Effect of Perineural Dexamethasone as an Adjuvant to Ropivacaine in Rectus Sheath Block for Radical Cystectomy: A Randomized Controlled Trial
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
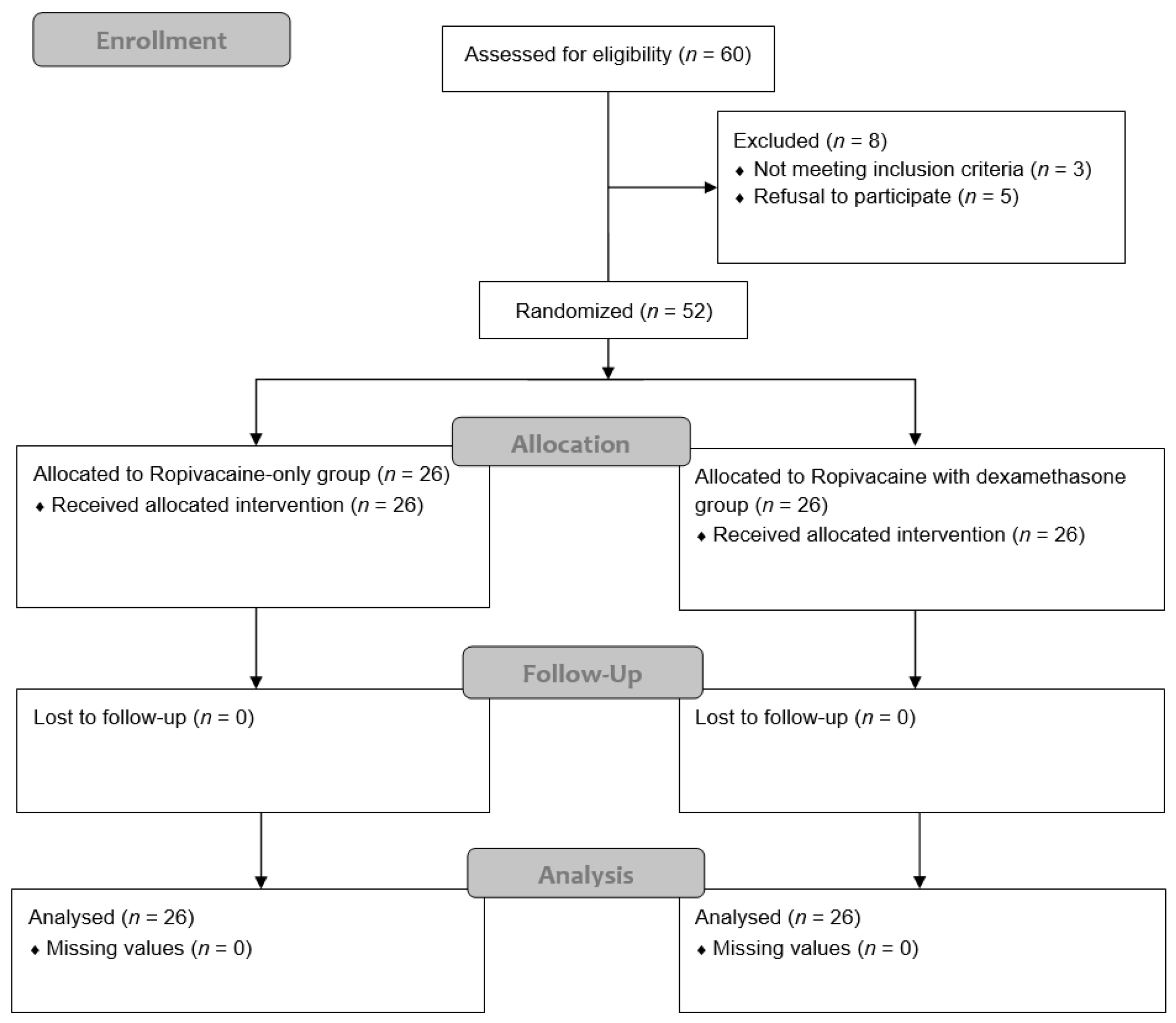
2.2. Randomization and Blinding
2.3. Anesthesia
2.4. Rectus Sheath Block Technique
2.5. Postoperative Pain Management
2.6. Outcome Assessment
2.7. Statistical Analysis
3. Results
3.1. Primary Outcomes
3.2. Secondary Outcomes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| NSAIDs | Nonsteroidal anti-inflammatory drugs |
| PNB | Peripheral nerve block |
| PONV | Postoperative nausea and vomiting |
| RSB | Rectus sheath block |
| TAP | Transversus abdominis plane |
| PACU | Post-anesthesia care unit |
| NRS | Numeric rating scale |
| IV-PCA | Intravenous patient-controlled analgesia |
References
- Howle, R.; Ng, S.C.; Wong, H.Y.; Onwochei, D.; Desai, N. Comparison of analgesic modalities for patients undergoing midline laparotomy: A systematic review and network meta-analysis. Can. J. Anaesth. 2022, 69, 140–176. [Google Scholar] [CrossRef]
- Smith, P.R.; Baig, M.A.; Brito, V.; Bader, F.; Bergman, M.I.; Alfonso, A. Postoperative pulmonary complications after laparotomy. Respiration 2010, 80, 269–274. [Google Scholar] [CrossRef] [PubMed]
- Bragg, D.; El-Sharkawy, A.M.; Psaltis, E.; Maxwell-Armstrong, C.A.; Lobo, D.N. Postoperative ileus: Recent developments in pathophysiology and management. Clin. Nutr. 2015, 34, 367–376. [Google Scholar] [CrossRef] [PubMed]
- Juneja, R. Opioids and cancer recurrence. Curr. Opin. Support. Palliat. Care 2014, 8, 91–101. [Google Scholar] [CrossRef] [PubMed]
- Snyder, G.L.; Greenberg, S. Effect of anaesthetic technique and other perioperative factors on cancer recurrence. Br. J. Anaesth. 2010, 105, 106–115. [Google Scholar] [CrossRef]
- Gavi, F.; Foschi, N.; Fettucciari, D.; Russo, P.; Giannarelli, D.; Ragonese, M.; Gandi, C.; Balocchi, G.; Francocci, A.; Bizzarri, F.P.; et al. Assessing Trifecta and Pentafecta Success Rates between Robot-Assisted vs. Open Radical Cystectomy: A Propensity Score-Matched Analysis. Cancers 2024, 16, 1270. [Google Scholar] [CrossRef]
- Jin, F.; Chung, F. Minimizing perioperative adverse events in the elderly. Br. J. Anaesth. 2001, 87, 608–624. [Google Scholar] [CrossRef]
- Fredrickson, M.J.; Krishnan, S.; Chen, C.Y. Postoperative analgesia for shoulder surgery: A critical appraisal and review of current techniques. Anaesthesia 2010, 65, 608–624. [Google Scholar] [CrossRef]
- Abdallah, F.W.; Halpern, S.H.; Aoyama, K.; Brull, R. Will the Real Benefits of Single-Shot Interscalene Block Please Stand Up? A Systematic Review and Meta-Analysis. Anesth. Analg. 2015, 120, 1114–1129. [Google Scholar] [CrossRef]
- Lavand’homme, P. Rebound pain after regional anesthesia in the ambulatory patient. Curr. Opin. Anaesthesiol. 2018, 31, 679–684. [Google Scholar] [CrossRef]
- Barry, G.S.; Bailey, J.G.; Sardinha, J.; Brousseau, P.; Uppal, V. Factors associated with rebound pain after peripheral nerve block for ambulatory surgery. Br. J. Anaesth. 2021, 126, 862–871. [Google Scholar] [CrossRef]
- Swain, A.; Nag, D.S.; Sahu, S.; Samaddar, D.P. Adjuvants to local anesthetics: Current understanding and future trends. World J. Clin. Cases 2017, 5, 307–323. [Google Scholar] [CrossRef]
- Choi, S.; Rodseth, R.; McCartney, C.J. Effects of dexamethasone as a local anaesthetic adjuvant for brachial plexus block: A systematic review and meta-analysis of randomized trials. Br. J. Anaesth. 2014, 112, 427–439. [Google Scholar] [CrossRef]
- Huynh, T.M.; Marret, E.; Bonnet, F. Combination of dexamethasone and local anaesthetic solution in peripheral nerve blocks: A meta-analysis of randomised controlled trials. Eur. J. Anaesthesiol. 2015, 32, 751–758. [Google Scholar] [CrossRef] [PubMed]
- Kawanishi, R.; Yamamoto, K.; Tobetto, Y.; Nomura, K.; Kato, M.; Go, R.; Tsutsumi, Y.M.; Tanaka, K.; Takeda, Y. Perineural but not systemic low-dose dexamethasone prolongs the duration of interscalene block with ropivacaine: A prospective randomized trial. Local Reg. Anesth. 2014, 7, 5–9. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sakae, T.M.; Marchioro, P.; Schuelter-Trevisol, F.; Trevisol, D.J. Dexamethasone as a ropivacaine adjuvant for ultrasound-guided interscalene brachial plexus block: A randomized, double-blinded clinical trial. J. Clin. Anesth. 2017, 38, 133–136. [Google Scholar] [CrossRef]
- Liang, S.; Xing, M.; Jiang, S.; Zou, W. Effect of Intravenous Dexamethasone on Postoperative Pain in Patients Undergoing Total Knee Arthroplasty: A Systematic Review and Meta-Analysis. Pain Physician 2022, 25, E169–E183. [Google Scholar] [PubMed]
- Singh, N.P.; Makkar, J.K.; Yadav, N.; Goudra, B.G.; Singh, P.M. The analgesic efficacy of intravenous dexamethasone for post-caesarean pain: A systematic review with meta-analysis and trial sequential analysis. Eur. J. Anaesthesiol. 2022, 39, 498–510. [Google Scholar] [CrossRef]
- Waldron, N.H.; Jones, C.A.; Gan, T.J.; Allen, T.K.; Habib, A.S. Impact of perioperative dexamethasone on postoperative analgesia and side-effects: Systematic review and meta-analysis. Br. J. Anaesth. 2013, 110, 191–200. [Google Scholar] [CrossRef]
- Polderman, J.A.; Farhang-Razi, V.; Van Dieren, S.; Kranke, P.; DeVries, J.H.; Hollmann, M.W.; Preckel, B.; Hermanides, J. Adverse side effects of dexamethasone in surgical patients. Cochrane Database Syst. Rev. 2018, 11, CD011940. [Google Scholar] [CrossRef]
- Weiss, R.; Popping, D.M. Is epidural analgesia still a viable option for enhanced recovery after abdominal surgery. Curr. Opin. Anaesthesiol. 2018, 31, 622–629. [Google Scholar] [CrossRef]
- Popping, D.M.; Elia, N.; Van Aken, H.K.; Marret, E.; Schug, S.A.; Kranke, P.; Wenk, M.; Tramer, M.R. Impact of epidural analgesia on mortality and morbidity after surgery: Systematic review and meta-analysis of randomized controlled trials. Ann. Surg. 2014, 259, 1056–1067. [Google Scholar] [CrossRef] [PubMed]
- Bailey, J.G.; Morgan, C.W.; Christie, R.; Ke, J.X.C.; Kwofie, M.K.; Uppal, V. Continuous peripheral nerve blocks compared to thoracic epidurals or multimodal analgesia for midline laparotomy: A systematic review and meta-analysis. Korean J. Anesthesiol. 2021, 74, 394–408. [Google Scholar] [CrossRef]
- Barry, G.; Sehmbi, H.; Retter, S.; Bailey, J.G.; Tablante, R.; Uppal, V. Comparative efficacy and safety of non-neuraxial analgesic techniques for midline laparotomy: A systematic review and frequentist network meta-analysis of randomised controlled trials. Br. J. Anaesth. 2023, 131, 1053–1071. [Google Scholar] [CrossRef] [PubMed]
- Rozen, W.M.; Tran, T.M.; Ashton, M.W.; Barrington, M.J.; Ivanusic, J.J.; Taylor, G.I. Refining the course of the thoracolumbar nerves: A new understanding of the innervation of the anterior abdominal wall. Clin. Anat. 2008, 21, 325–333. [Google Scholar] [CrossRef]
- Sandeman, D.J.; Dilley, A.V. Ultrasound-guided rectus sheath block and catheter placement. ANZ J. Surg. 2008, 78, 621–623. [Google Scholar] [CrossRef]
- Osaka, Y.; Kashiwagi, M.; Nagatsuka, Y.; Oosaku, M.; Hirose, C. Ultrasound-guided rectus sheath block for upper abdominal surgery. Masui 2010, 59, 1039–1041. [Google Scholar] [PubMed]
- Miller, R.D.; Eriksson, L.I.; Fleisher, L.A.; Wiener-Kronish, J.P.; Cohen, N.H.U. Miller’s Anesthesia, 8th ed.; Elsevier Saunders: Philadelphia, PA, USA, 2015; p. 2. [Google Scholar]
- Fletcher, D.; Martinez, V. Opioid-induced hyperalgesia in patients after surgery: A systematic review and a meta-analysis. Br. J. Anaesth. 2014, 112, 991–1004. [Google Scholar] [CrossRef]
- Woo, J.H.; Lee, H.J.; Oh, H.W.; Lee, J.W.; Baik, H.J.; Kim, Y.J. Perineural dexamethasone reduces rebound pain after ropivacaine single injection interscalene block for arthroscopic shoulder surgery: A randomized controlled trial. Reg. Anesth. Pain Med. 2021, 46, 965–970. [Google Scholar] [CrossRef]
- Breivik, H.; Borchgrevink, P.C.; Allen, S.M.; Rosseland, L.A.; Romundstad, L.; Hals, E.K.; Kvarstein, G.; Stubhaug, A. Assessment of pain. Br. J. Anaesth. 2008, 101, 17–24. [Google Scholar] [CrossRef]
- Melesse, D.Y.; Chekol, W.B.; Tawuye, H.Y.; Denu, Z.A.; Agegnehu, A.F. Assessment of the analgesic effectiveness of rectus sheath block in patients who had emergency midline laparotomy: Prospective observational cohort study. Int. J. Surg. Open 2020, 24, 27–31. [Google Scholar] [CrossRef]
- Yassin, H.M.; Abd Elmoneim, A.T.; El Moutaz, H. The Analgesic Efficiency of Ultrasound-Guided Rectus Sheath Analgesia Compared with Low Thoracic Epidural Analgesia After Elective Abdominal Surgery with a Midline Incision: A Prospective Randomized Controlled Trial. Anesth. Pain Med. 2017, 7, e14244. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.; Kim, H.; Park, J. Analgesic effectiveness of rectus sheath block during open gastrectomy: A prospective double-blinded randomized controlled clinical trial. Medicine 2019, 98, e15159. [Google Scholar] [CrossRef] [PubMed]
- Elbahrawy, K.; El-Deeb, A. Rectus sheath block for postoperative analgesia in patients with mesenteric vascular occlusion undergoing laparotomy: A randomized single-blinded study. Anesth. Essays Res. 2016, 10, 516–520. [Google Scholar] [CrossRef] [PubMed]
- Bashandy, G.M.; Elkholy, A.H. Reducing postoperative opioid consumption by adding an ultrasound-guided rectus sheath block to multimodal analgesia for abdominal cancer surgery with midline incision. Anesth. Pain Med. 2014, 4, e18263. [Google Scholar] [CrossRef]
- Chen, Q.; An, R.; Zhou, J.; Yang, B. Clinical analgesic efficacy of dexamethasone as a local anesthetic adjuvant for transversus abdominis plane (TAP) block: A meta-analysis. PLoS ONE 2018, 13, e0198923. [Google Scholar] [CrossRef]
- Albrecht, E.; Kern, C.; Kirkham, K.R. A systematic review and meta-analysis of perineural dexamethasone for peripheral nerve blocks. Anaesthesia 2015, 70, 71–83. [Google Scholar] [CrossRef]
- Chin, K.J.; McDonnell, J.G.; Carvalho, B.; Sharkey, A.; Pawa, A.; Gadsden, J. Essentials of Our Current Understanding: Abdominal Wall Blocks. Reg. Anesth. Pain Med. 2017, 42, 133–183. [Google Scholar] [CrossRef]
- Nakazawa, M.; Fukushima, T.; Shoji, K.; Momosaki, R.; Mio, Y. Preoperative versus postoperative ultrasound-guided rectus sheath block for acute postoperative pain relief after laparoscopy: A retrospective cohort study. Medicine 2024, 103, e37597. [Google Scholar] [CrossRef]
- Moiniche, S.; Kehlet, H.; Dahl, J.B. A qualitative and quantitative systematic review of preemptive analgesia for postoperative pain relief: The role of timing of analgesia. Anesthesiology 2002, 96, 725–741. [Google Scholar] [CrossRef]
- Katz, J.; Clarke, H.; Seltzer, Z. Review article: Preventive analgesia: Quo vadimus? Anesth. Analg. 2011, 113, 1242–1253. [Google Scholar] [CrossRef]
- Brennan, T.J.; Taylor, B.K. Analgesic treatment before incision compared with treatment after incision provides no improvement in postoperative pain relief. J. Pain 2000, 1, 96–98. [Google Scholar] [CrossRef]
- Pogatzki-Zahn, E.M.; Zahn, P.K. From preemptive to preventive analgesia. Curr. Opin. Anaesthesiol. 2006, 19, 551–555. [Google Scholar] [CrossRef] [PubMed]
- Brennan, T.J.; Kehlet, H. Preventive analgesia to reduce wound hyperalgesia and persistent postsurgical pain: Not an easy path. Anesthesiology 2005, 103, 681–683. [Google Scholar] [CrossRef]
- Adhikary, S.D.; Armstrong, K.; Chin, K.J. Perineural entrapment of an interscalene stimulating catheter. Anaesth. Intensive Care 2012, 40, 527–530. [Google Scholar] [CrossRef] [PubMed]
- Bowens, C., Jr.; Briggs, E.R.; Malchow, R.J. Brachial plexus entrapment of interscalene nerve catheter after uncomplicated ultrasound-guided placement. Pain Med. 2011, 12, 1117–1120. [Google Scholar] [CrossRef]
- Hughes, M.S.; Matava, M.J.; Wright, R.W.; Brophy, R.H.; Smith, M.V. Interscalene brachial plexus block for arthroscopic shoulder surgery: A systematic review. J. Bone Joint Surg. Am. 2013, 95, 1318–1324. [Google Scholar] [CrossRef]
- Yang, Z.S.; Lai, H.C.; Jhou, H.J.; Chan, W.H.; Chen, P.H. Rebound pain prevention after peripheral nerve block: A network meta-analysis comparing intravenous, perineural dexamethasone, and control. J. Clin. Anesth. 2024, 99, 111657. [Google Scholar] [CrossRef]
- Pehora, C.; Pearson, A.M.; Kaushal, A.; Crawford, M.W.; Johnston, B. Dexamethasone as an adjuvant to peripheral nerve block. Cochrane Database Syst. Rev. 2017, 11, CD011770. [Google Scholar] [CrossRef]
- Albrecht, E.; Renard, Y.; Desai, N. Intravenous versus perineural dexamethasone to prolong analgesia after interscalene brachial plexus block: A systematic review with meta-analysis and trial sequential analysis. Br. J. Anaesth. 2024, 133, 135–145. [Google Scholar] [CrossRef]
- Shishido, H.; Kikuchi, S.; Heckman, H.; Myers, R.R. Dexamethasone decreases blood flow in normal nerves and dorsal root ganglia. Spine 2002, 27, 581–586. [Google Scholar] [CrossRef]
- Attardi, B.; Takimoto, K.; Gealy, R.; Severns, C.; Levitan, E.S. Glucocorticoid induced up-regulation of a pituitary K+ channel mRNA in vitro and in vivo. Recept. Channels 1993, 1, 287–293. [Google Scholar]
- Johansson, A.; Hao, J.; Sjolund, B. Local corticosteroid application blocks transmission in normal nociceptive C-fibres. Acta Anaesthesiol. Scand. 1990, 34, 335–338. [Google Scholar] [CrossRef]
- An, K.; Elkassabany, N.M.; Liu, J. Dexamethasone as adjuvant to bupivacaine prolongs the duration of thermal antinociception and prevents bupivacaine-induced rebound hyperalgesia via regional mechanism in a mouse sciatic nerve block model. PLoS ONE 2015, 10, e0123459. [Google Scholar] [CrossRef]
- Fallon, F.; Ramly, M.S.; Moorthy, A. Rebound Pain After Regional Anaesthesia. Medicina 2025, 61, 790. [Google Scholar] [CrossRef] [PubMed]
- Admassie, B.M.; Tegegne, B.A.; Alemu, W.M.; Getahun, A.B. Magnitude and severity of rebound pain after resolution of peripheral nerve block and associated factors among patients undergoes surgery at university of gondar comprehensive specialized hospital northwest, Ethiopia, 2022. Longitudinal cross-sectional study. Ann. Med. Surg. 2022, 84, 104915. [Google Scholar] [CrossRef] [PubMed]
- Zufferey, P.J.; Chaux, R.; Lachaud, P.A.; Capdevila, X.; Lanoiselee, J.; Ollier, E. Dose-response relationships of intravenous and perineural dexamethasone as adjuvants to peripheral nerve blocks: A systematic review and model-based network meta-analysis. Br. J. Anaesth. 2024, 132, 1122–1132. [Google Scholar] [CrossRef] [PubMed]
- Gao, Z.; Xiao, Y.; Wang, Q.; Li, Y. Comparison of dexmedetomidine and dexamethasone as adjuvant for ropivacaine in ultrasound-guided erector spinae plane block for video-assisted thoracoscopic lobectomy surgery: A randomized, double-blind, placebo-controlled trial. Ann. Transl. Med. 2019, 7, 668. [Google Scholar] [CrossRef]
- Singh, N.; Gupta, S.; Kathuria, S. Dexmedetomidine vs dexamethasone as an adjuvant to 0.5% ropivacaine in ultrasound-guided supraclavicular brachial plexus block. J. Anaesthesiol. Clin. Pharmacol. 2020, 36, 238–243. [Google Scholar] [CrossRef]
- Venkatraman, R.; Pushparani, A.; Karthik, K.; Nandhini, P. Comparison of morphine, dexmedetomidine and dexamethasone as an adjuvant to ropivacaine in ultrasound-guided supraclavicular brachial plexus block for postoperative analgesia-a randomized controlled trial. J. Anaesthesiol. Clin. Pharmacol. 2021, 37, 102–107. [Google Scholar] [CrossRef]
- Gan, T.J.; Belani, K.G.; Bergese, S.; Chung, F.; Diemunsch, P.; Habib, A.S.; Jin, Z.; Kovac, A.L.; Meyer, T.A.; Urman, R.D.; et al. Fourth Consensus Guidelines for the Management of Postoperative Nausea and Vomiting. Anesth. Analg. 2020, 131, 411–448. [Google Scholar] [CrossRef]
- Weibel, S.; Schaefer, M.S.; Raj, D.; Rucker, G.; Pace, N.L.; Schlesinger, T.; Meybohm, P.; Kienbaum, P.; Eberhart, L.H.J.; Kranke, P. Drugs for preventing postoperative nausea and vomiting in adults after general anaesthesia: An abridged Cochrane network meta-analysis. Anaesthesia 2021, 76, 962–973. [Google Scholar] [CrossRef]
- Schacke, H.; Docke, W.D.; Asadullah, K. Mechanisms involved in the side effects of glucocorticoids. Pharmacol. Ther. 2002, 96, 23–43. [Google Scholar] [CrossRef]
| Ropivacaine-Only Group (n = 26) | Ropivacaine with Dexamethasone Group (n = 26) | p-Value | |
|---|---|---|---|
| Age (y) | 68.50 [8.50] | 67.50 [9.25] | 0.275 |
| Height (cm) | 167.00 [9.65] | 167.10 [8.98] | 0.498 |
| Weight (kg) | 66.66 ± 11.86 | 65.66 ± 10.17 | 0.745 |
| Sex (M/F) | 21 (81)/5 (19) | 21 (81)/5 (19) | 1.000 |
| Operation duration (min) | 252.50 [61.25] | 225.00 [31.25] | 0.184 |
| Anesthesia duration (min) | 287.50 [67.50] | 265.00 [33.75] | 0.152 |
| ASA physical status (1/2/3) | 0 (0)/23 (88)/3 (12) | 3 (11)/21 (81)/2 (8) | 0.193 |
| Diabetes mellitus | 7 (27) | 9 (35) | 0.548 |
| Hypertension | 16 (62) | 13 (50) | 0.402 |
| Pulmonary disease | 1 (4) | 3 (12) | 0.298 |
| Intraoperative fentanyl (μg) | 100.00 [50.00] | 100.00 [50.00] | 0.627 |
| Ropivacaine-Only Group (n = 26) | Ropivacaine with Dexamethasone Group (n = 26) | p-Value | |
|---|---|---|---|
| Total fentanyl dose (µg) | |||
| Postoperative 3 h | 101.70 [43.05] | 84.44 [33.72] | 0.164 |
| Postoperative 6 h | 179.20 [138.60] | 148.50 [100.03] | 0.059 |
| Postoperative 12 h | 294.95 [173.48] | 247.95 [131.98] | 0.041 |
| Postoperative 18 h | 420.35 [259.30] | 359.80 [140.50] | 0.022 |
| Postoperative 24 h | 521.50 [332.58] | 459.80 [175.42] | 0.032 |
| Postoperative 48 h | 913.70 [246.95] | 806.30 [300.45] | 0.024 |
| Numeric rating scale | |||
| Postoperative 3 h | 2.00 [2.00] | 2.00 [1.00] | 0.113 |
| Postoperative 6 h | 6.00 [2.25] | 4.50 [2.25] | 0.002 |
| Postoperative 12 h | 5.00 [2.00] | 3.00 [2.00] | 0.000 |
| Postoperative 18 h | 4.00 [2.00] | 3.00 [2.00] | 0.057 |
| Postoperative 24 h | 3.00 [1.50] | 2.50 [1.25] | 0.024 |
| Postoperative 48 h | 2.00 [2.25] | 1.00 [1.00] | 0.003 |
| Ropivacaine-Only Group (n = 26) | Ropivacaine with Dexamethasone (n = 26) | p-Value | |
|---|---|---|---|
| Postoperative nausea and vomiting | 3 (12) | 2 (8) | 0.638 |
| Wound infection | 0 (0) | 0 (0) | 1.000 |
| Local anesthetic systemic toxicity | 0 (0) | 0 (0) | 1.000 |
| Perineural infection or abscess | 0 (0) | 0 (0) | 1.000 |
| Pruritus | 0 (0) | 0 (0) | 1.000 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yoo, S.H.; Beak, M.H.; Lee, D.H.; Kim, W.-J. Effect of Perineural Dexamethasone as an Adjuvant to Ropivacaine in Rectus Sheath Block for Radical Cystectomy: A Randomized Controlled Trial. J. Clin. Med. 2025, 14, 5186. https://doi.org/10.3390/jcm14155186
Yoo SH, Beak MH, Lee DH, Kim W-J. Effect of Perineural Dexamethasone as an Adjuvant to Ropivacaine in Rectus Sheath Block for Radical Cystectomy: A Randomized Controlled Trial. Journal of Clinical Medicine. 2025; 14(15):5186. https://doi.org/10.3390/jcm14155186
Chicago/Turabian StyleYoo, Seung Hee, Min Hyouk Beak, Dong Hyeon Lee, and Won-Joong Kim. 2025. "Effect of Perineural Dexamethasone as an Adjuvant to Ropivacaine in Rectus Sheath Block for Radical Cystectomy: A Randomized Controlled Trial" Journal of Clinical Medicine 14, no. 15: 5186. https://doi.org/10.3390/jcm14155186
APA StyleYoo, S. H., Beak, M. H., Lee, D. H., & Kim, W.-J. (2025). Effect of Perineural Dexamethasone as an Adjuvant to Ropivacaine in Rectus Sheath Block for Radical Cystectomy: A Randomized Controlled Trial. Journal of Clinical Medicine, 14(15), 5186. https://doi.org/10.3390/jcm14155186






