Impact of Tumor Budding in Head and Neck Cancers on Neck Lymph Node Metastasis and Prognosis
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Patient Selection
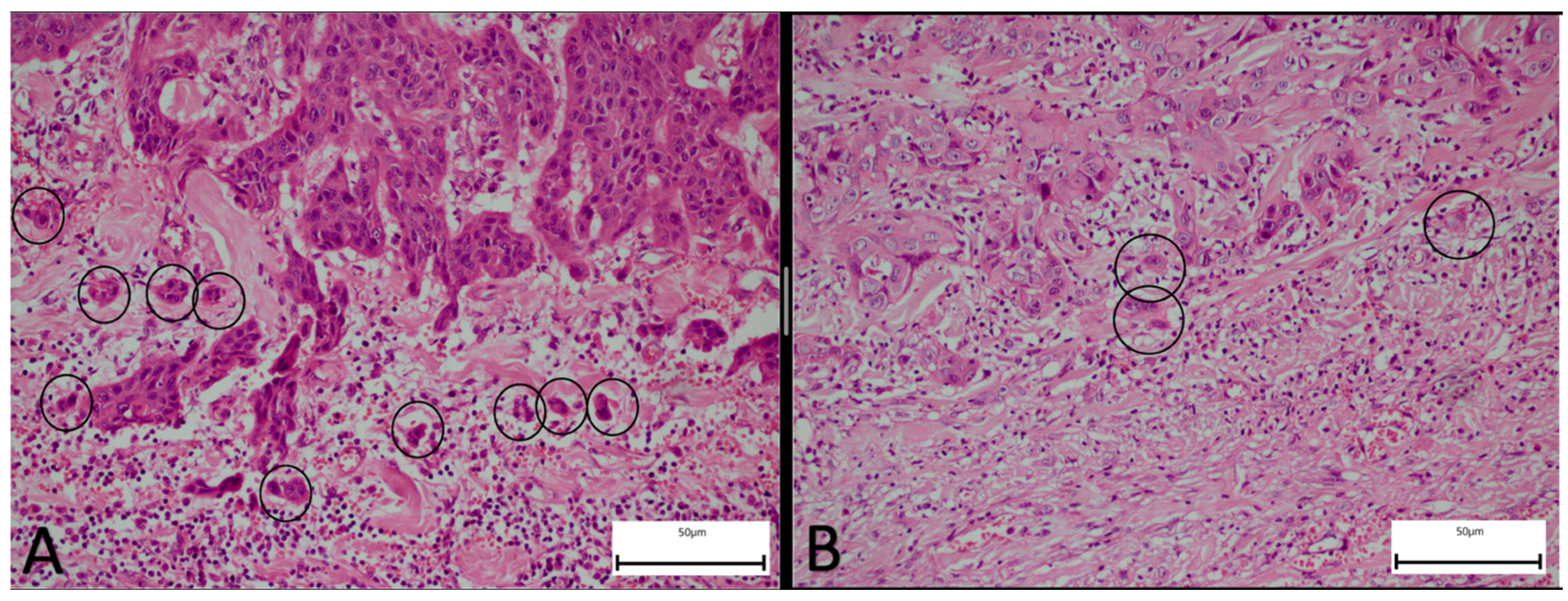
2.2. Pathological Assessment
2.3. Statistical Analysis
3. Results
3.1. Patient Cohort and Survival Outcomes
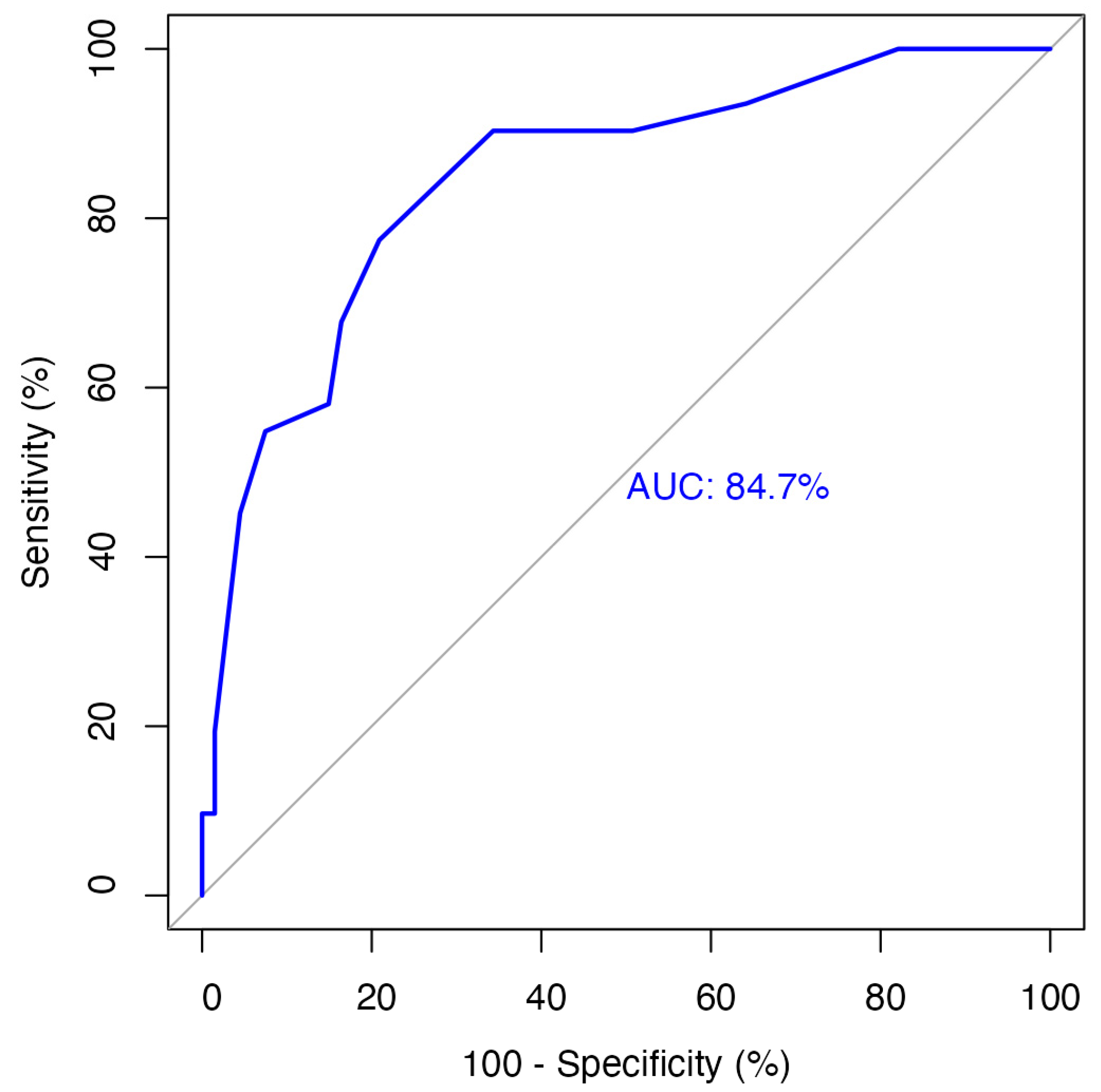
3.2. Tumor Budding Grouping
3.3. Patient and Tumor Characteristics
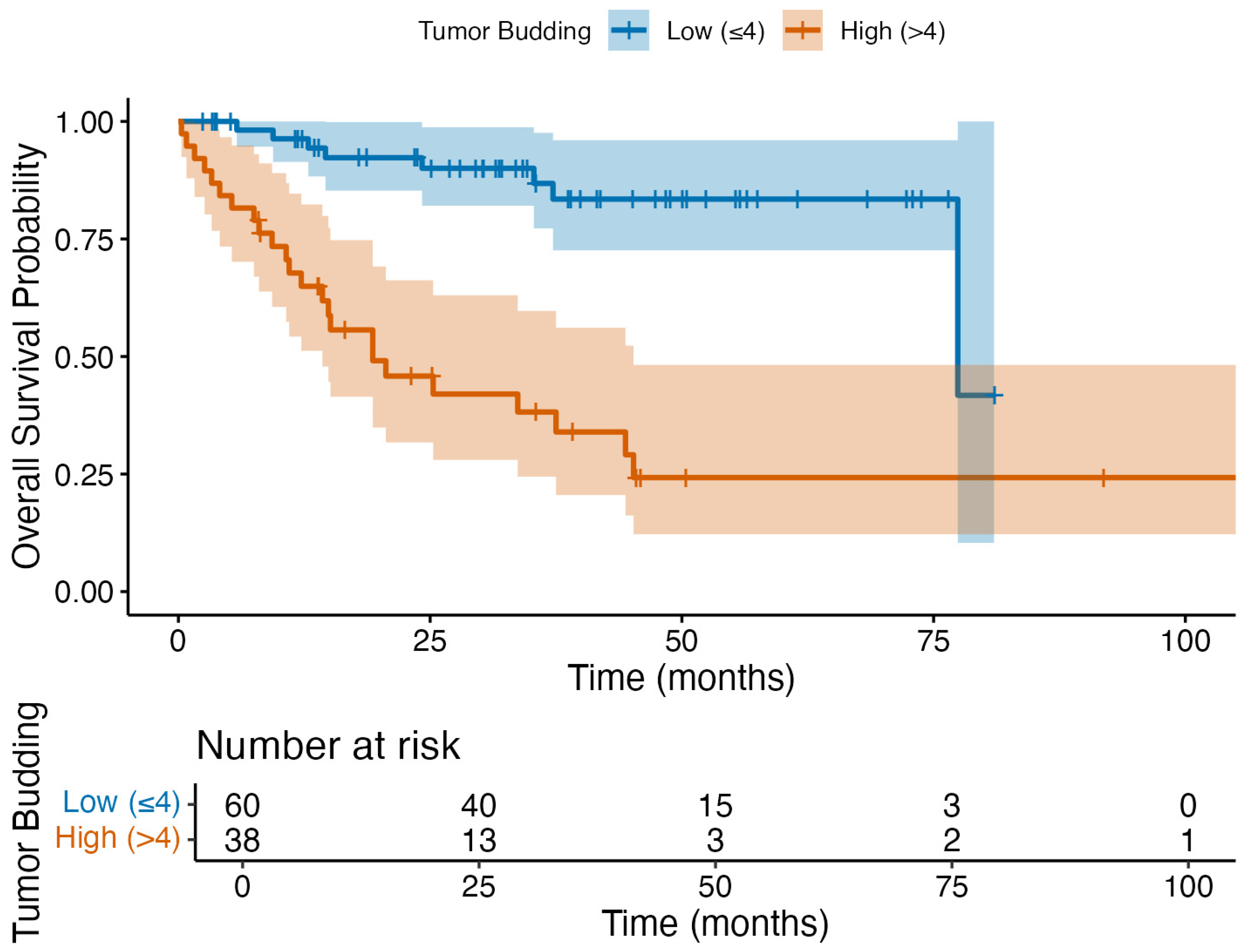
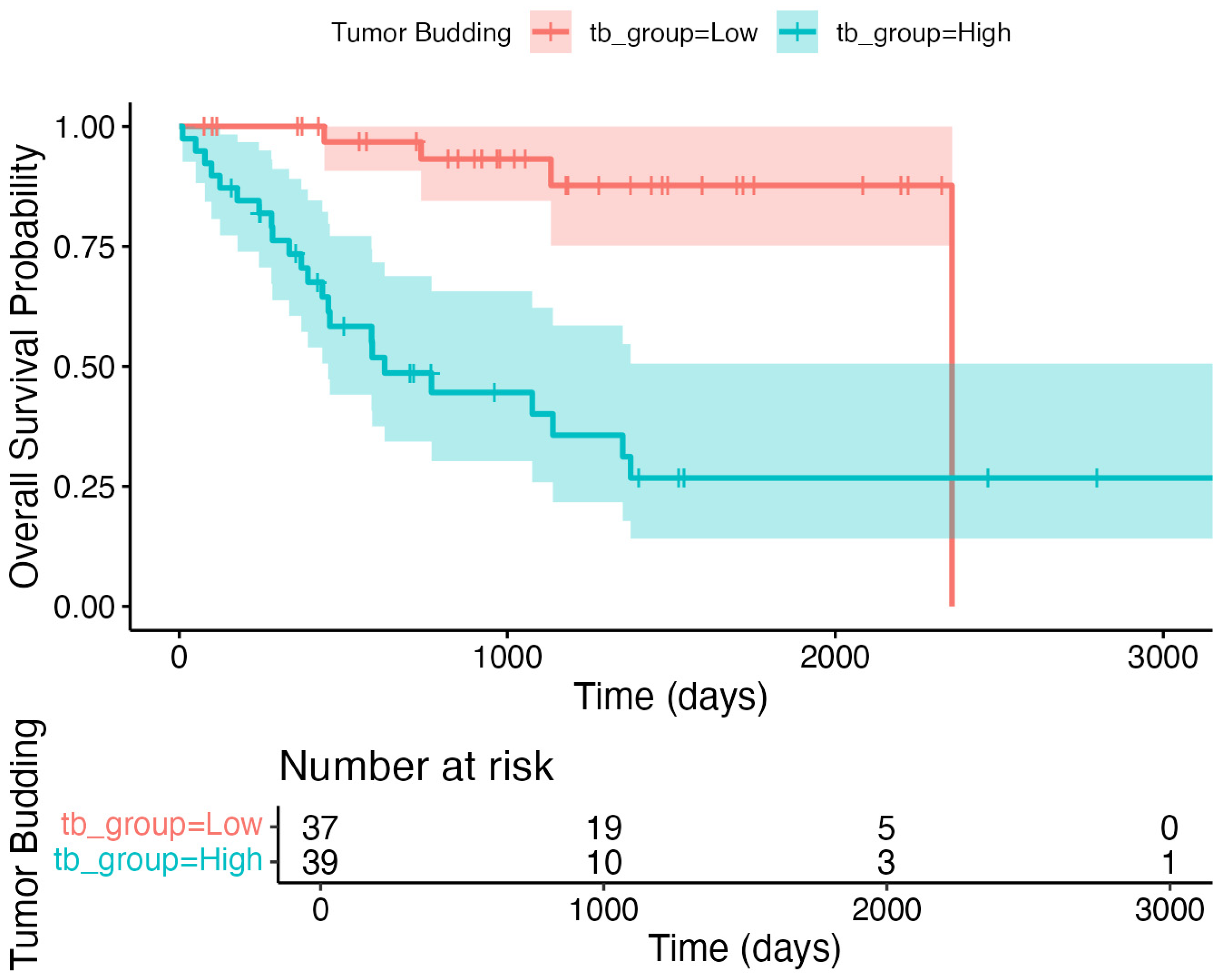
3.4. Primary Outcome: Overall Survival Analysis
3.5. Secondary Outcome: Neck Lymph Node Metastasis
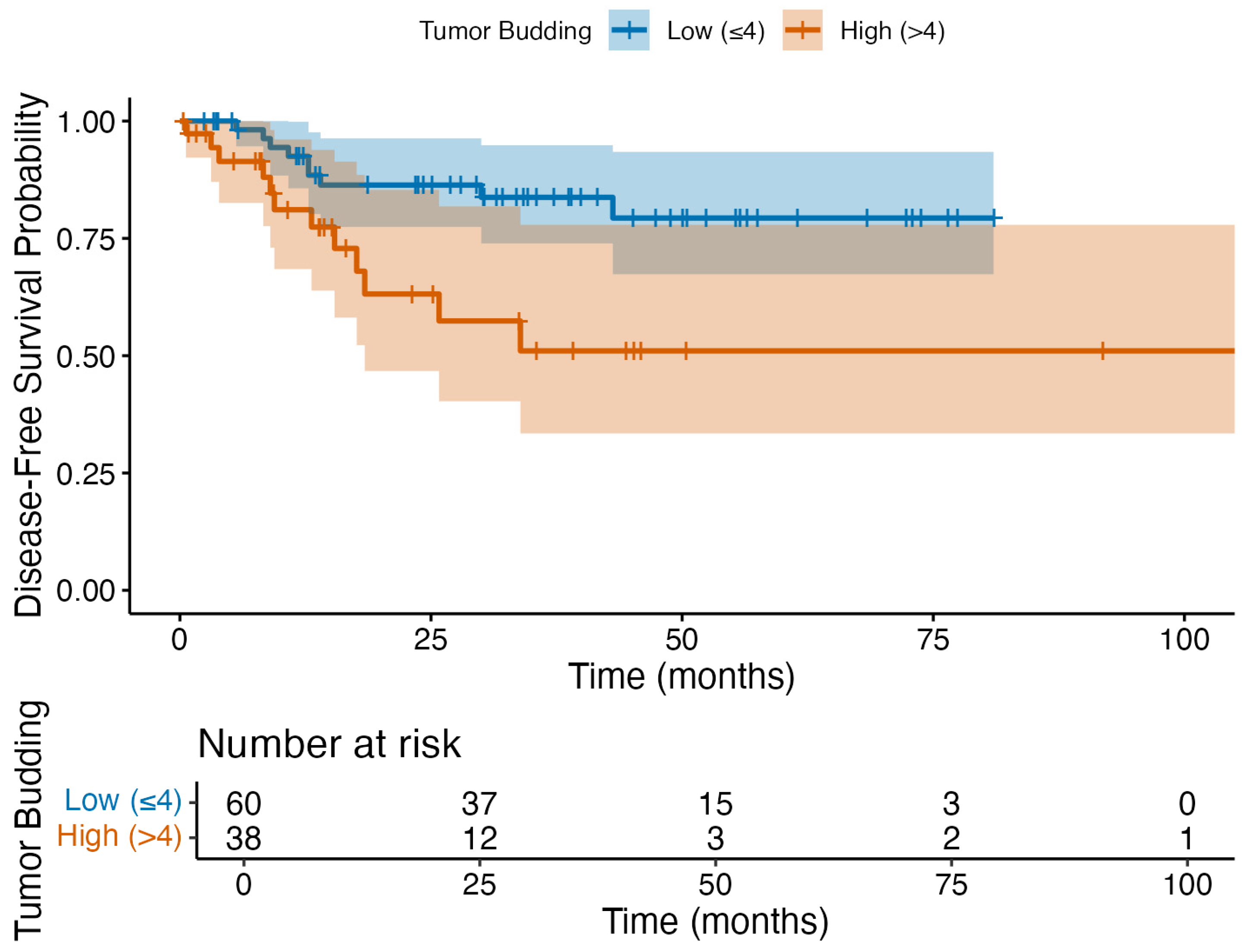
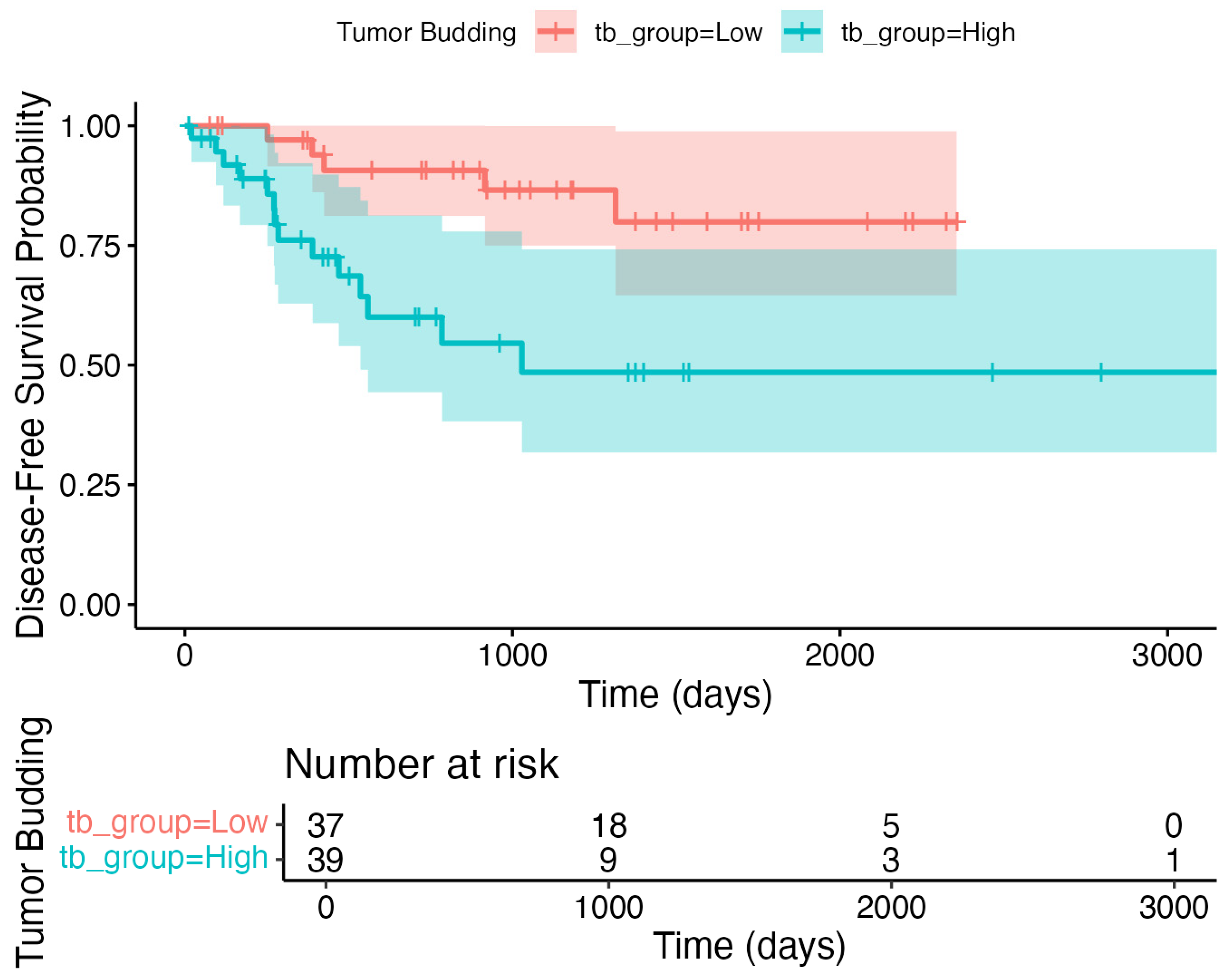
3.6. Exploratory Outcomes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| TB | Tumor budding |
| HNSCC | Head and neck squamous cell carcinoma |
| ITBCC | International Tumor Budding Consensus Conference |
| ROC | Receiver operating characteristics |
| OS | Overall survival |
| DFS | Disease-free survival |
| AUC | Area under the curve |
| CI | Confidence interval |
| HR | Hazard ratio |
| OR | Odds ratio |
| AJCC | American Joint Committee on Cancer |
| ENE | Extranodal extension |
| LVI | Lymphovascular invasion |
| PNI | Perineural invasion |
References
- Siegel, R.L.; Miller, K.D.; Wagle, N.S.; Jemal, A. Cancer statistics, 2023. CA Cancer J. Clin. 2023, 73, 17–48. [Google Scholar] [CrossRef] [PubMed]
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Mathers, C.; Parkin, D.M.; Piñeros, M.; Znaor, A.; Bray, F. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int. J. Cancer 2019, 144, 1941–1953. [Google Scholar] [CrossRef] [PubMed]
- Budach, V.; Tinhofer, I. Novel prognostic clinical factors and biomarkers for outcome prediction in head and neck cancer: A systematic review. Lancet Oncol. 2019, 20, e313–e326. [Google Scholar] [CrossRef] [PubMed]
- Leemans, C.R.; Tiwari, R.; Nauta, J.J.; van der Waal, I.; Snow, G.B. Regional lymph node involvement and its significance in the development of distant metastases in head and neck carcinoma. Cancer 1993, 71, 452–456. [Google Scholar] [CrossRef]
- Almangush, A.; Bello, I.O.; Keski-Säntti, H.; Mäkinen, L.K.; Kauppila, J.H.; Pukkila, M.; Hagström, J.; Laranne, J.; Tommola, S.; Nieminen, O.; et al. Depth of invasion, tumor budding, and worst pattern of invasion: Prognostic indicators in early-stage oral tongue cancer. Head Neck 2014, 36, 811–818. [Google Scholar] [CrossRef]
- Ueno, H.; Murphy, J.; Jass, J.R.; Mochizuki, H.; Talbot, I.C. Tumour ‘budding’ as an index to estimate the potential of aggressiveness in rectal cancer. Histopathology 2002, 40, 127–132. [Google Scholar] [CrossRef]
- Lugli, A.; Kirsch, R.; Ajioka, Y.; Bosman, F.; Cathomas, G.; Dawson, H.; El Zimaity, H.; Fléjou, J.F.; Hansen, T.P.; Hartmann, A.; et al. Recommendations for reporting tumor budding in colorectal cancer based on the International Tumor Budding Consensus Conference (ITBCC) 2016. Mod. Pathol. 2017, 30, 1299–1311. [Google Scholar] [CrossRef]
- Lamouille, S.; Xu, J.; Derynck, R. Molecular mechanisms of epithelial-mesenchymal transition. Nat. Rev. Mol. Cell Biol. 2014, 15, 178–196. [Google Scholar] [CrossRef]
- Kalluri, R.; Weinberg, R.A. The basics of epithelial-mesenchymal transition. J. Clin. Investig. 2009, 119, 1420–1428. [Google Scholar] [CrossRef]
- Hase, K.; Shatney, C.; Johnson, D.; Trollope, M.; Vierra, M. Prognostic value of tumor “budding” in patients with colorectal cancer. Dis. Colon Rectum 1993, 36, 627–635. [Google Scholar] [CrossRef]
- Ueno, H.; Price, A.B.; Wilkinson, K.H.; Jass, J.R.; Mochizuki, H.; Talbot, I.C. A new prognostic staging system for rectal cancer. Ann. Surg. 2004, 240, 832–839. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, T.; Mitomi, H.; Kikuchi, S.; Ohtani, Y.; Sato, K. Evaluation of the usefulness of tumor budding on the prediction of metastasis to the lung and liver after curative excision of colorectal cancer. Hepatogastroenterology 2005, 52, 1432–1435. [Google Scholar] [PubMed]
- Okcu, O.; Öztürk, Ç.; Şen, B.; Arpa, M.; Bedir, R. Tumor Budding is a reliable predictor for death and metastasis in invasive ductal breast cancer and correlates with other prognostic clinicopathological parameters. Ann. Diagn. Pathol. 2021, 54, 151792. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, N.J.; Jensen, D.H.; Lelkaitis, G.; Kiss, K.; Charabi, B.; Specht, L.; von Buchwald, C. Construction of a pathological risk model of occult lymph node metastases for prognostication by semi-automated image analysis of tumor budding in early-stage oral squamous cell carcinoma. Oncotarget 2017, 8, 18227–18237. [Google Scholar] [CrossRef]
- Chatterjee, D.; Bansal, V.; Malik, V.; Bhagat, R.; Punia, R.S.; Handa, U.; Gupta, A.; Dass, A. Tumor Budding and Worse Pattern of Invasion Can Predict Nodal Metastasis in Oral Cancers and Associated with Poor Survival in Early-Stage Tumors. Ear Nose Throat J. 2019, 98, E112–E119. [Google Scholar] [CrossRef]
- Joshi, P.; Pol, J.; Chougule, M.; Jadhav, K.; Patil, S.; Patil, S. Tumor budding—A promising prognostic histopathological parameter in oral squamous cell carcinoma—A comparative immunohistochemical study. J. Oral Maxillofac. Pathol. 2020, 24, 587. [Google Scholar] [CrossRef]
- Hayes, B.D.; Maguire, A.; Conlon, N.; Gibbons, D.; Wang, L.M.; Sheahan, K. Reproducibility of the rapid bud count method for assessment of tumor budding in stage II colorectal cancer. Am. J. Surg. Pathol. 2010, 34, 746–748. [Google Scholar] [CrossRef]
- De Smedt, L.; Palmans, S.; Sagaert, X. Tumour budding in colorectal cancer: What do we know and what can we do? Virchows Arch. 2016, 468, 397–408. [Google Scholar] [CrossRef]
- Kowalski, L.P.; Bagietto, R.; Lara, J.R.; Santos, R.L.; Silva, J.F.; Magrin, J. Prognostic significance of the distribution of neck node metastasis from oral carcinoma. Head Neck 2000, 22, 207–214. [Google Scholar] [CrossRef]
- Gil, Z.; Carlson, D.L.; Boyle, J.O.; Kraus, D.H.; Shah, J.P.; Shaha, A.R.; Singh, B.; Wong, B.R.; Patel, S.G. Lymph node density is a significant predictor of outcome in patients with oral cancer. Cancer 2009, 115, 5700–5710. [Google Scholar] [CrossRef]
- D’Cruz, A.K.; Vaish, R.; Kapre, N.; Dandekar, M.; Gupta, S.; Hawaldar, R.; Agarwal, J.P.; Pantvaidya, G.; Chaukar, D.; Deshmukh, A.; et al. Head and Neck Disease Management Group. Elective versus Therapeutic Neck Dissection in Node-Negative Oral Cancer. N. Engl. J. Med. 2015, 373, 521–529. [Google Scholar] [CrossRef]
- Ren, Z.H.; Xu, J.L.; Li, B.; Fan, T.F.; Ji, T.; Zhang, C.P. Elective versus therapeutic neck dissection in node-negative oral cancer: Evidence from five randomized controlled trials. Oral Oncol. 2015, 51, 976–981. [Google Scholar] [CrossRef]
- Fasunla, A.J.; Greene, B.H.; Timmesfeld, N.; Wiegand, S.; Werner, J.A.; Sesterhenn, A.M. A meta-analysis of the randomized controlled trials on elective neck dissection versus therapeutic neck dissection in oral cavity cancers with clinically node-negative neck. Oral Oncol. 2011, 47, 320–324. [Google Scholar] [CrossRef]
- Kumar, A.; Ghai, S.; Mhaske, S.; Singh, R. Elective Neck Dissection Versus Therapeutic Neck Dissection in Clinically Node-Negative Early Stage Oral Cancer: A Meta-analysis of Randomized Controlled Trials. J. Maxillofac. Oral Surg. 2022, 21, 340–349. [Google Scholar] [CrossRef]
- Kowalski, L.P.; Sanabria, A. Elective neck dissection in oral carcinoma: A critical review of the evidence. Acta Otorhinolaryngol. Ital. 2007, 27, 113–117. [Google Scholar] [PubMed]
- Gane, E.M.; Michaleff, Z.A.; Cottrell, M.A.; McPhail, S.M.; Hatton, A.L.; Panizza, B.J.; O’Leary, S.P. Prevalence, incidence, and risk factors for shoulder and neck dysfunction after neck dissection: A systematic review. Eur. J. Surg. Oncol. 2017, 43, 1199–1218. [Google Scholar] [CrossRef] [PubMed]
- Bjerkli, I.H.; Laurvik, H.; Nginamau, E.S.; Søland, T.M.; Costea, D.; Hov, H.; Uhlin-Hansen, L.; Hadler-Olsen, E.; Steigen, S.E. Tumor budding score predicts lymph node status in oral tongue squamous cell carcinoma and should be included in the pathology report. PLoS ONE 2020, 15, e0239783. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.H.; O’Sullivan, B. Overview of the 8th Edition TNM Classification for Head and Neck Cancer. Curr. Treat. Options Oncol. 2017, 18, 40. [Google Scholar] [CrossRef]
- Su, Z.; Duan, Z.; Pan, W.; Wu, C.; Jia, Y.; Han, B.; Li, C. Predicting extracapsular spread of head and neck cancers using different imaging techniques: A systematic review and meta-analysis. Int. J. Oral Maxillofac. Surg. 2016, 45, 413–421. [Google Scholar] [CrossRef]
- Dhanda, J.; Triantafyllou, A.; Liloglou, T.; Kalirai, H.; Lloyd, B.; Hanlon, R.; Shaw, R.J.; Sibson, D.R.; Risk, J.M. SERPINE1 and SMA expression at the invasive front predict extracapsular spread and survival in oral squamous cell carcinoma. Br. J. Cancer 2014, 111, 2114–2121. [Google Scholar] [CrossRef]
- Sarioglu, S.; Acara, C.; Akman, F.C.; Dag, N.; Ecevit, C.; Ikiz, A.O.; Cetinayak, O.H.; Ada, E.; Dokuz Eylül Head and Neck Tumour Group (DEHNTG). Tumor budding as a prognostic marker in laryngeal carcinoma. Pathol. Res. Pract. 2010, 206, 88–92. [Google Scholar] [CrossRef]
- Ekmekci, S.; Kucuk, U.; Kokkoz, S.; Cakir, E.; Gumussoy, M. Tumor budding in laryngeal carcinoma. Indian. J. Pathol. Microbiol. 2019, 62, 7–10. [Google Scholar] [CrossRef]
- Karpathiou, G.; Vieville, M.; Gavid, M.; Camy, F.; Dumollard, J.M.; Magné, N.; Froudarakis, M.; Prades, J.M.; Peoc’h, M. Prognostic significance of tumor budding, tumor-stroma ratio, cell nests size, and stroma type in laryngeal and pharyngeal squamous cell carcinomas. Head Neck 2019, 41, 1918–1927. [Google Scholar] [CrossRef]
| Variables | Total (n = 98) | Tumor Budding Count | p-Value | |
|---|---|---|---|---|
| Low (≤4) (n = 60) | High (>4) (n = 38) | |||
| Age | 65 (19–92) | 63 (19–92) | 68 (33–92) | 0.105 |
| Gender | 0.327 | |||
| Female | 19 (19.4%) | 14 (23.3%) | 5 (13.2%) | |
| Male | 79 (80.6%) | 46 (76.7%) | 33 (86.8%) | |
| Primary Region | 0.323 | |||
| Larynx | 48 (49%) | 29 (48.3%) | 19 (50%) | |
| Oral cavity | 28 (28.6%) | 19 (31.7%) | 9 (23.7%) | |
| Skin | 10 (10.2%) | 3 (5%) | 7 (18.5%) | |
| Lips | 7 (7.1%) | 6 (10%) | 1 (2.6%) | |
| Oropharynx | 3 (3.1%) | 2 (3.3%) | 1 (2.6%) | |
| Parotid | 2 (2%) | 1 (1.7%) | 1 (2.6%) | |
| Tumor grade | 0.189 | |||
| Mild | 8 (8.2%) | 7 (11.7%) | 1 (2.6%) | |
| Moderate | 85 (86.7%) | 51 (85%) | 34 (89.5%) | |
| Severe | 5 (5.1%) | 2 (3.3%) | 3 (7.9%) | |
| Perineural invasion | 42 (42.9%) | 21 (35%) | 21 (55.3%) | 0.077 |
| Lymphovascular invasion | 37 (37.8%) | 17 (28.3%) | 20 (52.6%) | 0.028 |
| Depth of Invasion | 9 (2–25) | 9 (2–22) | 11 (2–25) | 0.186 |
| ≤5 mm | 20 (23.8%) | 14 (26.4%) | 6 (19.4%) | 0.104 |
| 5–10 mm | 25 (29.8%) | 19 (35.8%) | 6 (19.4%) | |
| >10 mm | 39 (46.4%) | 20 (37.7%) | 19 (61.3%) | |
| Anterior commissure involvement | 25 (89.3%) | 14 (82.4%) | 11 (100%) | 0.258 |
| Inner perichondrium involvement * | 30 (62.5%) | 15 (51.7%) | 15 (78.9%) | 0.110 |
| External perichondrium involvement * | 19 (39.6%) | 9 (31.0%) | 10 (52.6%) | 0.232 |
| Extralaryngeal spread | 12 (25.5%) | 5 (17.2%) | 7 (38.9%) | 0.168 |
| Metastatic lymph node | 44 (44.9%) | 19 (31.7%) | 25 (65.8%) | 0.002 |
| Extranodal extension | 24 (24.5%) | 5 (8.3%) | 19 (50%) | <0.001 |
| Post-op adjuvant chemotherapy | 34 (35.8%) | 17 (29.3%) | 17 (45.9%) | 0.153 |
| Post-op adjuvant radiotherapy | 50 (52.6%) | 26 (44.8%) | 24 (64.9%) | 0.090 |
| Metastasis | 4 (4.1%) | 1 (1.7%) | 3 (7.9%) | 0.296 |
| Variables | Total (n = 98) | Tumor Budding Count | p-Value | |
|---|---|---|---|---|
| Low (≤4) (n = 60) | High (>4) (n = 38) | |||
| T Category | 0.021 | |||
| T1 | 16 (16.3%) | 15 (25%) | 1 (2.6%) | |
| T2 | 28 (28.6%) | 17 (28.3%) | 11 (28.9%) | |
| T3 | 34 (34.7%) | 19 (31.7%) | 15 (39.5%) | |
| T4 | 20 (20.4%) | 9 (15%) | 11 (28.9%) | |
| N Category | <0.001 | |||
| N0 | 54 (55.1%) | 41 (68.3%) | 13 (34.2%) | |
| N1 | 13 (13.3%) | 8 (13.3%) | 5 (13.2%) | |
| N2 | 9 (9.2%) | 7 (11.7%) | 2 (5.3%) | |
| N3 | 22 (22.4%) | 4 (6.7%) | 18 (47.4%) | |
| N2 | n = 9 | n = 7 | n = 2 | 0.413 |
| N2A | 2 (22.2%) | 1 (14.3%) | 1 (50%) | |
| N2B | 4 (44.4%) | 3 (42.9%) | 1 (50%) | |
| N2C | 3 (33.3%) | 3 (42.9%) | - | |
| AJCC 8 Stage | 0.006 | |||
| Stage 1 | 12 (12.2%) | 12 (20%) | - | |
| Stage 2 | 17 (17.3%) | 11 (18.3%) | 6 (15.8%) | |
| Stage 3 | 27 (27.6%) | 18 (30%) | 9 (23.7%) | |
| Stage 4 | 42 (42.9%) | 19 (31.7%) | 23 (60.5%) | |
| Univariate | Multivariate | |||
|---|---|---|---|---|
| Variables | Hazard Ratio (95% CI) | p-Value | Hazard Ratio (95% CI) | p-Value |
| Tumor budding (low–high) | 6.9 (3.1–15.4) | <0.001 | 3.4 (1.3–9.2) | 0.013 |
| Perineural invasion | 3.2 (1.6–6.7) | 0.002 | 1.3 (0.5–3.6) | 0.626 |
| Lymphovascular invasion | 2.4 (1.2–4.7) | 0.017 | 0.3 (0.1–1.0) | 0.051 |
| Metastatic lymph node involvement | 3.5 (1.6–7.6) | 0.001 | 1.1 (0.2–5.1) | 0.901 |
| Univariate | Multivariate | |||
|---|---|---|---|---|
| Variables | Odds Ratio (95% CI) | p-Value | Odds Ratio (95% CI) | p-Value |
| Tumor budding (low–high) | 4.2 (1.8–9.8) | 0.001 | 4.9 (1.2–20.5) | 0.029 |
| Perineural invasion | 4.1 (1.8–9.7) | 0.001 | 2.2(0.5–10.0) | 0.304 |
| Lymphovascular invasion | 57.8 (14.8–225.5) | <0.001 | 74.2 (12.9–426.8) | <0.001 |
| Depth of invasion > 10 mm | 5.4 (1.6–17.9) | 0.006 | 0.5 (0.1–2.5) | 0.303 |
| Univariate | Multivariate | |||
|---|---|---|---|---|
| Variables | Hazard Ratio (95% CI) | p-Value | Hazard Ratio (95% CI) | p-Value |
| Tumor budding (low–high) | 2.2 (0.9–5.3) | 0.071 | ||
| Perineural invasion | 1.3 (0.5–3.0) | 0.600 | ||
| Lymphovascular invasion | 1.4 (0.6–3.4) | 0.416 | ||
| Metastatic lymph node involvement | 3.1 (1.2–8.1) | 0.018 | 1.8 (0.5–6.3) | 0.379 |
| Extranodal extension | 3.8 (1.6–9.0) | 0.002 | 2.6 (0.8–8.2) | 0.100 |
| Univariate | Multivariate | |||
|---|---|---|---|---|
| Variables | Hazard Ratio (95% CI) | p-Value | Hazard Ratio (95% CI) | p-Value |
| Tumor budding (low–high) | 7.5 (1.6–35.6) | 0.011 | 3.5 (0.6–19.0) | 0.142 |
| Thyroid internal cartilage involvement | 6.5 (1.8–51.3) | 0.046 | 3.8 (0.5–31.0) | 0.214 |
| Metastatic lymph node involvement | 5.8 (1.2–27.4) | 0.026 | 1.7 (0.2–12.9) | 0.596 |
| Extranodal extension | 6.2 (1.7–22.0) | 0.005 | 2.4 (0.5–12.1) | 0.294 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gül, O.; Erdivanlı, Ö.Ç.; Birinci, M.; Terzi, S.; Çeliker, M.; Okçu, O.; Öztürk, Ç.; Yemiş, T.; Çeliker, F.B.; Özergin Coşkun, Z.; et al. Impact of Tumor Budding in Head and Neck Cancers on Neck Lymph Node Metastasis and Prognosis. J. Clin. Med. 2025, 14, 5224. https://doi.org/10.3390/jcm14155224
Gül O, Erdivanlı ÖÇ, Birinci M, Terzi S, Çeliker M, Okçu O, Öztürk Ç, Yemiş T, Çeliker FB, Özergin Coşkun Z, et al. Impact of Tumor Budding in Head and Neck Cancers on Neck Lymph Node Metastasis and Prognosis. Journal of Clinical Medicine. 2025; 14(15):5224. https://doi.org/10.3390/jcm14155224
Chicago/Turabian StyleGül, Oğuz, Özlem Çelebi Erdivanlı, Mehmet Birinci, Suat Terzi, Metin Çeliker, Oğuzhan Okçu, Çiğdem Öztürk, Tuğba Yemiş, Fatma Beyazal Çeliker, Zerrin Özergin Coşkun, and et al. 2025. "Impact of Tumor Budding in Head and Neck Cancers on Neck Lymph Node Metastasis and Prognosis" Journal of Clinical Medicine 14, no. 15: 5224. https://doi.org/10.3390/jcm14155224
APA StyleGül, O., Erdivanlı, Ö. Ç., Birinci, M., Terzi, S., Çeliker, M., Okçu, O., Öztürk, Ç., Yemiş, T., Çeliker, F. B., Özergin Coşkun, Z., & Dursun, E. (2025). Impact of Tumor Budding in Head and Neck Cancers on Neck Lymph Node Metastasis and Prognosis. Journal of Clinical Medicine, 14(15), 5224. https://doi.org/10.3390/jcm14155224






