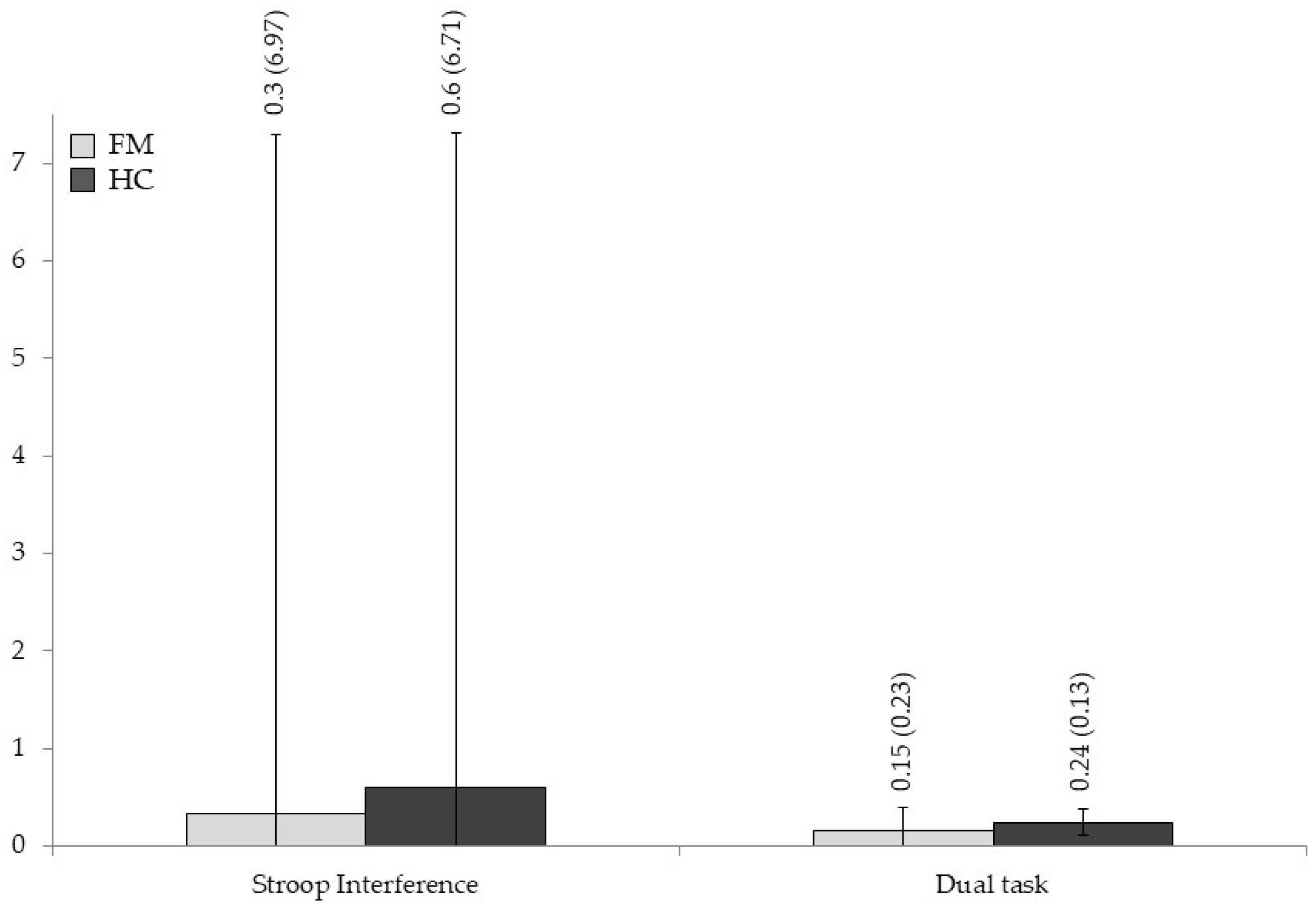
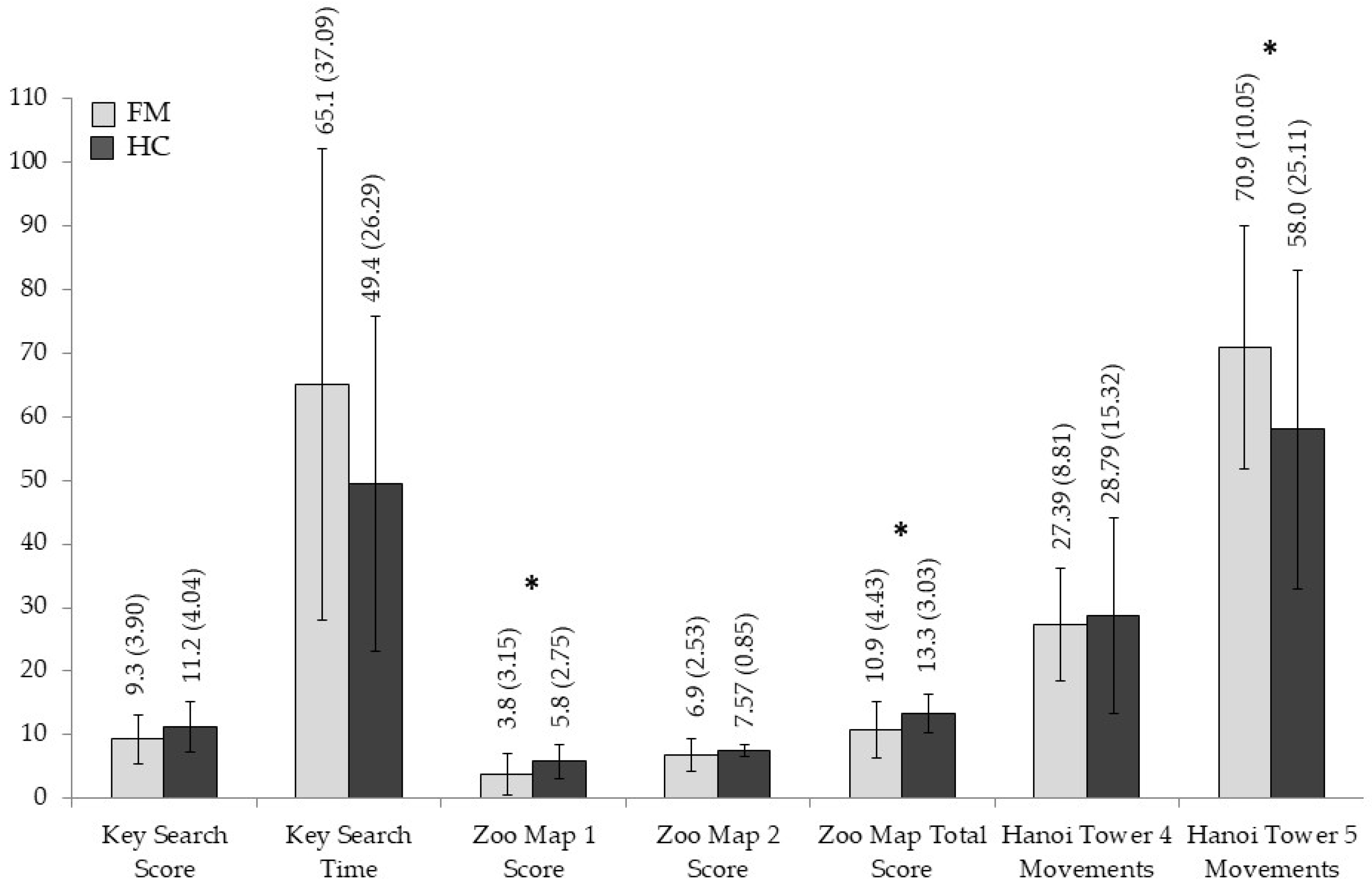
3.2. Group Differences in Neuropsychological Measures
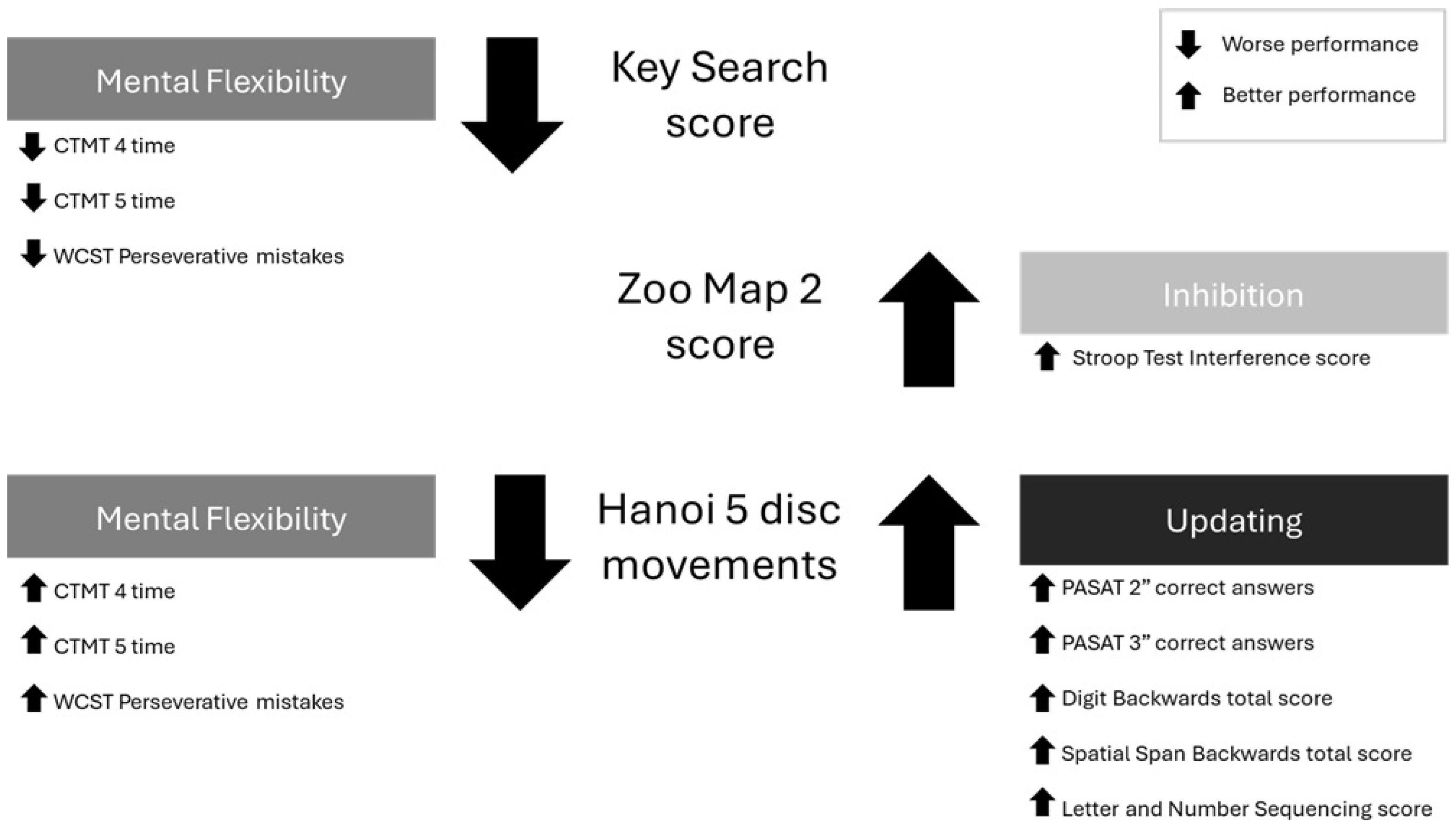
3.3. Predictive Value of Simple EF on Planning and Problem-Solving
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
Appendix A
| FMS N = 30 (Mean/SD) | HC N = 30 (Mean/SD) | F for Group | p for Group | η2p for Group | |
|---|---|---|---|---|---|
| WM (maintaining, manipulating, updating) | |||||
| Digit Span Forward Score | 7.86 (2.177) | 8.10 (2.294) | 0.163 | 0.688 | 0.003 |
| Digit Span Backwards Score | 5.40 (2.328) | 5.70 (2.135) | 0.270 | 0.605 | 0.005 |
| Digit Total Score | 13.30 (4.112) | 13.67 (3.845) | 0.127 | 0.723 | 0.002 |
| Spatial Span Forward Score | 7.80 (1.919) | 8.50 (1.697) | 2.240 | 0.140 | 0.037 |
| Spatial Span Backwards Score | 6.10 (2.023) | 7.37 (1.608) | 7.208 | 0.009 | 0.111 |
| Spatial Span Total Score | 13.90 (3.575) | 15.87 (2.776) | 5.663 | 0.021 | 0.089 |
| Letter and Number | 9.07 (2.348) | 9.97 (2.859) | 1.776 | 0.188 | 0.030 |
| PASAT 3” Correct | 37.50 (14.176) | 42.20 (10.226) | 2.169 | 0.146 | 0.036 |
| PASAT 2” Correct | 26.47 (10.298) | 30.97 (8.656) | 3.357 | 0.072 | 0.55 |
| Inhibition/interference control | |||||
| Stroop Interference | 0.33 (6.970) | 0.61 (6.714) | 0.024 | 0.878 | 0.000 |
| Access to memory information | |||||
| Letter fluency (FAS) | 34.80 (13.309) | 38.53 (12.899) | 1.217 | 0.274 | 0.021 |
| Semantic fluency (Animals) | 26.63 (7.885) | 28.47 (6.791) | 0.931 | 0.339 | 0.016 |
| Mental flexibility/Shifting | |||||
| CTMT 4 Time | 52.43 (24.384) | 48.83 (17.825) | 0.426 | 0.516 | 0.007 |
| CTMT 5 Time | 90.56 (40.098) | 83.93 (39.773) | 0.413 | 0.523 | 0.007 |
| WCST Perseverative Mistakes | 19.07 (13.362) | 17.10 (12.319) | 0.480 | 0.491 | 0.008 |
| WCST Failures maintaining set | 1.27 (1.258) | 0.87 (1.279) | 1.491 | 0.227 | 0.025 |
| Dual task | 0.15 (0.236) | 0.24 (0.137) | 3.359 | 0.072 | 0.055 |
| Planning and problem solving | |||||
| Key Search Score | 9.30 (3.905) | 11.27 (4.042) | 3.673 | 0.060 | 0.060 |
| Key Search Time | 65.03 (37.096) | 49.47 (26.296) | 3.294 | 0.075 | 0.054 |
| Zoo Map 1 Score | 3.80 (3.156) | 5.80 (2.759) | 6.829 | 0.011 | 0.105 |
| Zoo Map 2 Score | 6.90 (2.537) | 7.57 (0.858) | 1.858 | 0.178 | 0.031 |
| Zoo Map Total Score | 10.90 (4.436) | 13.37 (3.034) | 6.319 | 0.015 | 0.098 |
| Hanoi Tower 4 Disc Movements | 27.39 (8.814) | 28.79 (15.325) | 0.190 | 0.665 | 0.003 |
| Hanoi Tower 5 Disc Movements | 70.97 (19.059) | 58.00 (25.118) | 5.079 | 0.028 | 0.081 |
References
- Moriarty, O.; Finn, D.P. Cognition and Pain. Curr. Opin. Support. Palliat. Care 2014, 8, 130–136. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Gao, R.; Zhang, C.; Chen, H.; Wang, R.; Zhao, Q.; Zhu, T.; Chen, C. Evidence for Cognitive Decline in Chronic Pain: A Systematic Review and Meta-Analysis. Front. Neurosci. 2021, 15, 737874. [Google Scholar] [CrossRef] [PubMed]
- Sarzi-Puttini, P.; Giorgi, V.; Marotto, D.; Atzeni, F. Fibromyalgia: An Update on Clinical Characteristics, Aetiopathogenesis and Treatment. Nat. Rev. Rheumatol. 2020, 16, 645–660. [Google Scholar] [CrossRef] [PubMed]
- Gayà, T.F.; Ferrer, C.B.; Mas, A.J.; Seoane-Mato, D.; Reyes, F.Á.; Sánchez, M.D.; Dubois, C.M.; Sánchez-Fernández, S.A.; Vargas, L.M.R.; Morales, P.V.G.; et al. Prevalence of Fibromyalgia and Associated Factors in Spain. Clin. Exp. Rheumatol. 2020, 123, 47–52. [Google Scholar]
- Gelonch, O.; Garolera, M.; Valls, J.; Rosselló, L.; Pifarré, J. Executive Function in Fibromyalgia: Comparing Subjective and Objective Measures. Compr. Psychiatry 2016, 66, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Gelonch, O.; Garolera, M.; Valls, J.; Rosselló, L.; Pifarré, J. Cognitive Complaints in Women with Fibromyalgia: Are They Due to Depression or to Objective Cognitive Dysfunction? J. Clin. Exp. Neuropsychol. 2017, 39, 1013–1025. [Google Scholar] [CrossRef] [PubMed]
- Jacobsen, H.B.; Brun, A.; Stubhaug, A.; Reme, S.E. Stress Specifically Deteriorates Working Memory in Peripheral Neuropathic Pain and Fibromyalgia. Brain Commun. 2023, 5, fcad194. [Google Scholar] [CrossRef] [PubMed]
- Katz, R.S.; Heard, A.R.; Mills, M.; Leavitt, F. The Prevalence and Clinical Impact of Reported Cognitive Difficulties (Fibrofog) in Patients With Rheumatic Disease With and Without Fibromyalgia. JCR J. Clin. Rheumatol. 2004, 10, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Pidal-Miranda, M.; González-Villar, A.J.; Carrillo-de-la-Peña, M.T.; Andrade, E.; Rodríguez-Salgado, D. Broad Cognitive Complaints but Subtle Objective Working Memory Impairment in Fibromyalgia Patients. PeerJ 2018, 6, e5907. [Google Scholar] [CrossRef] [PubMed]
- Bell, T.; Trost, Z.; Buelow, M.T.; Clay, O.; Younger, J.; Moore, D.; Crowe, M. Meta-Analysis of Cognitive Performance in Fibromyalgia. J. Clin. Exp. Neuropsychol. 2018, 40, 698–714. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.-L.; Huang, C.-J.; Fang, S.-C.; Ko, L.-H.; Tsai, P.-S. Cognitive Impairment in Fibromyalgia: A Meta-Analysis of Case–Control Studies. Psychosom. Med. 2018, 80, 432–438. [Google Scholar] [CrossRef] [PubMed]
- Muñoz Ladrón De Guevara, C.; Fernández-Serrano, M.J.; Reyes Del Paso, G.A.; Duschek, S. Executive Function Impairments in Fibromyalgia Syndrome: Relevance of Clinical Variables and Body Mass Index. PLoS ONE 2018, 13, e0196329. [Google Scholar] [CrossRef] [PubMed]
- Verdejo-Garcia, A.; Lopez-Torrecillas, F.; Calandre, E.P.; Delgado-Rodriguez, A.; Bechara, A. Executive Function and Decision-Making in Women with Fibromyalgia. Arch. Clin. Neuropsychol. 2009, 24, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Walteros, C.; Sánchez-Navarro, J.P.; Muñoz, M.A.; Martínez-Selva, J.M.; Chialvo, D.; Montoya, P. Altered Associative Learning and Emotional Decision Making in Fibromyalgia. J. Psychosom. Res. 2011, 70, 294–301. [Google Scholar] [CrossRef] [PubMed]
- Galvez-Sánchez, C.M.; Reyes Del Paso, G.A.; Duschek, S. Cognitive Impairments in Fibromyalgia Syndrome: Associations With Positive and Negative Affect, Alexithymia, Pain Catastrophizing and Self-Esteem. Front. Psychol. 2018, 9, 377. [Google Scholar] [CrossRef] [PubMed]
- Galvez-Sánchez, C.M.; Muñoz Ladrón De Guevara, C.; Montoro, C.I.; Fernández-Serrano, M.J.; Duschek, S.; Reyes Del Paso, G.A. Cognitive Deficits in Fibromyalgia Syndrome Are Associated with Pain Responses to Low Intensity Pressure Stimulation. PLoS ONE 2018, 13, e0201488. [Google Scholar] [CrossRef] [PubMed]
- Galvez-Sánchez, C.M.; Reyes Del Paso, G.A.; Montoro, C.I. Revealing the Role of Social Support on Cognitive Deficits in Fibromyalgia Syndrome. Behav. Neurol. 2022, 2022, 3852746. [Google Scholar] [CrossRef] [PubMed]
- Wilson, B.A.; Evans, J.J.; Alderman, N.; Burgess, P.W.; Emslie, H. Behavioural Assessment of the Dysexecutive Syndrome. In Methodology of Frontal And Executive Function; Routledge: Hove, UK, 1997; ISBN 978-0-203-34418-7. [Google Scholar]
- Di Tella, M.; Castelli, L.; Colonna, F.; Fusaro, E.; Torta, R.; Ardito, R.B.; Adenzato, M. Theory of Mind and Emotional Functioning in Fibromyalgia Syndrome: An Investigation of the Relationship between Social Cognition and Executive Function. PLoS ONE 2015, 10, e0116542. [Google Scholar] [CrossRef] [PubMed]
- Mendonça, B.T.V.D.; Silva, G.G.; Busatto, L.M.; Dias, N.M. Executive Functions in Fibromyalgia: A Systematic Review. Psychol. Neurosci. 2021, 14, 413–437. [Google Scholar] [CrossRef]
- Alfeo, F.; Decarolis, D.; Clemente, L.; Delussi, M.; De Tommaso, M.; Curci, A.; Lanciano, T. Decision Making and Fibromyalgia: A Systematic Review. Brain Sci. 2022, 12, 1452. [Google Scholar] [CrossRef] [PubMed]
- Cuevas-Toro, A.M.; López-Torrecillas, F.; Díaz-Batanero, M.C.; Pérez-Marfil, M.N. Neuropsychological Function, Anxiety, Depression and Pain Impact in Fibromyalgia Patients. Span. J. Psychol. 2014, 17, E78. [Google Scholar] [CrossRef] [PubMed]
- Dick, B.D.; Verrier, M.J.; Harker, T.K.; Rashiq, S. Disruption of Cognitive Function in Fibromyalgia Syndrome. Pain 2008, 139, 610–616. [Google Scholar] [CrossRef] [PubMed]
- Tesio, V.; Torta, D.M.E.; Colonna, F.; Leombruni, P.; Ghiggia, A.; Fusaro, E.; Geminiani, G.C.; Torta, R.; Castelli, L. Are Fibromyalgia Patients Cognitively Impaired? Objective and Subjective Neuropsychological Evidence. Arthritis Care Res. 2015, 67, 143–150. [Google Scholar] [CrossRef] [PubMed]
- Friedman, N.P.; Miyake, A. Unity and Diversity of Executive Functions: Individual Differences as a Window on Cognitive Structure. Cortex 2017, 86, 186–204. [Google Scholar] [CrossRef] [PubMed]
- Miyake, A.; Friedman, N.P.; Emerson, M.J.; Witzki, A.H.; Howerter, A.; Wager, T.D. The Unity and Diversity of Executive Functions and Their Contributions to Complex “Frontal Lobe” Tasks: A Latent Variable Analysis. Cognit. Psychol. 2000, 41, 49–100. [Google Scholar] [CrossRef] [PubMed]
- Friedman, N.P.; Robbins, T.W. The Role of Prefrontal Cortex in Cognitive Control and Executive Function. Neuropsychopharmacology 2022, 47, 72–89. [Google Scholar] [CrossRef] [PubMed]
- Diamond, A. Executive Functions. Annu. Rev. Psychol. 2013, 64, 135–168. [Google Scholar] [CrossRef] [PubMed]
- De Melo, L.F.; Da-Silva, S.L. Neuropsychological Assessment of Cognitive Disorders in Patients with Fibromyalgia, Rheumatoid Arthritis, and Systemic Lupus Erythematosus. Rev. Bras. Reumatol. 2012, 52, 181–188. [Google Scholar] [PubMed]
- Kim, S.-H.; Kim, S.-H.; Kim, S.-K.; Nam, E.J.; Han, S.W.; Lee, S.J. Spatial versus Verbal Memory Impairments in Patients with Fibromyalgia. Rheumatol. Int. 2012, 32, 1135–1142. [Google Scholar] [CrossRef] [PubMed]
- Mercado, F.; Ferrera, D.; Fernandes-Magalhaes, R.; Peláez, I.; Barjola, P. Altered Subprocesses of Working Memory in Patients with Fibromyalgia: An Event-Related Potential Study Using N-Back Task. Pain Med. 2022, 23, 475–487. [Google Scholar] [CrossRef] [PubMed]
- Medina, L.D.; Hirshberg, L.; Taylor, M.J.; Gilbert, P.E.; Heaton, R.K. Rates of Neuropsychological Dysfunction in Fibromyalgia and Rheumatoid Arthritis: An Automated Clinical Rating Approach. JCR J. Clin. Rheumatol. 2019, 25, 252–257. [Google Scholar] [CrossRef] [PubMed]
- Barceló-Martinez, E.; Gelves-Ospina, M.; Lechuga, E.N.; Allegri, R.F.; Orozco-Acosta, E.; Benítez-Agudelo, J.C.; León-Jacobus, A.; Román, N.F. Serum Cortisol Levels and Neuropsychological Impairments in Patients Diagnosed with Fibromyalgia. Actas Esp. Psiquiatr. 2018, 46, 1–11. [Google Scholar] [PubMed]
- Bilgici, A.; Terzi, M.; Güz, H.; Kuru, O. Comparison of The Cognitive Performance Between Healthy Controls, Rheumatoid Arthritis and Fibromyalgia Patients Without Depression. J. Clin. Anal. Med. 2014, 5, 214–219. [Google Scholar] [CrossRef]
- Cherry, B.J.; Zettel-Watson, L.; Shimizu, R.; Roberson, I.; Rutledge, D.N.; Jones, C.J. Cognitive Performance in Women Aged 50 Years and Older With and Without Fibromyalgia. J. Gerontol. B Psychol. Sci. Soc. Sci. 2014, 69, 199–208. [Google Scholar] [CrossRef] [PubMed]
- Duschek, S.; Werner, N.S.; Limbert, N.; Winkelmann, A.; Montoya, P. Attentional Bias Toward Negative Information in Patients with Fibromyalgia Syndrome. Pain Med. 2014, 15, 603–612. [Google Scholar] [CrossRef] [PubMed]
- Mercado, F.; González, J.L.; Barjola, P.; Fernández-Sánchez, M.; López-López, A.; Alonso, M.; Gómez-Esquer, F. Brain Correlates of Cognitive Inhibition in Fibromyalgia: Emotional Intrusion of Symptom-Related Words. Int. J. Psychophysiol. 2013, 88, 182–192. [Google Scholar] [CrossRef] [PubMed]
- Duschek, S.; De Guevara, C.M.L.; Serrano, M.J.F.; Montoro, C.I.; López, S.P.; Reyes Del Paso, G.A. Variability of Reaction Time as a Marker of Executive Function Impairments in Fibromyalgia. Behav. Neurol. 2022, 1821684. [Google Scholar] [CrossRef] [PubMed]
- Goulart, R.; Pessoa, C.; Lombardi Junior, I. Neuropsychological Assessment of Patients with Fibromyalgia. Estud. Psicol. 2017, 22, 264–273. [Google Scholar] [CrossRef]
- Serrano, P.V.; Zortea, M.; Alves, R.L.; Beltran, G.; Deliberali, C.B.; Maule, A.; Torres, I.L.S.; Fregni, F.; Caumo, W. Association between Descending Pain Modulatory System and Cognitive Impairment in Fibromyalgia: A Cross-Sectional Exploratory Study. Front. Behav. Neurosci. 2022, 169, 17554. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Henderson, M.; Calderón, C.; Toro-Roa, I.; Aguilera-Choppelo, R.; Palominos, D.; Soto-Añari, M.; López, N.; Domic-Siede, M. The Cumulative Effect of Fibromyalgia Symptoms on Cognitive Performance: The Mediating Role of Pain. Appl. Neuropsychol. Adult 2022, 31, 1302–1312. [Google Scholar] [CrossRef] [PubMed]
- Walitt, B.; Čeko, M.; Khatiwada, M.; Gracely, J.L.; Rayhan, R.; VanMeter, J.W.; Gracely, R.H. Characterizing “Fibrofog”: Subjective Appraisal, Objective Performance, and Task-Related Brain Activity during a Working Memory Task. NeuroImage Clin. 2016, 11, 173–180. [Google Scholar] [CrossRef] [PubMed]
- Castel, A.; Cascón-Pereira, R.; Boada, S. Memory Complaints and Cognitive Performance in Fibromyalgia and Chronic Pain: The Key Role of Depression. Scand. J. Psychol. 2021, 62, 328–338. [Google Scholar] [CrossRef] [PubMed]
- Smith, A. Cognitive Impairments in Fibromyalgia Patients: Psychomotor Performance, Selective Attention and Memory. World J. Pharm. Med. Res. 2022, 8, 226–232. [Google Scholar]
- Muñoz Ladrón De Guevara, C.; Reyes Del Paso, G.A.; Fernández Serrano, M.J.; Montoro, C.I. Fibromyalgia Syndrome and Cognitive Decline: The Role of Body Mass Index and Clinical Symptoms. J. Clin. Med. 2022, 11, 3404. [Google Scholar] [CrossRef] [PubMed]
- Del Missier, F.; Mäntylä, T.; Bruine de Bruin, W. Executive Functions in Decision Making: An Individual Differences Approach. Think. Reason. 2010, 16, 69–97. [Google Scholar] [CrossRef]
- Corbo, I.; Troisi, G.; Marselli, G.; Casagrande, M. The Role of Cognitive Flexibility on Higher Level Executive Functions in Mild Cognitive Impairment and Healthy Older Adults. BMC Psychol. 2024, 12, 317. [Google Scholar] [CrossRef] [PubMed]
- Johann, V.E.; Karbach, J. Educational Application of Cognitive Training. In Cognitive Training; Strobach, T., Karbach, J., Eds.; Springer: Cham, Switzerland, 2021. [Google Scholar]
- Schäfer, J.; Reuter, T.; Leuchter, M.; Karbach, J. Executive Functions and Problem-Solving—The Contribution of Inhibition, Working Memory, and Cognitive Flexibility to Science Problem-Solving Performance in Elementary School Students. J. Exp. Child Psychol. 2024, 244, 105962. [Google Scholar] [CrossRef] [PubMed]
- Biringer, E.; Rongve, A.; Lund, A. A Review of Modern Antidepressants Effects on Neurocognitive Function. Curr. Psychiatry Rev. 2009, 5, 164–174. [Google Scholar] [CrossRef]
- Herrera-Guzmán, I.; Herrera-Abarca, J.E.; Gudayol-Ferré, E.; Herrera-Guzmán, D.; Gómez-Carbajal, L.; Peña-Olvira, M.; Villuendas-González, E.; Joan, G.-O. Effects of Selective Serotonin Reuptake and Dual Serotonergic–Noradrenergic Reuptake Treatments on Attention and Executive Functions in Patients with Major Depressive Disorder. Psychiatry Res. 2010, 177, 323–329. [Google Scholar] [CrossRef] [PubMed]
- Iwamoto, K.; Takahashi, M.; Nakamura, Y.; Kawamura, Y.; Ishihara, R.; Uchiyama, Y.; Ebe, K.; Noda, A.; Noda, Y.; Yoshida, K.; et al. The Effects of Acute Treatment with Paroxetine, Amitriptyline, and Placebo on Driving Performance and Cognitive Function in Healthy Japanese Subjects: A Double-blind Crossover Trial. Hum. Psychopharmacol. Clin. Exp. 2008, 23, 399–407. [Google Scholar] [CrossRef] [PubMed]
- Mohs, R.; Mease, P.; Arnold, L.M.; Wang, F.; Ahl, J.; Gaynor, P.J.; Wohlreich, M.M. The Effect of Duloxetine Treatment on Cognition in Patients With Fibromyalgia. Psychosom. Med. 2012, 74, 628–634. [Google Scholar] [CrossRef] [PubMed]
- Ojeda, B.; Dueñas, M.; Salazar, A.; Mico, J.A.; Torres, L.M.; Failde, I. Factors Influencing Cognitive Impairment in Neuropathic and Musculoskeletal Pain and Fibromyalgia. Pain Med. 2018, 19, 499–510. [Google Scholar] [CrossRef] [PubMed]
- Prado, C.E.; Watt, S.; Crowe, S.F. A Meta-Analysis of the Effects of Antidepressants on Cognitive Functioning in Depressed and Non-Depressed Samples. Neuropsychol. Rev. 2018, 28, 32–72. [Google Scholar] [CrossRef] [PubMed]
- Rose, E.J.; Simonotto, E.; Spencer, E.P.; Ebmeier, K.P. The Effects of Escitalopram on Working Memory and Brain Activity in Healthy Adults during Performance of the N-Back Task. Psychopharmacology 2006, 185, 339–347. [Google Scholar] [CrossRef] [PubMed]
- Wolfe, F.; Smythe, H.A.; Yunus, M.B.; Bennett, R.M.; Bombardier, C.; Goldenberg, D.L.; Tugwell, P.; Campbell, S.M.; Abeles, M.; Clark, P.; et al. The American College of Rheumatology 1990 Criteria for the Classification of Fibromyalgia. Arthritis Rheum. 1990, 33, 160–172. [Google Scholar] [CrossRef] [PubMed]
- Wolfe, F.; Clauw, D.J.; Fitzcharles, M.; Goldenberg, D.L.; Katz, R.S.; Mease, P.; Russell, A.S.; Russell, I.J.; Winfield, J.B.; Yunus, M.B. The American College of Rheumatology Preliminary Diagnostic Criteria for Fibromyalgia and Measurement of Symptom Severity. Arthritis Care Res. 2010, 62, 600–610. [Google Scholar] [CrossRef] [PubMed]
- Jiménez Bascuñán, A.; Fuentes-Durá, I.; Dasí, C. Short Forms of the Wechsler Adult Scales: A Systematic Review. Psychol. Soc. Educ. 2020, 12, 187–200. [Google Scholar] [CrossRef]
- Wechsler, D. Wechsler Adult Intelligence Scale III (WAIS-III), 3rd ed.; The Psychological Corporation: Marrickville, Australia, 1997. [Google Scholar] [CrossRef]
- Wechsler, D. Wechsler Memory Scale III (WMS-III), 3rd ed.; The Psychological Corporation: Marrickville, Australia, 1997; Available online: https://www.scirp.org/reference/referencespapers?referenceid=456843 (accessed on 22 July 2025).
- Gronwall, D.M.A. Paced Auditory Serial-Addition Task: A Measure of Recovery from Concussion. Percept. Mot. Skills 1977, 44, 367–373. [Google Scholar] [CrossRef] [PubMed]
- Golden, C.J. Stroop: Test de Colores y Palabras, 3rd ed.; TEA Ediciones: Madrid, Spain, 2001. [Google Scholar]
- Reynolds, C.R. Comprehensive Trail-Making Test: CTMT: Examiner’s Manual; PRO-ED: Austin, TX, USA, 2002. [Google Scholar]
- Heaton, R.K. Wisconsin Card Sorting Test Manual; Psychological Assessment Resources: Lutz, FL, USA, 1981. [Google Scholar]
- Strauss, E.; Sherman, E.M.; Spreen, O. A Compendium of Neuropsychological Tests: Administration, Norms, and Commentary; American Chemical Society: Washington, DC, USA, 2006. [Google Scholar]
- Tirapu-Ustárroz, J.; Luna-Lario, P. Neuropsicología de las Funciones Ejecutivas; Manual De Neuropsicología: Miami, FL, USA, 2008. [Google Scholar]
- Rey, A. Rey: Test de Copia y de Reproducción de Memoria de Figuras Geométricas Complejas; TEA Ediciones: Madrid, Spain, 1997. [Google Scholar]
- Simon, H.A. The Functional Equivalence of Problem Solving Skills. Cognit. Psychol. 1975, 7, 268–288. [Google Scholar] [CrossRef]
- Beck, A.T.; Steer, R.A. Beck Depression Inventory. Manual; The Psychological Corporation: Marrickville, Australia, 1993. [Google Scholar]
- Spielberger, C.D.; Gorsuch, R.L.; Lushene, R.E.; Cubero, N.S. STAI: Cuestionario de Ansiedad Estado-Rasgo; TEA Ediciones: Madrid, Spain, 1999. [Google Scholar]
- Macías, J.A.; Royuela, A. La Versión Española Del Índice de Calidad de Sueño de Pittsburgh. Inf. Psiquiátr. 1996, 146, 465–472. [Google Scholar]
- García Campayo, J.; Rodero, B.; Alda, M.; Sobradiel, N.; Montero, J.; Moreno, S. Validación de la versión española de la escala de la catastrofización ante el dolor (Pain Catastrophizing Scale) en la fibromialgia. Med. Clín. 2008, 131, 487–492. [Google Scholar] [CrossRef] [PubMed]
- Miyake, A.; Emerson, M.J.; Friedman, N.P. Assessment of Executive Functions in Clinical Settings: Problems and Recommendations. Semin. Speech Lang. 2000, 21, 169–183. [Google Scholar] [CrossRef] [PubMed]
- Eccleston, C. Chronic Pain and Distraction: An Experimental Investigation into the Role of Sustained and Shifting Attention in the Processing of Chronic Persistent Pain. Behav. Res. Ther. 1995, 33, 391–405. [Google Scholar] [CrossRef] [PubMed]
- Grace, G.M.; Nielson, W.R.; Hopkins, M.; Berg, M.A. Concentration and Memory Deficits in Patients with Fibromyalgia Syndrome. J. Clin. Exp. Neuropsychol. 1999, 21, 477–487. [Google Scholar] [CrossRef] [PubMed]
- Eccleston, C.; Crombez, G. Pain Demands Attention: A Cognitive–Affective Model of the Interruptive Function of Pain. Psychol. Bull. 1999, 125, 356–366. [Google Scholar] [CrossRef] [PubMed]
- Kendall, P.C.; Hollon, S.D.; Beck, A.T.; Hammen, C.L.; Ingram, R.E. Issues and Recommendations Regarding Use of the Beck Depression Inventory. Cogn. Ther. Res. 1987, 11, 289–299. [Google Scholar] [CrossRef]
- Salkind, M.R. Beck Depression Inventory in General Practice. J. R. Coll. Gen. Pract. 1969, 18, 267–271. [Google Scholar] [PubMed]
- Muñoz Ladrón De Guevara, C.; Reyes Del Paso, G.A.; Fernández-Serrano, M.J.; Duschek, S. Facial Emotion Recognition and Executive Functions in Fibromyalgia. Pain Med. 2021, 22, 1619–1629. [Google Scholar] [CrossRef] [PubMed]
- Gelonch, O.; Garolera, M.; Valls, J.; Castellà, G.; Varela, O.; Rosselló, L.; Pifarre, J. The Effect of Depressive Symptoms on Cognition in Patients with Fibromyalgia. PLoS ONE 2018, 13, e0200057. [Google Scholar] [CrossRef] [PubMed]
- Veldhuijzen, D.S.; Sondaal, S.F.V.; Oosterman, J.M. Intact Cognitive Inhibition in Patients With Fibromyalgia but Evidence of Declined Processing Speed. J. Pain 2012, 13, 507–515. [Google Scholar] [CrossRef] [PubMed]
- Filipiak, S.; Hawrot, A. Negative Affect and Planning Ability in Preschool Children: A Mediation Model of Working Memory, Inhibition, and Cognitive Flexibility. Scand. J. Psychol. 2024, 66, 277–287. [Google Scholar] [CrossRef] [PubMed]
- Bull, R.; Espy, K.A.; Senn, T.E. A Comparison of Performance on the Towers of London and Hanoi in Young Children. J. Child Psychol. Psychiatry 2004, 45, 743–754. [Google Scholar] [CrossRef] [PubMed]
- Cooper, R.P.; Marsh, V. Set-Shifting as a Component Process of Goal-Directed Problem-Solving. Psychol. Res. 2016, 80, 307–323. [Google Scholar] [CrossRef] [PubMed]
- Welsh, M.C. Rule-Guided Behavior and Self-Monitoring on the Tower of Hanoi Disk-Transfer Task. Cogn. Dev. 1991, 6, 59–76. [Google Scholar] [CrossRef]
- D’Antuono, G.; La Torre, F.R.; Marin, D.; Antonucci, G.; Piccardi, L.; Guariglia, C. Role of Working Memory, Inhibition, and Fluid Intelligence in the Performance of the Tower of London Task. Appl. Neuropsychol. Adult 2017, 24, 548–558. [Google Scholar] [CrossRef] [PubMed]
- Zook, N.A.; Davalos, D.B.; DeLosh, E.L.; Davis, H.P. Working Memory, Inhibition, and Fluid Intelligence as Predictors of Performance on Tower of Hanoi and London Tasks. Brain Cogn. 2004, 56, 286–292. [Google Scholar] [CrossRef] [PubMed]
- Altamirano, L.J.; Miyake, A.; Whitmer, A.J. When Mental Inflexibility Facilitates Executive Control: Beneficial Side Effects of Ruminative Tendencies on Goal Maintenance. Psychol. Sci. 2010, 21, 1377–1382. [Google Scholar] [CrossRef] [PubMed]
- Oosterman, J.M.; Wijers, M.; Kessels, R.P.C. Planning or Something Else? Examining Neuropsychological Predictors of Zoo Map Performance. Appl. Neuropsychol. Adult 2013, 20, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Norris, G.; Tate, R.L. The Behavioural Assessment of the Dysexecutive Syndrome (BADS): Ecological, Concurrent and Construct Validity. Neuropsychol. Rehabil. 2000, 10, 33–45. [Google Scholar] [CrossRef]
- Emmanouel, A.; Kessels, R.P.C.; Mouza, E.; Fasotti, L. Sensitivity, Specificity and Predictive Value of the BADS to Anterior Executive Dysfunction. Neuropsychol. Rehabil. 2014, 24, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Duggan, E.C.; Garcia-Barrera, M.A. Executive Functioning and Intelligence. In Handbook of Intelligence: Evolutionary Theory, Historical Perspective, and Current Concepts; Goldstein, S., Princiotta, D., Naglieri, J.A., Eds.; Springer: New York, NY, USA, 2015; pp. 435–458. [Google Scholar]
- Friedman, N.P.; Miyake, A.; Corley, R.P.; Young, S.E.; DeFries, J.C.; Hewitt, J.K. Not All Executive Functions Are Related to Intelligence. Psychol. Sci. 2006, 17, 172–179. [Google Scholar] [CrossRef] [PubMed]

| FMS Patients N = 30 | HC Participants N = 30 | t/x2 | p-Value | |
|---|---|---|---|---|
| Age (M ± SD) | 49.73 (9.09) | 48.60 (8.50) | 0.487 | 0.628 |
| Marital status (N/%) | 3.498 | 0.321 | ||
| Married | 21 (70) | 17 (56.66) | ||
| Single | 1 (3.33) | 5 (16.67) | ||
| Widow | 1 (3.33) | 2 (6.67) | ||
| Separated/Divorced | 7 (23.34) | 6 (20) | ||
| Educational level (N/%) | 1.529 | 0.821 | ||
| Elemental education | 1 (3.33) | 1 (3.33) | ||
| Primary Education | 6 (20) | 3 (10) | ||
| Basic Education | 10 (33.33) | 10 (33.33) | ||
| High School | 7 (23.34) | 10 (33.33) | ||
| Higher Education | 6 (20) | 6 (20) | ||
| Estimated Intelligence Quotient (M ± SD) | ||||
| Vocabulary | 40.97 (10.13) | 44.37 (8.05) | −1.439 | 0.155 |
| Block Design | 35.40 (12.82) | 38.03 (9.17) | −0.915 | 0.364 |
| Tobacco consumers (N/%) | 0.341 | 0.559 | ||
| Yes | 7 (23.33) | 9 (30) | ||
| No | 23 (76.67) | 21 (70) | ||
| Cigarettes/day (M ± SD) | 3.43 (6.76) | 4.27 (8.67) | −0.415 | 0.680 |
| Alcohol consumers (N/%) | 7.177 | 0.007 | ||
| Yes | 6 (20) | 16 (53.33) | ||
| No | 24 (80) | 14 (46.67) | ||
| Alcoholic drinks/day (M ± SD) | 0.37 (0.89) | 0.89 (1.21) | −1.902 | 0.062 |
| Years since diagnosis (M ± SD) | 6.97 (5.16) | |||
| Medication (N/%) | ||||
| NSAIDs | 16 (53.33) | 0 | 21.818 | 0.000 |
| SSRIs | 5 (16.67) | 1 (3.33) | 2.963 | 0.085 |
| Dual ADs | 4 (13.33) | 0 | 4.286 | 0.038 |
| Non-Opioid Analgesics | 12 (40) | 0 | 15.000 | 0.000 |
| Dopamine Agonists | 1 (3.33) | 0 | 1.017 | 0.313 |
| Other | 9 (30) | 1 (3.33) | 7.680 | 0.006 |
| Pain level (PNS) (M ± SD) | 7.71 (1.42) | 1.63 (1.13) | 16.773 | 0.000 |
| Fatigue level (FNS) (M ± SD) | 5.60 (2.42) | 1.73 (1.36) | 7.636 | 0.000 |
| Depressive symptoms (BDI) (M ± SD) | 16.70 (9.11) | 5.70 (5.48) | 5.670 | 0.000 |
| Trait anxiety (STAI-T) (M ± SD) | 29.90 (11.27) | 16.47 (9.39) | 5.017 | 0.000 |
| Pain worrying (PCS) (M ± SD) | 21.10 (1.33) | 15.83 (11.58) | 1.687 | 0.097 |
| Sleep disturbances (PSQI) (M ± SD) | 1.70 (0.57) | 0.91 (0.62) | 5.200 | 0.000 |
| Pain Effect (d.f. = 1, 57) | Fatigue Effect (d.f. = 1, 57) | Sleeping Disturbances Effect (d.f. = 1, 57) | Depression Effect (d.f. = 1, 57) | Anxiety Effect (d.f. = 1, 57) | |
|---|---|---|---|---|---|
| WM (maintaining, manipulating, updating) | |||||
| Spatial Span Backwards Score | F = 0.86; p = 0.358 | F = 1.205; p = 0.277 | F = 0.513; p = 0.477 | F = 1.892; p = 0.174 | F = 5.182; p = 0.027, η2p = 0.083 |
| Spatial Span Total Score | F = 0.744; p = 0.392 | F = 0.824; p = 0.368 | F = 0.594; p = 0.444 | F = 1.021; p = 0.316 | F = 3.039; p = 0.087 |
| Planning and problem-solving | |||||
| Zoo Map 1 Score | F = 0.619; p = 0.435 | F = 3.405; p = 0.070 | F = 3.662; p = 0.061 | F = 4.897; p = 0.031, η2p = 0.079 | F = 4.794; p = 0.033, η2p = 0.078 |
| Zoo Map Total Score | F = 0.261; p = 0.611 | F = 4.657; p = 0.035, η2p = 0.076 | F = 3.541; p = 0.065 | F = 2.923; p = 0.093 | F = 3.578; p = 0.064 |
| Hanoi Tower 5 Disc Movements | F = 0.477; p = 0.493 | F = 1.943; p = 0.169 | F = 5.513; p = 0.022, η2p = 0.088 | F = 4.882; p = 0.031, η2p = 0.079 | F = 4.608; p = 0.036, η2p = 0.075 |
| Complex EF Test | Fibromyalgia Patients | Healthy Controls | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Key Search Score | Model | R | r2 | SE | F | d.f. | p | R | r2 | SE | F | d.f. | p |
| 1 | 0.622 | 0.387 | 0.794 | 5.466 | (3,26) | 0.005 | 0.420 | 0.177 | 0.953 | 1.859 | (3,26) | 0.161 | |
| Predictors | Β | SE | 95% CI | β | t | p | Β | SE | 95% CI | β | t | p | |
| Constant | −0.198 | 0.150 | [−0.507, 0.111] | −1.317 | 0.199 | 0.187 | 0.183 | [−0.188, 0.563] | 1.026 | 0.314 | |||
| Mental Flexibility | −0.218 | 0.080 | [−0.383, −0.053] | −0.599 | −2.710 | 0.012 | −0.168 | 0.108 | [−0.391, 0.055] | −0.387 | −1.548 | 0.134 | |
| Inhibition | 0.218 | 0.146 | [−0.082, 0.518] | 0.233 | 1.492 | 0.148 | 0.022 | 0.194 | [−0.378, 0.421] | 0.022 | 0.112 | 0.912 | |
| Updating | −0.017 | 0.052 | [−0.123, 0.089] | −0.071 | −0.324 | 0.748 | 0.011 | 0.073 | [−0.139, 0.162] | 0.040 | 0.151 | 0.881 | |
| Zoo Map 1 Score | Model | R | r2 | SE | F | d.f. | p | R | r2 | SE | F | d.f. | p |
| 1 | 0.266 | 0.071 | 1.033 | 0.661 | (3,26) | 0.584 | 0.556 | 0.309 | 0.779 | 3.869 | (3,26) | 0.021 | |
| Predictors | Β | SE | 95% CI | β | t | p | Β | SE | 95% CI | β | t | p | |
| Constant | −0.316 | 0.196 | [−0.718, 0.086] | −1.617 | 0.118 | 0.257 | 0.149 | [−0.050, 0.564] | 1.718 | 0.098 | |||
| Mental Flexibility | −0.128 | 0.105 | [−0.343, 0.086] | −0.334 | −1.229 | 0.230 | −0.169 | 0.089 | [−0.351, 0.014] | −0.436 | −1.902 | 0.068 | |
| Inhibition | −0.046 | 0.190 | [−0.436, 0.345] | −0.046 | −0.240 | 0.812 | −0.298 | 0.159 | [−0.625, 0.028] | −0.332 | −1.877 | 0.072 | |
| Updating | −0.027 | 0.067 | [−0.165, 0.111] | −0.109 | −0.401 | 0.692 | 0.028 | 0.060 | [−0.095, 0.151] | 0.113 | 0.466 | 0.645 | |
| Zoo Map 2 Score | Model | R | r2 | SE | F | d.f. | p | R | r2 | SE | F | d.f. | p |
| 1 | 0.606 | 0.368 | 1.116 | 5.039 | (3,26) | 0.007 | 0.276 | 0.076 | 0.456 | 0.717 | (3,26) | 0.551 | |
| Predictors | Β | SE | 95% CI | β | t | p | Β | SE | 95% CI | β | t | p | |
| Constant | −0.084 | 0.211 | [−0.519, 0.350] | −0.399 | 0.693 | 0.194 | 0.087 | [0.014, 0.374] | 2.215 | 0.036 | |||
| Mental Flexibility | −0.068 | 0.113 | [−0.300, 0.164] | −0.135 | −0.599 | 0.554 | −0.074 | 0.052 | [−0.181, 0.033] | −0.377 | −1.425 | 0.166 | |
| Inhibition | 0.674 | 0.205 | [0.253, 1.096] | 0.521 | 3.288 | 0.003 | −0.002 | 0.093 | [−0.193, 0.189] | −0.004 | −0.022 | 0.983 | |
| Updating | 0.060 | 0.073 | [−0.089, 0.209] | 0.185 | 0.828 | 0.415 | −0.039 | 0.035 | [−0.111, 0.034] | −0.309 | −1.099 | 0.282 | |
| Hanoi Tower 4 Discs Movements | Model | R | r2 | SE | F | d.f. | p | R | r2 | SE | F | d.f. | p |
| 1 | 0.322 | 0.104 | 0.709 | 1.001 | (3,26) | 0.408 | 0.226 | 0.051 | 1.269 | 0.468 | (3,26) | 0.707 | |
| Predictors | Β | SE | 95% CI | β | t | p | Β | SE | 95% CI | β | t | p | |
| Constant | −0.055 | 0.134 | [−0.331, 0.221] | −0.408 | 0.687 | 0.011 | 0.243 | [−0.489, 0.511] | 0.044 | 0.965 | |||
| Mental Flexibility | 0.053 | 0.072 | [−0.094, 0.201] | 0.199 | 0.745 | 0.463 | 0.057 | 0.144 | [−0.239, 0.354] | 0.107 | 0.398 | 0.694 | |
| Inhibition | −0.184 | 0.130 | [−0.452, 0.084] | −0.266 | −1.409 | 0.171 | −0.297 | 0.259 | [−0.829, 0.235] | −0.238 | −1.146 | 0.262 | |
| Updating | 0.020 | 0.046 | [−0.075, 0.114] | 0.113 | 0.423 | 0.676 | 0.067 | 0.098 | [−0.133, 0.268] | 0.197 | 0.691 | 0.496 | |
| Hanoi Tower 5 Discs Movements | Model | R | r2 | SE | F | d.f. | p | R | r2 | SE | F | d.f. | p |
| 1 | 0.525 | 0.275 | 0.743 | 3.292 | (3,26) | 0.036 | 0.289 | 0.083 | 1.101 | 0.789 | (3,26) | 0.511 | |
| Predictors | Β | SE | 95% CI | β | t | p | Β | SE | 95% CI | β | t | p | |
| Constant | 0.190 | 0.141 | [−0.099, 0.479] | 1.348 | 0.189 | −0.312 | 0.211 | [−0.746, 0.122] | −1.479 | 0.151 | |||
| Mental Flexibility | −0.208 | 0.075 | [−0.363, −0.054] | −0.667 | −2.775 | 0.010 | −0.136 | 0.125 | [−0.394, 0.121] | −0.287 | −1.089 | 0.286 | |
| Inhibition | −0.068 | 0.136 | [−0.348, 0.213] | −0.084 | −0.496 | 0.624 | 0.066 | 0.225 | [−0.395, 0.528] | 0.060 | 0.295 | 0.770 | |
| Updating | −0.146 | 0.048 | [−0.245, −0.046] | −0.722 | −3.017 | 0.006 | −0.006 | 0.085 | [−0.180, 0.168] | −0.019 | −0.068 | 0.946 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fernández-Sánchez, M.; Martín-Plasencia, P.; Fernandes-Magalhaes, R.; Barjola, P.; del Pino, A.B.; Martínez-Íñigo, D.; Peláez, I.; Mercado, F. Planning and Problem-Solving Impairments in Fibromyalgia: The Predictive Role of Updating, Inhibition, and Mental Flexibility. J. Clin. Med. 2025, 14, 5263. https://doi.org/10.3390/jcm14155263
Fernández-Sánchez M, Martín-Plasencia P, Fernandes-Magalhaes R, Barjola P, del Pino AB, Martínez-Íñigo D, Peláez I, Mercado F. Planning and Problem-Solving Impairments in Fibromyalgia: The Predictive Role of Updating, Inhibition, and Mental Flexibility. Journal of Clinical Medicine. 2025; 14(15):5263. https://doi.org/10.3390/jcm14155263
Chicago/Turabian StyleFernández-Sánchez, Marisa, Pilar Martín-Plasencia, Roberto Fernandes-Magalhaes, Paloma Barjola, Ana Belén del Pino, David Martínez-Íñigo, Irene Peláez, and Francisco Mercado. 2025. "Planning and Problem-Solving Impairments in Fibromyalgia: The Predictive Role of Updating, Inhibition, and Mental Flexibility" Journal of Clinical Medicine 14, no. 15: 5263. https://doi.org/10.3390/jcm14155263
APA StyleFernández-Sánchez, M., Martín-Plasencia, P., Fernandes-Magalhaes, R., Barjola, P., del Pino, A. B., Martínez-Íñigo, D., Peláez, I., & Mercado, F. (2025). Planning and Problem-Solving Impairments in Fibromyalgia: The Predictive Role of Updating, Inhibition, and Mental Flexibility. Journal of Clinical Medicine, 14(15), 5263. https://doi.org/10.3390/jcm14155263







