Effects of Photobiomodulation Therapy (PBMT) in the Management of Postoperative Pain After Third Lower Molar Extraction: A Narrative Review
Abstract
1. Introduction
1.1. Low-Level Laser Therapy (LLLT) on Tissues and Safety
1.2. Low-Level Laser Therapy (LLLT) Effects on Pain
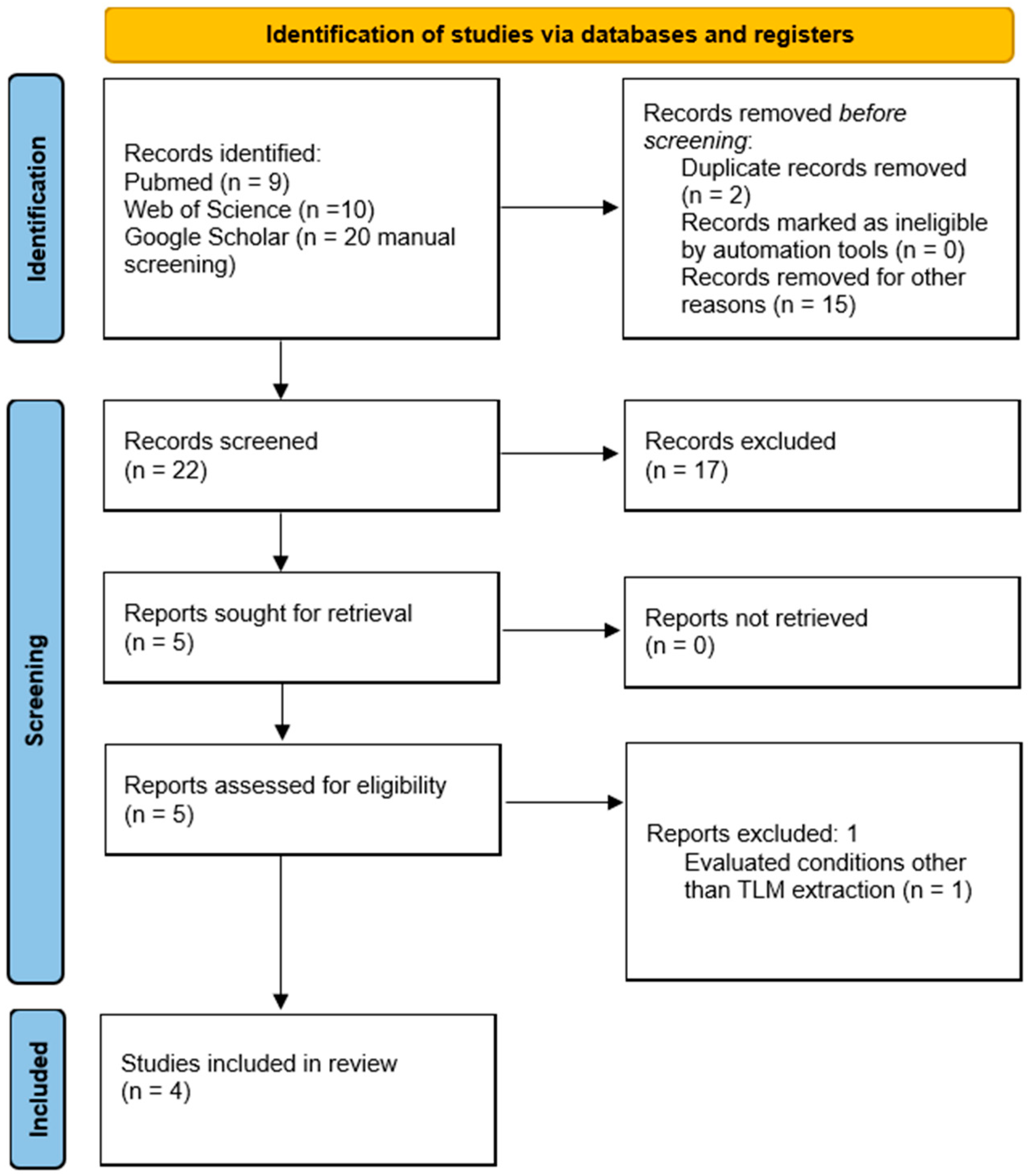
2. Materials and Methods
- Studies focused on the clinical effects of PBMT in pain reduction after TLM extraction;
- Studies performed in vivo on humans;
- Randomized controlled trials (RCTs).
- Studies that use lasers to treat other oral conditions;
- Studies not available in English.
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| TLM | third lower molar |
| PBMT | photobiomodulation therapy |
| M2M | mandibular second molars |
| AIN | alveolar inferior nerve |
| IL | interleukin |
| MCP | monocyte chemoattractant protein |
| TNF | tumor necrosis factor |
| EMI | electromagnetic interference |
| NF-kB | nuclear factor kappa B |
| LLLT | low-level laser therapy |
References
- Blondeau, F.; Daniel, N.G. Extraction of Impacted Mandibular Third Molars: Postoperative Complications and Their Risk Factors. J. Can. Dent. Assoc. 2007, 73, 325. [Google Scholar] [PubMed]
- Mummolo, S.; Gallusi, G.; Strappa, E.M.; Grilli, F.; Mattei, A.; Fiasca, F.; Bambini, F.; Memè, L. Prediction of Mandibular Third Molar Impaction Using Linear and Angular Measurements in Young Adult Orthopantomograms. Appl. Sci. 2023, 13, 4637. [Google Scholar] [CrossRef]
- Al-Gunaid, T.H.; Bukhari, A.K.; El Khateeb, S.M.; Yamaki, M. Relationship of Mandibular Ramus Dimensions to Lower Third Molar Impaction. Eur. J. Dent. 2019, 13, 213–221. [Google Scholar] [CrossRef] [PubMed]
- Ye, Z.X.; Qian, W.H.; Wu, Y.B.; Yang, C. Pathologies Associated with the Mandibular Third Molar Impaction. Sci. Prog. 2021, 104, 00368504211013247. [Google Scholar] [CrossRef] [PubMed]
- Achararit, P.; Manaspon, C.; Jongwannasiri, C.; Kulthanaamondhita, P.; Itthichaisri, C.; Chantarangsu, S.; Osathanon, T.; Phattarataratip, E.; Sappayatosok, K. Impacted Lower Third Molar Classification and Difficulty Index Assessment: Comparisons among Dental Students, General Practitioners and Deep Learning Model Assistance. BMC Oral. Health 2025, 25, 152. [Google Scholar] [CrossRef] [PubMed]
- Isola, G.; Matarese, M.; Ramaglia, L.; Cicciù, M.; Matarese, G. Evaluation of the Efficacy of Celecoxib and Ibuprofen on Postoperative Pain, Swelling, and Mouth Opening after Surgical Removal of Impacted Third Molars: A Randomized, Controlled Clinical Trial. Int. J. Oral. Maxillofac. Surg. 2019, 48, 1348–1354. [Google Scholar] [CrossRef] [PubMed]
- Briguglio, F.; Zenobio, E.G.; Isola, G.; Briguglio, R.; Briguglio, E.; Farronato, D.; Shibli, J.A. Complications in Surgical Removal of Impacted Mandibular Third Molars in Relation to Flap Design: Clinical and Statistical Evaluations. Quintessence Int. 2011, 42, 445–453. [Google Scholar] [PubMed]
- Bailey, E.; Worthington, H.V.; van Wijk, A.; Yates, J.M.; Coulthard, P.; Afzal, Z. Ibuprofen and/or Paracetamol (Acetaminophen) for Pain Relief after Surgical Removal of Lower Wisdom Teeth. Cochrane Database Syst. Rev. 2013, 2013, CD004624. [Google Scholar] [CrossRef] [PubMed]
- Miroshnychenko, A.; Azab, M.; Ibrahim, S.; Roldan, Y.; Diaz Martinez, J.P.; Tamilselvan, D.; He, L.; Urquhart, O.; Verdugo-Paiva, F.; Tampi, M.; et al. Corticosteroids for Managing Acute Pain Subsequent to Surgical Extraction of Mandibular Third Molars: A Systematic Review and Meta-Analysis. J. Am. Dent. Assoc. 2023, 154, 727–741.e10. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, S.; Meer, M.; George, R. Efficacy of Photobiomodulation on Accelerating Bone Healing after Tooth Extraction: A Systematic Review. Lasers Med. Sci. 2019, 34, 685–692. [Google Scholar] [CrossRef] [PubMed]
- Camolesi, G.C.V.; El Kattan, A.S.; Lopez-Lopez, J.O.S.É.; Blanco-Carrión, A.; García-García, A.B.E.L.; Gándara-Vila, P.; Pérez-Sayáns, M. Pain, Oedema and trismus responses following photobiomodulation therapy immediately after lower third molar extraction: Results of a randomized, doble-blind and split mouth clinical trial. J. Evid.-Based Dent. Pract. 2025, 25, 102080. [Google Scholar] [CrossRef] [PubMed]
- de Barros, D.D.; dos Santos Barros Catão, J.S.; Ferreira, A.C.D.; Simões, T.M.S.; Almeida, R.d.A.C.; de Vasconcelos Catão, M.H.C. Low-Level Laser Therapy Is Effective in Controlling Postoperative Pain in Lower Third Molar Extractions: A Systematic Review and Meta-Analysis. Lasers Med. Sci. 2022, 37, 2363–2377. [Google Scholar] [CrossRef] [PubMed]
- Lacerda-Santos, J.T.; Granja, G.L.; Firmino, R.T.; Dias, R.d.F.; de Melo, D.P.; Granville-Garcia, A.F.; Martins, C.C. Use of Photobiomodulation to Reduce Postoperative Pain, Edema, and Trismus After Third Molar Surgery: A Systematic Review and Meta-Analysis. J. Oral Maxillofac. Surg. 2023, 81, 1135–1145. [Google Scholar] [CrossRef] [PubMed]
- Thorat, S.; Nilesh, K. Efficacy of Low-Level Laser Therapy in the Management of Postoperative Surgical Sequelae after Surgical Removal of Impacted Mandibular Third Molars. Natl. J. Maxillofac. Surg. 2022, 13, S52–S56. [Google Scholar] [CrossRef] [PubMed]
- Peavy, G.M. Lasers and Laser–Tissue Interaction. Vet. Clin. N. Am. Small Anim. Pract. 2002, 32, 517–534. [Google Scholar] [CrossRef] [PubMed]
- Ansari, M.A.; Erfanzadeh, M.; Mohajerani, E. Mechanisms of Laser-Tissue Interaction: II. Tissue Thermal Properties. J. Lasers Med. Sci. 2013, 4, 99–106. [Google Scholar] [PubMed]
- Kahraman, S.A. Low-Level Laser Therapy in Oral and Maxillofacial Surgery. Oral. Maxillofac. Surg. Clin. N. Am. 2004, 16, 277–288. [Google Scholar] [CrossRef] [PubMed]
- Sliney, D.H. Laser Safety. Lasers Surg. Med. 1995, 16, 215–225. [Google Scholar] [CrossRef] [PubMed]
- Amarillas-Escobar, E.D.; Toranzo-Fernández, J.M.; Martínez-Rider, R.; Noyola-Frías, M.A.; Hidalgo-Hurtado, J.A.; Serna, V.M.F.; Gordillo-Moscoso, A.; Pozos-Guillén, A.J. Use of Therapeutic Laser After Surgical Removal of Impacted Lower Third Molars. J. Oral Maxillofac. Surg. 2010, 68, 319–324. [Google Scholar] [CrossRef] [PubMed]
- Lister, T.; Grant, L.; Lee, S.-M.; Cole, R.P.; Jones, A.; Taylor, T.; Mayo, A.; Wright, P.A. Electromagnetic Interference from Lasers and Intense Light Sources in the Treatment of Patients with Artificial Pacemakers and Other Implantable Cardiac Devices. Lasers Med. Sci. 2015, 30, 1619–1622. [Google Scholar] [CrossRef] [PubMed]
- Ferrante, M.; Petrini, M.; Trentini, P.; Perfetti, G.; Spoto, G. Effect of Low-Level Laser Therapy after Extraction of Impacted Lower Third Molars. Lasers Med. Sci. 2013, 28, 845–849. [Google Scholar] [CrossRef] [PubMed]
- Landucci, A.; Wosny, A.C.; Uetanabaro, L.C.; Moro, A.; Araujo, M.R. Efficacy of a Single Dose of Low-Level Laser Therapy in Reducing Pain, Swelling, and Trismus Following Third Molar Extraction Surgery. Int. J. Oral. Maxillofac. Surg. 2016, 45, 392–398. [Google Scholar] [CrossRef] [PubMed]
- Pereira, D.A.; Mendes, P.G.J.; de Souza Santos, S.; de Rezende Barbosa, G.L.; Pessoa, R.S.E.; de Oliveira, G.J.P.L. Effect of the Association of Infra-Red and Red Wavelength Photobiomodulation Therapy on the Healing of Post-Extraction Sockets of Third Lower Molars: A Split-Mouth Randomized Clinical Trial. Lasers Med. Sci. 2022, 37, 2479–2487. [Google Scholar] [CrossRef] [PubMed]
- Fernando, S.; Hill, C.M.; Walker, R. A Randomised Double Blind Comparative Study of Low Level Laser Therapy Following Surgical Extraction of Lower Third Molar Teeth. Br. J. Oral Maxillofac. Surg. 1993, 31, 170–172. [Google Scholar] [CrossRef] [PubMed]
- Kahraman, S.A.; Cetiner, S.; Strauss, R.A. The Effects of Transcutaneous and Intraoral Low-Level Laser Therapy After Extraction of Lower Third Molars: A Randomized Single Blind, Placebo Controlled Dual-Center Study. Photomed. Laser Surg. 2017, 35, 401–407. [Google Scholar] [CrossRef] [PubMed]
- de Paula, L.M.; de Andrade Fernandes, A.C.; Evangelista, B.C.; do Couto Lima-Moreira, F.; Andrade, G.; de Andrade Fernandes, J.V.; de Castro, F.L.A.; Roriz, V.M. Clinical and Thermographic Evaluation after Lower Third Molar Extractions and the Application of Different Photobiomodulation Protocols: Double-Blind Randomised Clinical Trial. Clin. Oral. Investig. 2024, 28, 203. [Google Scholar] [CrossRef] [PubMed]
- Ahrari, F.; Eshghpour, M.; Zare, R.; Ebrahimi, S.; Fallahrastegar, A.; Khaki, H. Effectiveness of Low-Level Laser Irradiation in Reducing Pain and Accelerating Socket Healing after Undisturbed Tooth Extraction. J. Lasers Med. Sci. 2020, 11, 274–279. [Google Scholar] [CrossRef] [PubMed]
- Giansiracusa, A.; Parrini, S.; Baldini, N.; Bartali, E.; Chisci, G. The Effect of Photobiomodulation on Third Molar Wound Recovery: A Systematic Review with Meta-Analysis. J. Clin. Med. 2024, 13, 5402. [Google Scholar] [CrossRef] [PubMed]
- Iijima, K.; Shimoyama, N.; Shimoyama, M.; Mizuguchi, T. Effect of Low-Power He-Ne Laser on Deformability of Stored Human Erythrocytes. J. Clin. Laser Med. Surg. 1993, 11, 185–189. [Google Scholar] [CrossRef] [PubMed]
- Lemes, C.H.J.; da Rosa, W.L.d.O.; Sonego, C.L.; Lemes, B.J.; Moraes, R.R.; da Silva, A.F. Does Laser Therapy Improve the Wound Healing Process after Tooth Extraction? A Systematic Review. Wound Repair. Regen. 2019, 27, 102–113. [Google Scholar] [CrossRef] [PubMed]
- Brignardello-Petersen, R.; Carrasco-Labra, A.; Araya, I.; Yanine, N.; Beyene, J.; Shah, P.S. Is Adjuvant Laser Therapy Effective for Preventing Pain, Swelling, and Trismus After Surgical Removal of Impacted Mandibular Third Molars? A Systematic Review and Meta-Analysis. J. Oral Maxillofac. Surg. 2012, 70, 1789–1801. [Google Scholar] [CrossRef] [PubMed]
- Duarte de Oliveira, F.J.; Brasil, G.M.L.C.; Araújo Soares, G.P.; Fernandes Paiva, D.F.; de Assis de Souza Júnior, F. Use of Low-Level Laser Therapy to Reduce Postoperative Pain, Edema, and Trismus Following Third Molar Surgery: A Systematic Review and Meta-Analysis. J. Cranio-Maxillofac. Surg. 2021, 49, 1088–1096. [Google Scholar] [CrossRef] [PubMed]
- Raja, S.N.; Carr, D.B.; Cohen, M.; Finnerup, N.B.; Flor, H.; Gibson, S.; Keefe, F.J.; Mogil, J.S.; Ringkamp, M.; Sluka, K.A.; et al. The Revised International Association for the Study of Pain Definition of Pain: Concepts, Challenges, and Compromises. Pain 2020, 161, 1976–1982. [Google Scholar] [CrossRef] [PubMed]
- Malamed, S.F. Pain Management Following Dental Trauma and Surgical Procedures. Dent. Traumatol. 2023, 39, 295–303. [Google Scholar] [CrossRef] [PubMed]
| Authors | Study Design | Setting | N° of Patients | Gender (Male/ Female) | Mean Age (Years) | Type of Laser Used | Application | Treatment | Pain Scale Used and Results | Time Evaluation (Days) |
|---|---|---|---|---|---|---|---|---|---|---|
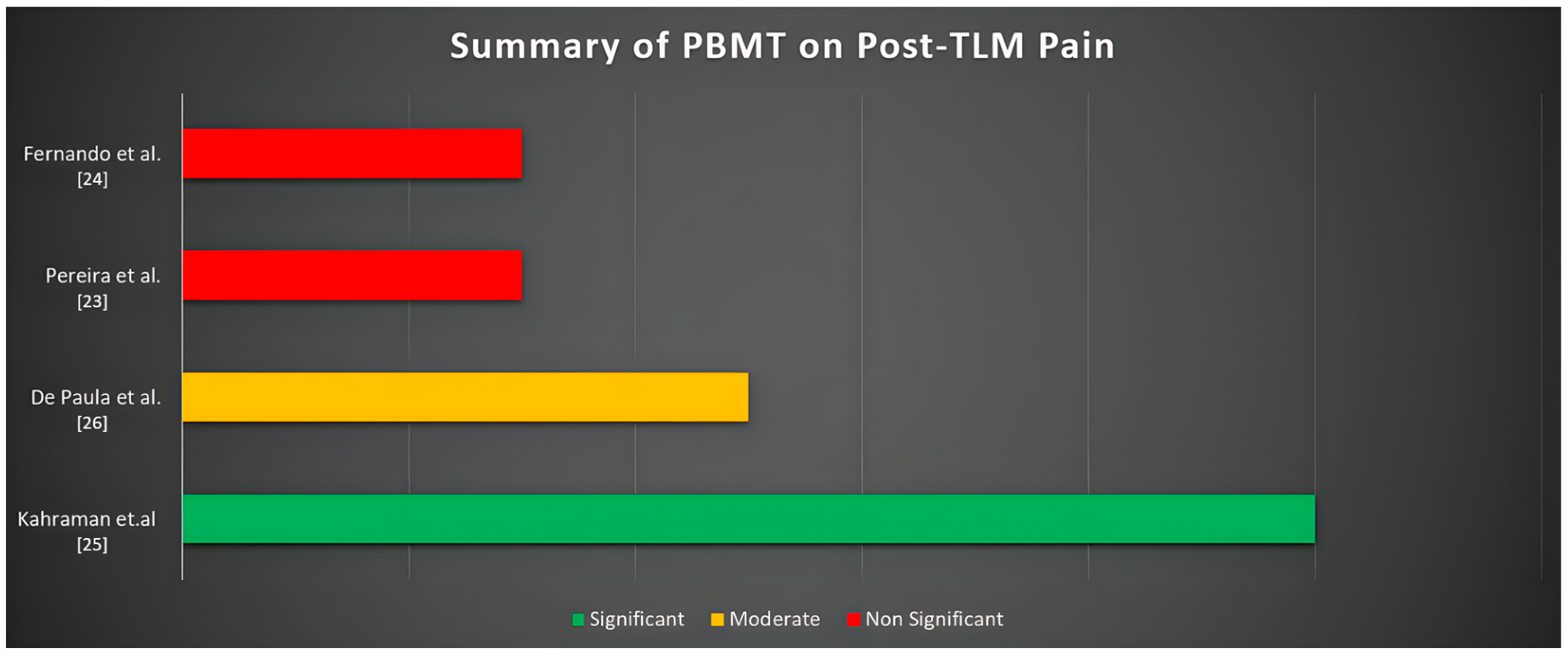
| Fernando et al. (1993) [24] | RCT | University | 64 | N.A. | From 18 to 50 | CBM Master 3 semiconductor laser of 830 nm wavelength and 30 mW mean power of beam | The laser was inserted into the post-extraction socket and used for 132 s for each extracted tooth | Test Group: third lower molar extraction plus laser application Control group: third lower molar extraction plus placebo | A verbal digital pain scale described by Giles (1984). No statistically significant difference was found at 1, 3, and 7 days. (p = 0.174; 0.772.; 0.884) | 1 to 7 days after surgery |
| Kahraman et al. (2017) [25] | RCT | University | 60 | 24M/36F | 21.88 ± 4.32 | Lumenis (Lumenis, Inc., Santa Clara, CA, USA) gallium aluminum arsenide (GaAlAs) 830 nm diode lasers set at 100 mW for extraoral Using and BTL 2000 (Medictinedic, Denmark) GaAlAs 830 nm diode lasers set at 100 mW for intraoral use | The laser was administered immediately before and after the extraction procedure. The applications lasted for 15 s. (3 J/cm2 of energy, 10.0 Hz, 63 mW) in continuous mode | Test Group 1: third lower molar extraction plus laser transcutaneous application Test Group 2: third lower molar extraction plus laser intraoral application Control group: third lower molar extraction plus placebo | VAS (visual analogic scale) was used. A statistically significant result was recorded between the intraoral laser application and the placebo group (p = 0.001) between the intraoral and the transcutaneous group (p = 0.001) | 1 to 7 days after surgery |
| Pereira et al. (2022) [23] | RCT | University | 20 | N.A. | Over 18 years old | The GaAlAs laser (TheraLase, λ 660 nm and λ 808 nm 100 mW, ϕ ∼ 0.600 μm, tip divergence = 0.37 rad, CW, spot area 0.0283 cm2, DMC Equipamentos, São Carlos, SP, Brazil) | Two irradiations were performed intraorally in the middle of the extraction socket, at the buccal and lingual sides in contact with the alveolar sockets. The laser was irradiated immediately after tooth extraction and 3 and 7 days after surgery for 40 s in each session. | Test Group: third lower molar extraction plus laser application Control group: third lower molar extraction | VAS (visual analogic scale) was used. No statistically significant differences were found regarding the pain felt among the test and control groups | After tooth extraction and 3 and 7 days after surgery for 40 s in each session |
| De Paula et al. (2024) [26] | RCT | University | 31 | 11M/20F | 21 years (minimum 16; maximum 28) | Diode laser device (Therapy ECDMC Equipment, São Paulo, Brazil) used with a power of 100 milliwatts (mW) in continuous mode | The irradiation of dental sockets in the 808 (infrared laser) group was applied at three intraoral points with 2 Joules (J), for 20 s for each point. The dental sockets in the 808 + 606 (infrared + red laser) were irradiated in the same way. | Test Group: third lower molar extraction plus laser application with a wavelength of 808 nm Control group: third lower molar extraction plus laser application with a wavelength of 808 + 606 nm | VAS (visual analogic scale) was used. The pain assessment on the 7th day showed a statistically significant difference, being higher in the 808 + 660 group (p = 0.031). | After tooth extraction and 3 and 7 days after surgery for 40 s in each session |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mauriello, L.; Cuozzo, A.; Pezzella, V.; Iorio-Siciliano, V.; Isola, G.; Spagnuolo, G.; Ramaglia, L.; Blasi, A. Effects of Photobiomodulation Therapy (PBMT) in the Management of Postoperative Pain After Third Lower Molar Extraction: A Narrative Review. J. Clin. Med. 2025, 14, 5210. https://doi.org/10.3390/jcm14155210
Mauriello L, Cuozzo A, Pezzella V, Iorio-Siciliano V, Isola G, Spagnuolo G, Ramaglia L, Blasi A. Effects of Photobiomodulation Therapy (PBMT) in the Management of Postoperative Pain After Third Lower Molar Extraction: A Narrative Review. Journal of Clinical Medicine. 2025; 14(15):5210. https://doi.org/10.3390/jcm14155210
Chicago/Turabian StyleMauriello, Leopoldo, Alessandro Cuozzo, Vitolante Pezzella, Vincenzo Iorio-Siciliano, Gaetano Isola, Gianrico Spagnuolo, Luca Ramaglia, and Andrea Blasi. 2025. "Effects of Photobiomodulation Therapy (PBMT) in the Management of Postoperative Pain After Third Lower Molar Extraction: A Narrative Review" Journal of Clinical Medicine 14, no. 15: 5210. https://doi.org/10.3390/jcm14155210
APA StyleMauriello, L., Cuozzo, A., Pezzella, V., Iorio-Siciliano, V., Isola, G., Spagnuolo, G., Ramaglia, L., & Blasi, A. (2025). Effects of Photobiomodulation Therapy (PBMT) in the Management of Postoperative Pain After Third Lower Molar Extraction: A Narrative Review. Journal of Clinical Medicine, 14(15), 5210. https://doi.org/10.3390/jcm14155210