Fragility Fractures of the Pelvis—Current Understanding and Open Questions
Abstract
1. Introduction
2. Materials and Methods
3. Definitions/Epidemiology
Risk Factors
4. Diagnostic Approach
4.1. Clinical Presentation
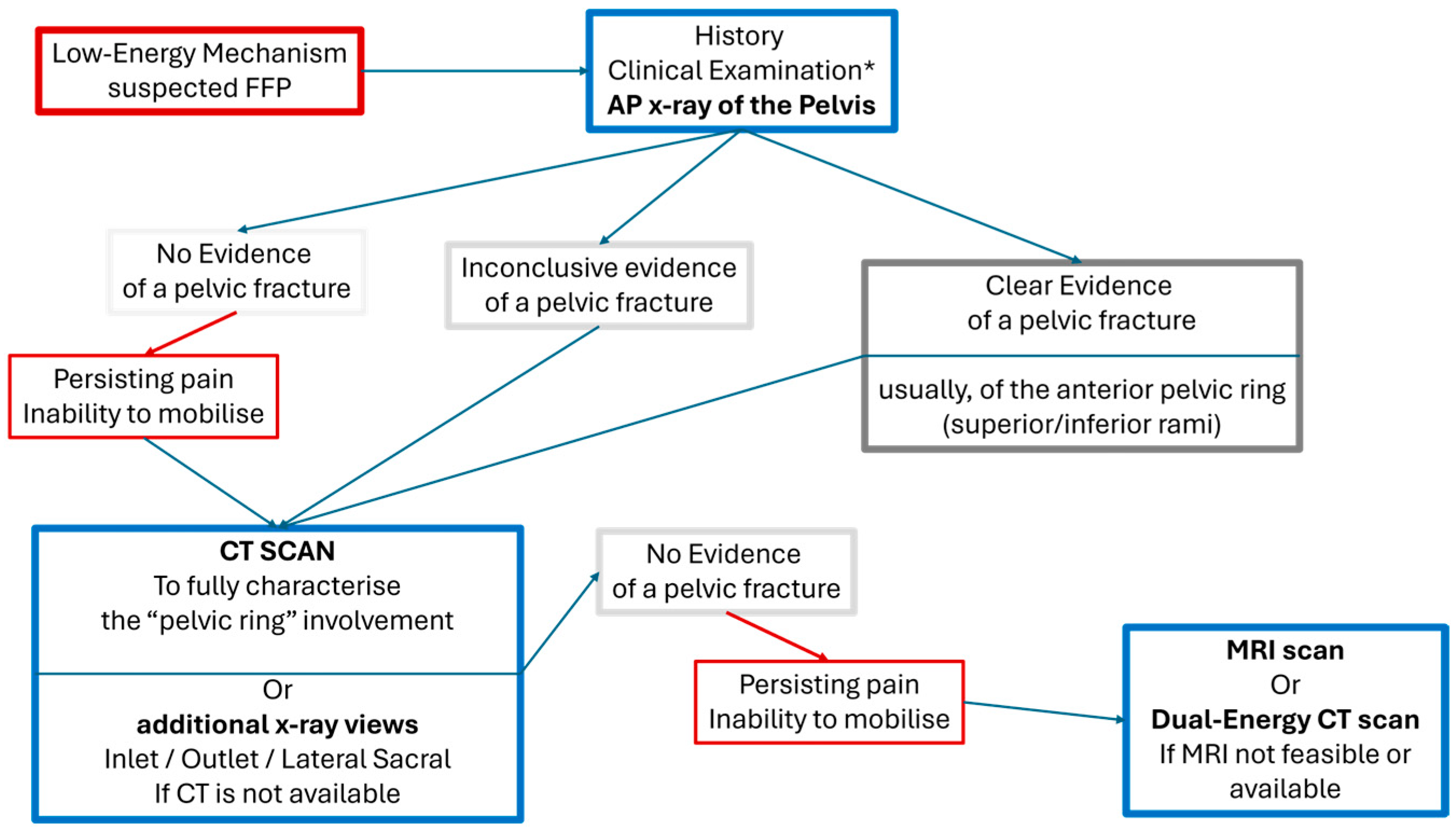
4.2. Imaging
4.3. Classification Systems
- FFPs type I: Stable non-displaced fractures that affect only the anterior pelvic ring (Ia lesion: unilateral; Ib lesion: bilateral).
- FFPs type II: Partially unstable and non-displaced fractures involving the anterior and/or posterior pelvic ring (IIa: unilateral and non-displaced sacral fractures; IIb: fractures of the pubic rami and the sacrum (ventral cortex); IIc: fractures of the pubic rami and complete (ventral and dorsal cortices) sacral fractures).
- FFPs type III: Unstable and displaced fractures with complete disruption of the pelvic ring. (IIIa: pubic rami and iliac wing fractures; IIIb: pubic rami and iliosacral crescent fracture/dislocations; IIIc: pubic rami and sacral wing displaced fractures).
- FFPs type IV: Highly unstable fractures, with complete dissociation between the spine and the pelvic ring. (IVa: bilateral iliac wing/crescent fractures; IVb: bilateral sacral fractures/U-shaped fractures with transverse sacral fracture line at S1/S2; IVc: different lesions between the two sides with combinations of trans-iliac/trans-sacral/transilio-sacral instability).
5. Management Strategies
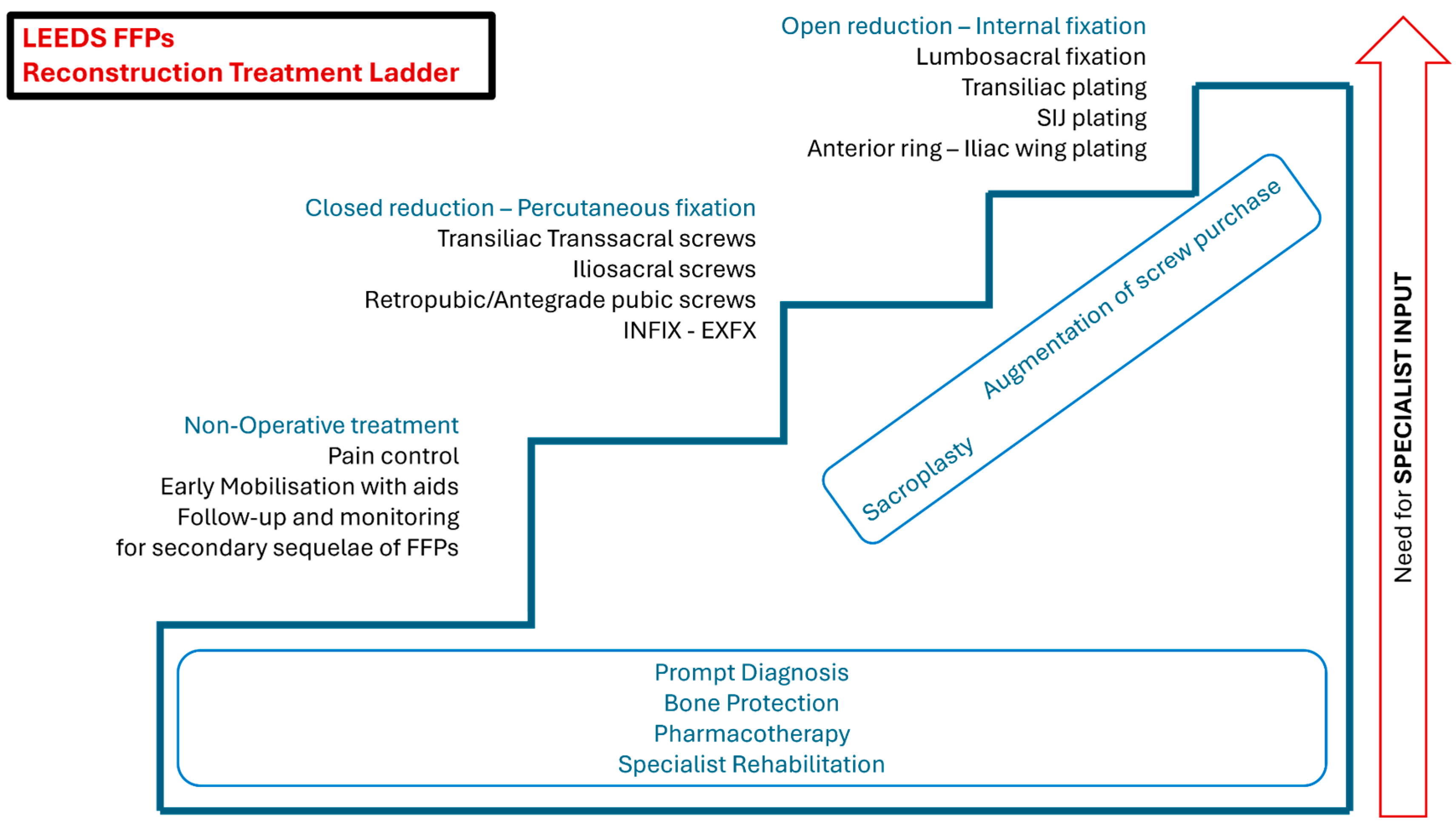
5.1. Non-Operative Treatment
5.2. Operative Treatment
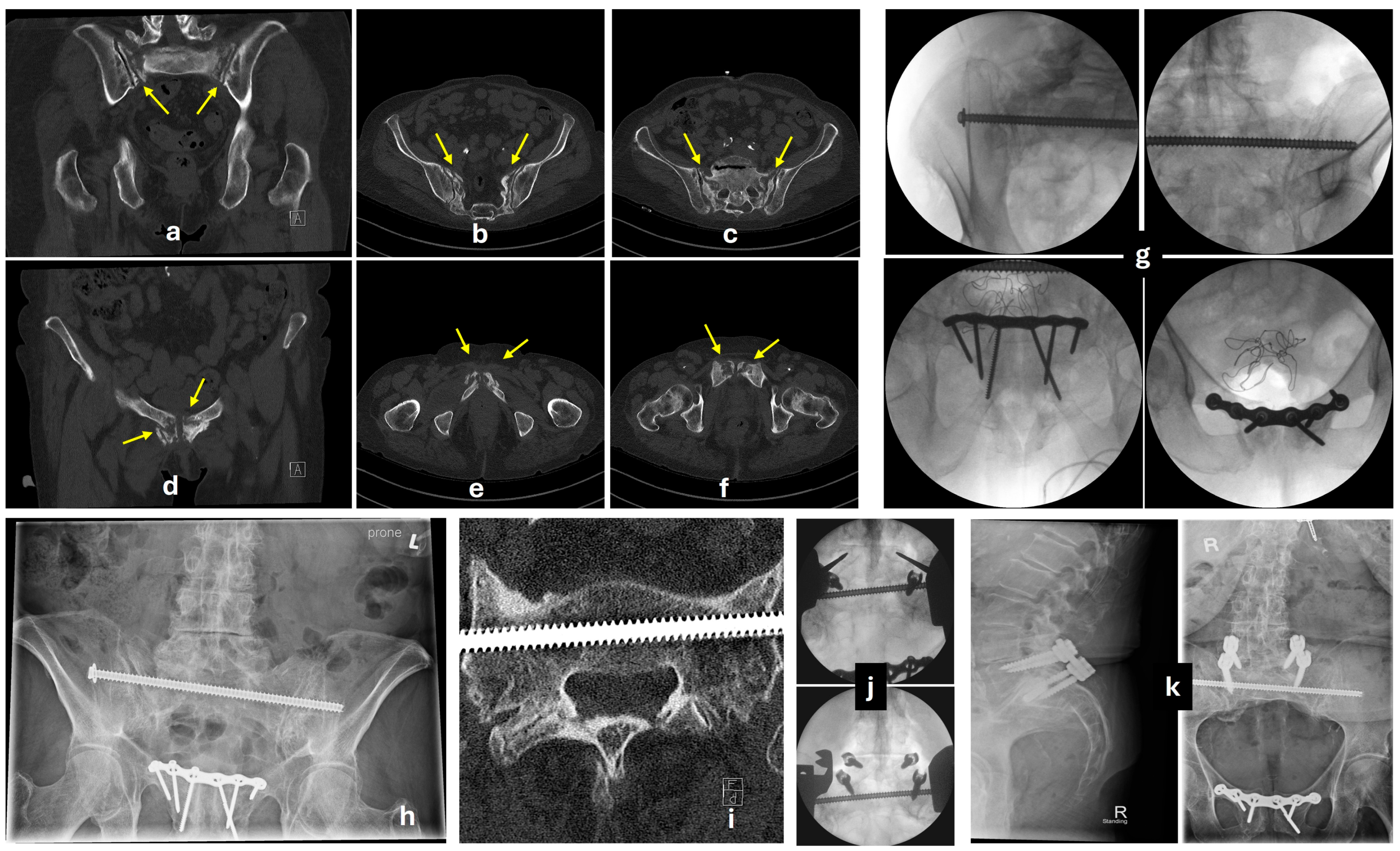
5.3. Posterior Fixation
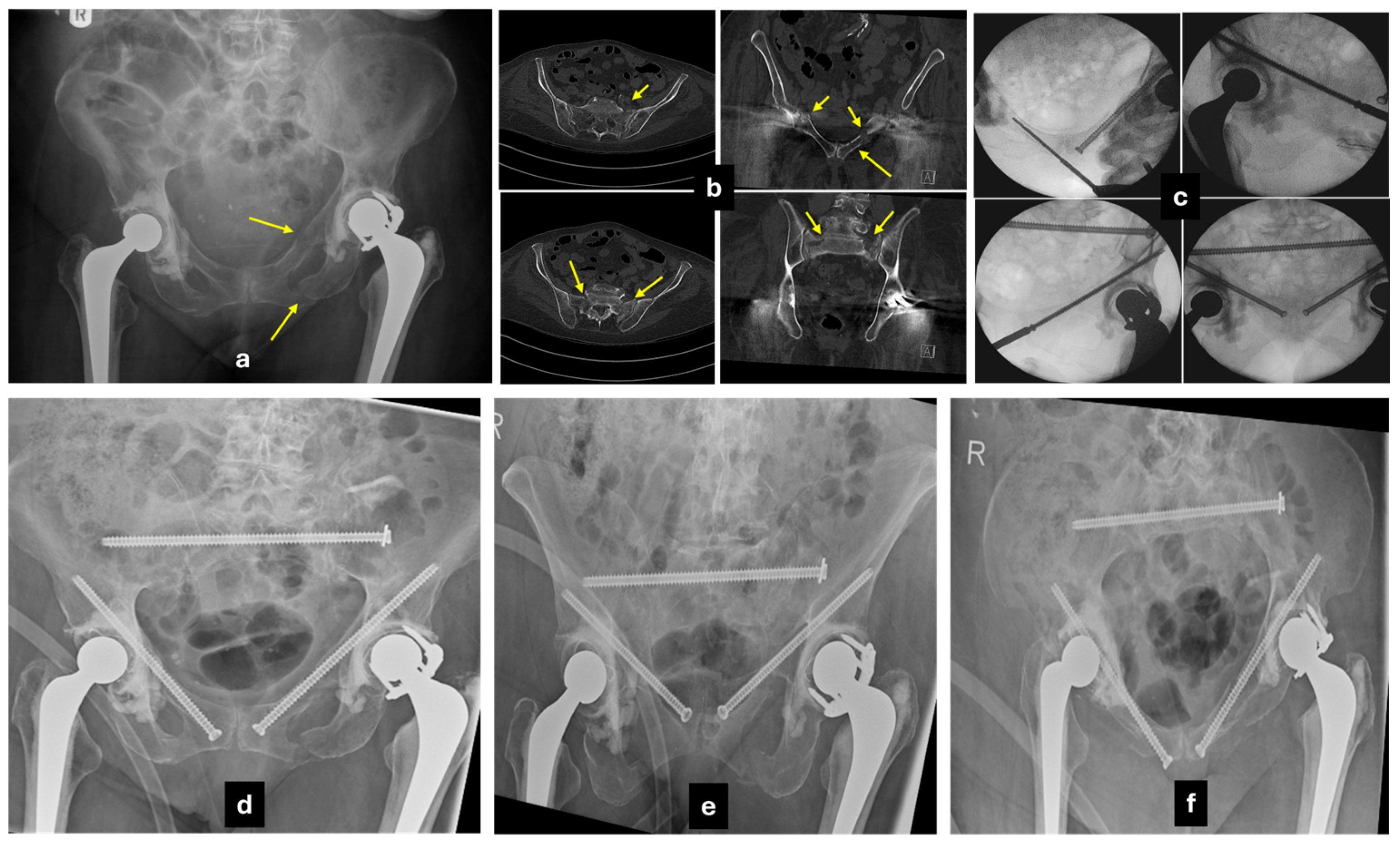
5.3.1. Transiliac-Transsacral Screws
5.3.2. Transiliac Bridge Plating
5.3.3. Lumbopelvic Fixation
5.4. Anterior Fixation
5.4.1. Conventional Anterior Plating
5.4.2. Percutaneous Screw Fixation
5.4.3. External Fixator
5.5. Augmentation Techniques
5.6. Orthobiologic Adjuncts
6. Reported Outcomes
6.1. Mortality
6.2. Return to Activities
7. Conclusions
| ROMMENS HOFFMAN [46] 2013 | Description | OF-Pelvis ** [47] 2021 | YOUNG BURGESS [45] 1986 | Tile/AO/OTA [43] 2018 | AO SPINE SACRAL [75] 2020 | STARR NAKATANI [80] 2008 | DENIS [41] 1988 | ROY-CAMILLE [44] 1985 | ISLER [42] 1990 |
|---|---|---|---|---|---|---|---|---|---|
| FFP I | only Anterior ring frxs | OF2 | n/a | 61A2 | n/a | zones 1/2/3 | n/a | n/a | n/a |
| Ia | Unilateral | OF2 | n/a | 61A2.2 | n/a | zones 1/2/3 | n/a | n/a | n/a |
| Ib | Bilateral | OF2 | n/a | 61A2.3 | n/a | zones 1/2/3 | n/a | n/a | n/a |
| FFP II | Undisplaced Posterior ring frxs +/− anterior frxs | OF3 | LC1 | 61B2.1 | B | n/a | zones 1/2/3 | n/a | (type a) |
| IIa | sacral frx without anterior ring frx | OF3 | LC1 | n/a | B | n/a | zone 1 | n/a | (type a) |
| IIb | incomplete sacral frx with anterior ring frx | OF3 | LC1 (stable) | 61B2.1 | B | n/a | zones 1/2 | n/a | (type a) |
| IIc | complete sacral frx with anterior ring frx | OF3 | LC1 (unstable) | 61C1.3 | B | n/a | zones 1/2/3 | n/a | (type a) |
| FFP III | Displaced Unilateral Posterior ring frxs +/− anterior frxs | OF3/5 | LC2 | 61B/C | (B) | zones 1/2/3 | (zones 1/2/3) | n/a | (types a/b/c) |
| IIIa | Iliac wing frx | OF5 | LC2 | 61B2.2 | n/a | zones 1/2/3 | n/a | n/a | n/a |
| IIIb | Iliac wing frx involving the SIJ (crescent) | OF5 | LC2 | 61B2.2 | n/a | zones 1/2/3 | n/a | n/a | n/a |
| IIIc | Sacral frx | OF3 | LC1 | 61C1.3 | B | zones 1/2/3 | zones 1/2/3c | n/a | types a/b/c |
| FFP IV | Displaced Bilateral Posterior ring frxs +/− anterior frxs | OF4 | LC1-2-3/VS | 61B/C | (B/C) | n/a | zones 3a/b/d | (types I/II/III) | types a/b/c |
| Iva | Iliac wing frxs | OF5 | LC2 | n/a | n/a | n/a | n/a | n/a | n/a |
| Ivb * | Sacral frxs | OF4 | LC1/VS | 61B3.2/C3.3 | C | n/a | zones 3a/b/d | types I/II/III | types a/b/c |
| Ivc * | Combinations of posterior frxs | OF5 | LC3/VS | 61B3.1/C3.2 | B/C | n/a | zones 3a/b/d | types I/II/III | types a/b/c |
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kanakaris, N.K.; Bouamra, O.; Lecky, F.; Giannoudis, P.V. Severe trauma with associated pelvic fractures: The impact of regional trauma networks on clinical outcome. Injury 2023, 54, 1670–1676. [Google Scholar] [CrossRef] [PubMed]
- Kuper, M.A.; Trulson, A.; Stuby, F.M.; Stockle, U. Pelvic ring fractures in the elderly. EFORT Open Rev. 2019, 4, 313–320. [Google Scholar] [CrossRef] [PubMed]
- Moldovan, F.; Moldovan, L. A Modeling Study for Hip Fracture Rates in Romania. J. Clin. Med. 2025, 14, 3162. [Google Scholar] [CrossRef] [PubMed]
- Kjeldgaard, H.K.; Holvik, K.; Abrahamsen, B.; Tell, G.S.; Meyer, H.E.; O’Flaherty, M. Explaining declining hip fracture rates in Norway: A population-based modelling study. Lancet Reg. Health-Eur. 2023, 30, 100643. [Google Scholar] [CrossRef] [PubMed]
- Oberkircher, L.; Ruchholtz, S.; Rommens, P.M.; Hofmann, A.; Bucking, B.; Kruger, A. Osteoporotic Pelvic Fractures. Dtsch. Arztebl. Int. 2018, 115, 70–80. [Google Scholar] [CrossRef] [PubMed]
- Bazire, L.; Xu, H.; Foy, J.P.; Amessis, M.; Malhaire, C.; Cao, K.; De La Rochefordiere, A.; Kirova, Y.M. Pelvic insufficiency fracture (PIF) incidence in patients treated with intensity-modulated radiation therapy (IMRT) for gynaecological or anal cancer: Single-institution experience and review of the literature. Br. J. Radiol. 2017, 90, 20160885. [Google Scholar] [CrossRef] [PubMed]
- Soles, G.L.; Ferguson, T.A. Fragility fractures of the pelvis. Curr. Rev. Musculoskelet. Med. 2012, 5, 222–228. [Google Scholar] [CrossRef] [PubMed]
- Sassara, G.M.; Smakaj, A.; De Mauro, D.; Righini, R.; Arnone, A.; Rovere, G.; El Ezzo, O.; Farsetti, P.; Tarantino, U.; Liuzza, F. Evaluating Treatment Outcomes for Pelvic Insufficiency Fractures: A Systematic Review. J. Clin. Med. 2024, 13, 3176. [Google Scholar] [CrossRef] [PubMed]
- Omichi, T.; Takegami, Y.; Tokutake, K.; Saito, Y.; Ito, O.; Ando, T.; Imagama, S. Mortality and functional outcomes of fragility fractures of the pelvis by fracture type with conservative treatment: A retrospective, multicenter TRON study. Eur. J. Trauma Emerg. Surg. 2022, 48, 2897–2904. [Google Scholar] [CrossRef] [PubMed]
- Kanakaris, N.K.; Greven, T.; West, R.M.; Van Vugt, A.B.; Giannoudis, P.V. Implementation of a standardized protocol to manage elderly patients with low energy pelvic fractures: Can service improvement be expected? Int. Orthop. 2017, 41, 1813–1824. [Google Scholar] [CrossRef] [PubMed]
- Gewiess, J.; Albers, C.E.; Keel, M.J.B.; Frihagen, F.; Rommens, P.M.; Bastian, J.D. Chronic pelvic insufficiency fractures and their treatment. Arch. Orthop. Trauma Surg. 2024, 145, 76. [Google Scholar] [CrossRef] [PubMed]
- Svedbom, A.; Ivergard, M.; Hernlund, E.; Rizzoli, R.; Kanis, J.A. Epidemiology and economic burden of osteoporosis in Switzerland. Arch. Osteoporos. 2014, 9, 187. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, T.; Rottbeck, U.; Hofbauer, V.; Raschke, M.; Stange, R. Pelvic ring fractures in the elderly. Underestimated osteoporotic fracture. Der Unfallchirurg 2011, 114, 663–670. [Google Scholar] [CrossRef] [PubMed]
- Rossler, A.; Lukhaup, L.; Seidelmann, M.; Gaeth, C.; Dietz, S.O.; Audretsch, C.; Grutzner, P.; Windolf, J.; Neubert, A. Hemorrhage in Pelvic Ring Fractures After Low-Energy Trauma: A Systematic Review. J. Clin. Med. 2024, 13, 7223. [Google Scholar] [CrossRef] [PubMed]
- Rommens, P.M.; Hofmann, A. The FFP-classification: From eminence to evidence. Injury 2023, 54 (Suppl. 3), S10–S19. [Google Scholar] [CrossRef] [PubMed]
- Prieto-Alhambra, D.; Aviles, F.F.; Judge, A.; Van Staa, T.; Nogues, X.; Arden, N.K.; Diez-Perez, A.; Cooper, C.; Javaid, M.K. Burden of pelvis fracture: A population-based study of incidence, hospitalisation and mortality. Osteoporos. Int. 2012, 23, 2797–2803. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, M.P.; Baldwin, K.D.; Donegan, D.J.; Mehta, S.; Ahn, J. Geriatric fractures about the hip: Divergent patterns in the proximal femur, acetabulum, and pelvis. Orthopedics 2014, 37, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Osche, D.B.; Liodakis, E.; Huber, S.; Pohlemann, T.; Kleber, C.; Herath, S.C.; Hoch, A. Fragility Fractures of the Pelvic Ring: Analysis of Epidemiology, Treatment Concepts, and Surgical Strategies from the Registry of the German Pelvic Multicenter Study Group. J. Clin. Med. 2025, 14, 2935. [Google Scholar] [CrossRef] [PubMed]
- Sterneder, M.; Lang, P.; Riesner, H.J.; Hackenbroch, C.; Friemert, B.; Palm, H.G. Insufficiency Fractures vs. Low-Energy Pelvic Ring Fractures—Epidemiological, Diagnostic and Therapeutic Characteristics of Fragility Fractures of the Pelvic Ring. Z. Orthop. Unfallchirurgie 2022, 160, 497–506. [Google Scholar] [CrossRef] [PubMed]
- Scemama, C.; D’Astorg, H.; Guigui, P. Sacral stress fracture after lumbar and lumbosacral fusion. How to manage it? A proposition based on three cases and literature review. Orthop. Traumatol. Surg. Res. 2016, 102, 261–268. [Google Scholar] [CrossRef] [PubMed]
- Shono, Y.; Kaneda, K.; Abumi, K.; McAfee, P.C.; Cunningham, B.W. Stability of posterior spinal instrumentation and its effects on adjacent motion segments in the lumbosacral spine. Spine 1998, 23, 1550–1558. [Google Scholar] [CrossRef] [PubMed]
- Odate, S.; Shikata, J.; Kimura, H.; Soeda, T. Sacral fracture after instrumented lumbosacral fusion: Analysis of risk factors from spinopelvic parameters. Spine 2013, 38, E223–E229. [Google Scholar] [CrossRef] [PubMed]
- Zoulakis, M.; Johansson, L.; Litsne, H.; Axelsson, K.; Lorentzon, M. Type 2 Diabetes and Fracture Risk in Older Women. JAMA Netw. Open 2024, 7, e2425106. [Google Scholar] [CrossRef] [PubMed]
- Caffarelli, C.; Alessi, C.; Nuti, R.; Gonnelli, S. Divergent effects of obesity on fragility fractures. Clin. Interv. Aging 2014, 9, 1629–1636. [Google Scholar] [CrossRef] [PubMed]
- Hou, J.; He, C.; He, W.; Yang, M.; Luo, X.; Li, C. Obesity and Bone Health: A Complex Link. Front. Cell Dev. Biol. 2020, 8, 600181. [Google Scholar] [CrossRef] [PubMed]
- Sgarro, G.A.; Grilli, L.; Valenzano, A.A.; Moscatelli, F.; Monacis, D.; Toto, G.; De Maria, A.; Messina, G.; Polito, R. The Role of BIA Analysis in Osteoporosis Risk Development: Hierarchical Clustering Approach. Diagnostics 2023, 13, 2292. [Google Scholar] [CrossRef] [PubMed]
- Martiniakova, M.; Biro, R.; Penzes, N.; Sarocka, A.; Kovacova, V.; Mondockova, V.; Omelka, R. Links among Obesity, Type 2 Diabetes Mellitus, and Osteoporosis: Bone as a Target. Int. J. Mol. Sci. 2024, 25, 4827. [Google Scholar] [CrossRef] [PubMed]
- Ke, Y.; Hu, H.; Zhang, J.; Yuan, L.; Li, T.; Feng, Y.; Wu, Y.; Fu, X.; Wang, M.; Gao, Y.; et al. Alcohol Consumption and Risk of Fractures: A Systematic Review and Dose-Response Meta-Analysis of Prospective Cohort Studies. Adv. Nutr. 2023, 14, 599–611. [Google Scholar] [CrossRef] [PubMed]
- Dautel, A.; Eckert, T.; Gross, M.; Hauer, K.; Schaufele, M.; Lacroix, A.; Hendlmeier, I.; Abel, B.; Pomiersky, R.; Gugenhan, J.; et al. Multifactorial intervention for hip and pelvic fracture patients with mild to moderate cognitive impairment: Study protocol of a dual-centre randomised controlled trial (OF-CARE). BMC Geriatr. 2019, 19, 125. [Google Scholar] [CrossRef] [PubMed]
- Krappinger, D.; Zegg, M.; Jeske, C.; El Attal, R.; Blauth, M.; Rieger, M. Hemorrhage after low-energy pelvic trauma. J. Trauma Acute Care Surg. 2012, 72, 437–442. [Google Scholar] [CrossRef] [PubMed]
- Papadopoulos, I.N.; Kanakaris, N.; Bonovas, S.; Triantafillidis, A.; Garnavos, C.; Voros, D.; Leukidis, C. Auditing 655 fatalities with pelvic fractures by autopsy as a basis to evaluate trauma care. J. Am. Coll. Surg. 2006, 203, 30–43. [Google Scholar] [CrossRef] [PubMed]
- Al Ma’ani, M.; Nelson, A.; Castillo Diaz, F.; Specner, A.L.; Khurshid, M.H.; Anand, T.; Hejazi, O.; Ditillo, M.; Magnotti, L.J.; Joseph, B. A narrative review: Resuscitation of older adults with hemorrhagic shock. Transfusion 2025, 65 (Suppl. 1), S131–S139. [Google Scholar] [CrossRef] [PubMed]
- Bramos, A.; Velmahos, G.C.; Butt, U.M.; Fikry, K.; Smith, R.M.; Chang, Y. Predictors of bleeding from stable pelvic fractures. Arch. Surg. 2011, 146, 407–411. [Google Scholar] [CrossRef] [PubMed]
- Mennen, A.H.M.; Blokland, A.S.; Maas, M.; van Embden, D. Imaging of pelvic ring fractures in older adults and its clinical implications-a systematic review. Osteoporos. Int. 2023, 34, 1549–1559. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, V.; Lampart, A.; Bingisser, R.; Nickel, C.H. Accuracy of plain radiography in detecting fractures in older individuals after low-energy falls: Current evidence. Trauma Surg. Acute Care Open 2020, 5, e000560. [Google Scholar] [CrossRef] [PubMed]
- Mendel, T.; Ullrich, B.W.; Hofmann, G.O.; Schenk, P.; Goehre, F.; Schwan, S.; Klauke, F. Progressive instability of bilateral sacral fragility fractures in osteoporotic bone: A retrospective analysis of X-ray, CT, and MRI datasets from 78 cases. Eur. J. Trauma Emerg. Surg. 2021, 47, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Hackenbroch, C.; Riesner, H.J.; Lang, P.; Stuby, F.; Danz, B.; Friemert, B.; Palm, H.G.; AG Becken III der Deutschen Gesellschaft für Unfallchirurgie. Dual Energy CT—A Novel Technique for Diagnostic Testing of Fragility Fractures of the Pelvis. Z. Orthop. Unfallchirurgie 2017, 155, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, W.; Cagirici, R.; Al Askar, Y.; Spering, C. Diagnostics and treatment of insufficiency fractures of the pelvis. Unfallchirurgie 2024, 127, 273–282. [Google Scholar] [CrossRef] [PubMed]
- Wong, A.J.N.; Wong, M.; Kutschera, P.; Lau, K.K. Dual-energy CT in musculoskeletal trauma. Clin. Radiol. 2021, 76, 38–49. [Google Scholar] [CrossRef] [PubMed]
- Rommens, P.M.; Hofmann, A. Fragility fractures of the pelvis: An update. J. Musculoskelet. Surg. Res. 2023, 7, 1–10. [Google Scholar] [CrossRef]
- Denis, F.; Davis, S.; Comfort, T. Sacral fractures: An important problem. Retrospective analysis of 236 cases. Clin. Orthop. Relat. Res. 1988, 227, 67–81. [Google Scholar] [CrossRef] [PubMed]
- Isler, B.; Ganz, R. Classification of pelvic girdle injuries. Der Unfallchirurg 1990, 93, 289–302. [Google Scholar] [PubMed]
- Meinberg, E.G.; Agel, J.; Roberts, C.S.; Karam, M.D.; Kellam, J.F. Fracture and Dislocation Classification Compendium-2018. J. Orthop. Trauma 2018, 32 (Suppl. 1), S1–S170. [Google Scholar] [CrossRef] [PubMed]
- Roy-Camille, R.; Saillant, G.; Gagna, G.; Mazel, C. Transverse fracture of the upper sacrum. Suicidal jumper’s fracture. Spine 1985, 10, 838–845. [Google Scholar] [CrossRef] [PubMed]
- Young, J.W.; Burgess, A.R.; Brumback, R.J.; Poka, A. Pelvic fractures: Value of plain radiography in early assessment and management. Radiology 1986, 160, 445–451. [Google Scholar] [CrossRef] [PubMed]
- Rommens, P.M.; Hofmann, A. Comprehensive classification of fragility fractures of the pelvic ring: Recommendations for surgical treatment. Injury 2013, 44, 1733–1744. [Google Scholar] [CrossRef] [PubMed]
- Ullrich, B.W.; Schnake, K.J.; Spiegl, U.J.A.; Schenk, P.; Mendel, T.; Behr, L.; Bula, P.; Flucht, L.B.; Franck, A.; Gercek, E.; et al. OF-Pelvis classification of osteoporotic sacral and pelvic ring fractures. BMC Musculoskelet. Disord. 2021, 22, 992. [Google Scholar] [CrossRef] [PubMed]
- Mennen, A.H.M.; Lommerse, M.; Hemke, R.; Willems, H.C.; Maas, M.; Bloemers, F.W.; Ponsen, K.J.; Van Embden, D. Does regional implementation of a clinical pathway for older adult patients with pelvic fragility fractures after low-energy trauma improve patient outcomes (PELVIC): A multicentre, stepped-wedge, randomised controlled trial. BMJ Open 2024, 14, e083809. [Google Scholar] [CrossRef] [PubMed]
- Rommens, P.M.; Ossendorf, C.; Pairon, P.; Dietz, S.O.; Wagner, D.; Hofmann, A. Clinical pathways for fragility fractures of the pelvic ring: Personal experience and review of the literature. J. Orthop. Sci. 2015, 20, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Locke, S.; Doonan, J.; Jones, B. Advancements in the Management of Fragility Fractures in Orthopaedic Patients. Cureus 2024, 16, e74065. [Google Scholar] [CrossRef] [PubMed]
- Swenson, R.A.; Paull, T.Z.; Yates, R.A.; Foster, J.A.; Griffin, J.T.; Southall, W.G.S.; Aneja, A.; Nguyen, M.P. Comparison of Operative and Nonoperative Management of Elderly Fragility Pelvic Ring Fractures. J. Orthop. Trauma 2024, 38, 472–476. [Google Scholar] [CrossRef] [PubMed]
- Bruce, B.; Reilly, M.; Sims, S. OTA highlight paper predicting future displacement of nonoperatively managed lateral compression sacral fractures: Can it be done? J. Orthop. Trauma 2011, 25, 523–527. [Google Scholar] [CrossRef] [PubMed]
- Lindsay, A.; Tornetta, P., 3rd; Diwan, A.; Templeman, D. Is Closed Reduction and Percutaneous Fixation of Unstable Posterior Ring Injuries as Accurate as Open Reduction and Internal Fixation? J. Orthop. Trauma 2016, 30, 29–33. [Google Scholar] [CrossRef] [PubMed]
- Papakostidis, C.; Kanakaris, N.K.; Kontakis, G.; Giannoudis, P.V. Pelvic ring disruptions: Treatment modalities and analysis of outcomes. Int. Orthop. 2009, 33, 329–338. [Google Scholar] [CrossRef] [PubMed]
- Liuzza, F.; Silluzio, N.; Florio, M.; El Ezzo, O.; Cazzato, G.; Ciolli, G.; Perisano, C.; Maccauro, G. Comparison between posterior sacral plate stabilization versus minimally invasive transiliac-transsacral lag-screw fixation in fractures of sacrum: A single-centre experience. Int. Orthop. 2019, 43, 177–185. [Google Scholar] [CrossRef] [PubMed]
- Bordoni, B.; Varacallo, M.A. Anatomy, Bony Pelvis and Lower Limb, Iliopsoas Muscle. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Ricci, P.L.; Maas, S.; Kelm, J.; Gerich, T. Finite element analysis of the pelvis including gait muscle forces: An investigation into the effect of rami fractures on load transmission. J. Exp. Orthop. 2018, 5, 33. [Google Scholar] [CrossRef] [PubMed]
- Bodzay, T.; Sztrinkai, G.; Pajor, S.; Gal, T.; Jonas, Z.; Erdos, P.; Varadi, K. Does surgically fixation of pubic fracture increase the stability of the operated posterior pelvis? Eklem Hastalik. Cerrahisi 2014, 25, 91–95. [Google Scholar] [CrossRef] [PubMed]
- Moussa, I.S.; Sallam, A.M.; Mahmoud, A.K.; Elzaher, E.H.; Nagy, A.M.; Eid, A.S. Combined anterior and posterior ring fixation versus posterior ring fixation alone in the management of unstable Tile B and C pelvic ring injuries: A randomized controlled trial. Chin. J. Traumatol.=Zhonghua Chuang Shang Za Zhi/Chin. Med. Assoc. 2023, 26, 48–59. [Google Scholar] [CrossRef] [PubMed]
- Arand, C.; Mehler, D.; Sauer, A.; Hartung, C.; Gercek, E.; Rommens, P.M.; Wagner, D. Do we need to fix the anterior fracture component in insufficiency fractures of the pelvis? A biomechanical comparison on an FFP type IIIc fracture in an osteoporotic pelvic bone model. Injury 2023, 54, 111096. [Google Scholar] [CrossRef] [PubMed]
- Wagner, D.; Ossendorf, C.; Gruszka, D.; Hofmann, A.; Rommens, P.M. Fragility fractures of the sacrum: How to identify and when to treat surgically? Eur. J. Trauma Emerg. Surg. 2015, 41, 349–362. [Google Scholar] [CrossRef] [PubMed]
- Cintean, R.; Fritzsche, C.; Zderic, I.; Gueorguiev-Ruegg, B.; Gebhard, F.; Schutze, K. Sacroiliac versus transiliac-transsacral screw osteosynthesis in osteoporotic pelvic fractures: A biomechanical comparison. Eur. J. Trauma Emerg. Surg. 2023, 49, 2553–2560. [Google Scholar] [CrossRef] [PubMed]
- Hadeed, M.M.; Woods, D.; Koerner, J.; Strage, K.E.; Mauffrey, C.; Parry, J.A. Risk factors for screw breach and iatrogenic nerve injury in percutaneous posterior pelvic ring fixation. J. Clin. Orthop. Trauma 2022, 33, 101994. [Google Scholar] [CrossRef] [PubMed]
- Goncalves, R.M.; Freitas, A.; Aragao, V.A.D.; Azevedo, F.E.R.; Lopes, N.B.; Mangueira, L.J.A.; da Silva, L.H.P.; Pires, R.E.; Giordano, V. Comparison of sacroiliac screw techniques for unstable sacroiliac joint disruptions: A finite element model analysis. Injury 2023, 54 (Suppl. 6), 110783. [Google Scholar] [CrossRef] [PubMed]
- Heydemann, J.; Hartline, B.; Gibson, M.E.; Ambrose, C.G.; Munz, J.W.; Galpin, M.; Achor, T.S.; Gary, J.L. Do Transsacral-transiliac Screws Across Uninjured Sacroiliac Joints Affect Pain and Functional Outcomes in Trauma Patients? Clin. Orthop. Relat. Res. 2016, 474, 1417–1421. [Google Scholar] [CrossRef] [PubMed]
- Kaiser, S.P.; Gardner, M.J.; Liu, J.; Routt, M.L., Jr.; Morshed, S. Anatomic Determinants of Sacral Dysmorphism and Implications for Safe Iliosacral Screw Placement. J. Bone Jt. Surg. 2014, 96, e120. [Google Scholar] [CrossRef] [PubMed]
- Takao, M.; Hamada, H.; Sakai, T.; Sugano, N. Factors influencing the accuracy of iliosacral screw insertion using 3D fluoroscopic navigation. Arch. Orthop. Trauma Surg. 2019, 139, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Bastian, J.D.; Jost, J.; Cullmann, J.L.; Aghayev, E.; Keel, M.J.; Benneker, L.M. Percutaneous screw fixation of the iliosacral joint: Optimal screw pathways are frequently not completely intraosseous. Injury 2015, 46, 2003–2009. [Google Scholar] [CrossRef] [PubMed]
- Collinge, C.; Coons, D.; Tornetta, P.; Aschenbrenner, J. Standard multiplanar fluoroscopy versus a fluoroscopically based navigation system for the percutaneous insertion of iliosacral screws: A cadaver model. J. Orthop. Trauma 2005, 19, 254–258. [Google Scholar] [CrossRef] [PubMed]
- Routt, M.L., Jr.; Nork, S.E.; Mills, W.J. Percutaneous fixation of pelvic ring disruptions. Clin. Orthop. Relat. Res. 2000, 375, 15–29. [Google Scholar] [CrossRef] [PubMed]
- Banaszek, D.; Starr, A.J.; Lefaivre, K.A. Technical Considerations and Fluoroscopy in Percutaneous Fixation of the Pelvis and Acetabulum. J. Am. Acad. Orthop. Surg. 2019, 27, 899–908. [Google Scholar] [CrossRef] [PubMed]
- Miller, H.S.; Gardner, M. Curvafix: A novel implant for pelvic fragility fractures. Trauma Case Rep. 2023, 43, 100749. [Google Scholar] [CrossRef] [PubMed]
- Theologis, A.A.; Burch, S.; Pekmezci, M. Placement of iliosacral screws using 3D image-guided (O-Arm) technology and Stealth Navigation: Comparison with traditional fluoroscopy. Bone Jt. J. 2016, 98, 696–702. [Google Scholar] [CrossRef] [PubMed]
- Krappinger, D.; Larndorfer, R.; Struve, P.; Rosenberger, R.; Arora, R.; Blauth, M. Minimally invasive transiliac plate osteosynthesis for type C injuries of the pelvic ring: A clinical and radiological follow-up. J. Orthop. Trauma 2007, 21, 595–602. [Google Scholar] [CrossRef] [PubMed]
- Vaccaro, A.R.; Schroeder, G.D.; Divi, S.N.; Kepler, C.K.; Kleweno, C.P.; Krieg, J.C.; Wilson, J.R.; Holstein, J.H.; Kurd, M.F.; Firoozabadi, R.; et al. Description and Reliability of the AOSpine Sacral Classification System. J. Bone Jt. Surg. 2020, 102, 1454–1463. [Google Scholar] [CrossRef] [PubMed]
- Schroeder, G.D.; Karamian, B.A.; Canseco, J.A.; Vialle, L.R.; Kandziora, F.; Benneker, L.M.; Rajasekaran, S.; Holstein, J.H.; Schnake, K.J.; Kurd, M.F.; et al. Validation of the AO Spine Sacral Classification System: Reliability Among Surgeons Worldwide. J. Orthop. Trauma 2021, 35, e496–e501. [Google Scholar] [CrossRef] [PubMed]
- Gruneweller, N.; Leunig, J.; Zderic, I.; Gueorguiev, B.; Colcuc, C.; Wahnert, D.; Vordemvenne, T. Lumbopelvic Stabilization with Two Methods of Triangular Osteosynthesis: A Biomechanical Study. J. Clin. Med. 2024, 13, 4744. [Google Scholar] [CrossRef] [PubMed]
- Welle, K.; Khoury, M.; Prangenberg, C.; Tager, S.; Goost, H.; Kabir, K. Minimally invasive lumbopelvic stabilization of sacral fracture and sacroiliac injury. Oper. Orthop. Traumatol. 2021, 33, 538–545. [Google Scholar] [CrossRef] [PubMed]
- Herteleer, M.; Boudissa, M.; Hofmann, A.; Wagner, D.; Rommens, P.M. Plate fixation of the anterior pelvic ring in patients with fragility fractures of the pelvis. Eur. J. Trauma Emerg. Surg. 2022, 48, 3711–3719. [Google Scholar] [CrossRef] [PubMed]
- Starr, A.J.; Nakatani, T.; Reinert, C.M.; Cederberg, K. Superior pubic ramus fractures fixed with percutaneous screws: What predicts fixation failure? J. Orthop. Trauma 2008, 22, 81–87. [Google Scholar] [CrossRef] [PubMed]
- Acklin, Y.P.; Zderic, I.; Buschbaum, J.; Varga, P.; Inzana, J.A.; Grechenig, S.; Richards, R.G.; Gueorguiev, B.; Schmitz, P. Biomechanical comparison of plate and screw fixation in anterior pelvic ring fractures with low bone mineral density. Injury 2016, 47, 1456–1460. [Google Scholar] [CrossRef] [PubMed]
- Arand, C.; Hartung, C.; Mehler, D.; Gercek, E.; Wollstadter, J.; Wagner, D.; Rommens, P.M. Biomechanical evaluation of an experimental internal ring fixator (RingFix) for stabilization of pelvic ring injuries on an osteoporotic bone model. Sci. Rep. 2024, 14, 20823. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Wu, G.; Chen, C.Y.; Qiu, Y.Y.; Xie, Y. Percutaneous screw fixation assisted by hollow pedicle finder for superior pubic ramus fractures. BMC Surg. 2022, 22, 216. [Google Scholar] [CrossRef] [PubMed]
- Oikonomidis, S.; Alabsi, A.; Ashqar, G.; Graf, M.; Sobottke, R. Intramedullary Stabilization of Pubic Ramus Fractures in Elderly Patients With a Photodynamic Bone Stabilization System (IlluminOss). Geriatr. Orthop. Surg. Rehabil. 2019, 10, 2151459318824904. [Google Scholar] [CrossRef] [PubMed]
- Gansslen, A.; Hildebrand, F.; Kretek, C. Supraacetabular external fixation for pain control in geriatric type B pelvic injuries. Acta Chir. Orthop. Traumatol. Cech. 2013, 80, 101–105. [Google Scholar] [CrossRef] [PubMed]
- McDonald, C.; Firoozabadi, R.; Routt, M.L., Jr.; Kleweno, C. Complications Associated With Pelvic External Fixation. Orthopedics 2017, 40, e959–e963. [Google Scholar] [CrossRef] [PubMed]
- Cole, P.A.; Dyskin, E.A.; Gilbertson, J.A. Minimally-invasive fixation for anterior pelvic ring disruptions. Injury 2015, 46 (Suppl. 3), S27–S34. [Google Scholar] [CrossRef] [PubMed]
- Vigdorchik, J.M.; Esquivel, A.O.; Jin, X.; Yang, K.H.; Onwudiwe, N.A.; Vaidya, R. Biomechanical stability of a supra-acetabular pedicle screw internal fixation device (INFIX) vs external fixation and plates for vertically unstable pelvic fractures. J. Orthop. Surg. Res. 2012, 7, 31. [Google Scholar] [CrossRef] [PubMed]
- Vaidya, R.; Woodbury, D.; Nasr, K. Anterior Subcutaneous Internal Pelvic Fixation/INFIX: A Systemic Review. J. Orthop. Trauma 2018, 32 (Suppl. 6), S24–S30. [Google Scholar] [CrossRef] [PubMed]
- Hiesterman, T.G.; Hill, B.W.; Cole, P.A. Surgical technique: A percutaneous method of subcutaneous fixation for the anterior pelvic ring: The pelvic bridge. Clin. Orthop. Relat. Res. 2012, 470, 2116–2123. [Google Scholar] [CrossRef] [PubMed]
- Cole, P.A.; Hesse, D.K.; Dugarte, A.J.; Dyskin, E. The Pelvic Bridge: A Subcutaneous Approach for Anterior Pelvic Fixation. JBJS Essent. Surg. Tech. 2017, 7, e20. [Google Scholar] [CrossRef] [PubMed]
- Reichel, L.M.; MacCormick, L.M.; Dugarte, A.J.; Rizkala, A.R.; Graves, S.C.; Cole, P.A. Minimally invasive anterior pelvic internal fixation: An anatomic study comparing Pelvic Bridge to INFIX. Injury 2018, 49, 309–314. [Google Scholar] [CrossRef] [PubMed]
- Moazzam, C.; Heddings, A.A.; Moodie, P.; Cole, P.A. Anterior pelvic subcutaneous internal fixator application: An anatomic study. J. Orthop. Trauma 2012, 26, 263–268. [Google Scholar] [CrossRef] [PubMed]
- Muller, F.; Fuchtmeier, B. A systematic review of the transiliac internal fixator (TIFI) for posterior pelvic injuries. SICOT-J 2021, 7, 40. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.; Balmaceno-Criss, M.; Knebel, A.; Kuharski, M.; Sakr, I.; Daher, M.; McDonald, C.L.; Diebo, B.G.; Czerwein, J.K.; Daniels, A.H. Sacroplasty for Sacral Insufficiency Fractures: Narrative Literature Review on Patient Selection, Technical Approaches, and Outcomes. J. Clin. Med. 2024, 13, 1101. [Google Scholar] [CrossRef] [PubMed]
- Schwetje, D.; Wahd, Y.; Bornemann, R.; Jansen, T.R.; Pflugmacher, R.; Kasapovic, A. Balloon-assisted sacroplasty as a successful procedure for osteoporotic sacral insufficiency fractures after failure of the conservative treatment. Sci. Rep. 2020, 10, 18455. [Google Scholar] [CrossRef] [PubMed]
- Kao, F.C.; Hsu, Y.C.; Chen, T.S.; Liu, P.H.; Tu, Y.K. Combination of long- and short-axis alar sacroplasty techniques under fluoroscopic guidance for osteoporotic sacral insufficiency fracture. J. Orthop. Surg. Res. 2021, 16, 269. [Google Scholar] [CrossRef] [PubMed]
- Bastian, J.D.; Keel, M.J.; Heini, P.F.; Seidel, U.; Benneker, L.M. Complications related to cement leakage in sacroplasty. Acta Orthop. Belg. 2012, 78, 100–105. [Google Scholar] [PubMed]
- Suero, E.M.; Greiner, A.; Becker, C.A.; Cavalcanti Kussmaul, A.; Weidert, S.; Pfeufer, D.; Woiczinski, M.; Braun, C.; Flatz, W.; Bocker, W.; et al. Biomechanical stability of sacroiliac screw osteosynthesis with and without cement augmentation. Injury 2021, 52, 2707–2711. [Google Scholar] [CrossRef] [PubMed]
- Oberkircher, L.; Masaeli, A.; Hack, J.; Figiel, J.; Bliemel, C.; Ruchholtz, S.; Kruger, A. Pull-out strength evaluation of cement augmented iliac screws in osteoporotic spino-pelvic fixation. Orthop. Traumatol. Surg. Res. 2021, 107, 102945. [Google Scholar] [CrossRef] [PubMed]
- Schmitz, P.; Baumann, F.; Grechenig, S.; Gaensslen, A.; Nerlich, M.; Muller, M.B. The cement-augmented transiliacal internal fixator (caTIFI): An innovative surgical technique for stabilization of fragility fractures of the pelvis. Injury 2015, 46 (Suppl. 4), S114–S120. [Google Scholar] [CrossRef] [PubMed]
- Collinge, C.A.; Crist, B.D. Combined Percutaneous Iliosacral Screw Fixation With Sacroplasty Using Resorbable Calcium Phosphate Cement for Osteoporotic Pelvic Fractures Requiring Surgery. J. Orthop. Trauma 2016, 30, e217–e222. [Google Scholar] [CrossRef] [PubMed]
- Elder, S.; Frankenburg, E.; Goulet, J.; Yetkinler, D.; Poser, R.; Goldstein, S. Biomechanical evaluation of calcium phosphate cement-augmented fixation of unstable intertrochanteric fractures. J. Orthop. Trauma 2000, 14, 386–393. [Google Scholar] [CrossRef] [PubMed]
- Collinge, C.; Merk, B.; Lautenschlager, E.P. Mechanical evaluation of fracture fixation augmented with tricalcium phosphate bone cement in a porous osteoporotic cancellous bone model. J. Orthop. Trauma 2007, 21, 124–128. [Google Scholar] [CrossRef] [PubMed]
- Castro, H.; Carmona, M.; Zamora, T.; Klaber, I.; Botello, E.; Faundez, J.; Schweitzer, D. Augmented ilio-sacral screws for the treatment of fragility pelvic fractures: Review of literature, presentation of a novel low-cost technique, and clinical results of a case series. Eur. J. Orthop. Surg. Traumatol. 2024, 35, 29. [Google Scholar] [CrossRef] [PubMed]
- Ellmerer, A.E.; Kuper, M.A.; Rollmann, M.F.; Herath, S.C.; Histing, T. Cement augmentation in pelvic ring fractures. Unfallchirurgie 2022, 125, 443–451. [Google Scholar] [CrossRef] [PubMed]
- Giannoudis, P.V.; Psarakis, S.; Kanakaris, N.K.; Pape, H.C. Biological enhancement of bone healing with Bone Morphogenetic Protein-7 at the clinical setting of pelvic girdle non-unions. Injury 2007, 38 (Suppl. 4), S43–S48. [Google Scholar] [CrossRef] [PubMed]
- Smith, C.T.; Barton, D.W.; Piple, A.S.; Carmouche, J.J. Pelvic Fragility Fractures: An Opportunity to Improve the Undertreatment of Osteoporosis. J. Bone Jt. Surg. 2021, 103, 213–218. [Google Scholar] [CrossRef] [PubMed]
- Collinge, C.; Favela, J. Use of teriparatide in osteoporotic fracture patients. Injury 2016, 47 (Suppl. 1), S36–S38. [Google Scholar] [CrossRef] [PubMed]
- Peichl, P.; Holzer, L.A.; Maier, R.; Holzer, G. Parathyroid hormone 1-84 accelerates fracture-healing in pubic bones of elderly osteoporotic women. J Bone Jt. Surg. 2011, 93, 1583–1587. [Google Scholar] [CrossRef] [PubMed]
- Bovbjerg, P.; Hogh, D.; Froberg, L.; Schmal, H.; Kassem, M. Effect of PTH treatment on bone healing in insufficiency fractures of the pelvis: A systematic review. EFORT Open Rev. 2021, 6, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Hegde, V.; Jo, J.E.; Andreopoulou, P.; Lane, J.M. Effect of osteoporosis medications on fracture healing. Osteoporos. Int. 2016, 27, 861–871. [Google Scholar] [CrossRef] [PubMed]
- Yoo, J.I.; Ha, Y.C.; Ryu, H.J.; Chang, G.W.; Lee, Y.K.; Yoo, M.J.; Koo, K.H. Teriparatide Treatment in Elderly Patients With Sacral Insufficiency Fracture. J. Clin. Endocrinol. Metab. 2017, 102, 560–565. [Google Scholar] [CrossRef] [PubMed]
- Novikov, D.; Kelley, M.G.; Kain, M.S.; Tornetta, P., 3rd. Low Rate of Teriparatide Supplementation for the Treatment of Osteoporotic Pelvic Fractures in Elderly Females. Geriatr. Orthop. Surg. Rehabil. 2024, 15, 21514593241296396. [Google Scholar] [CrossRef] [PubMed]
- Moon, N.H.; Jang, J.H.; Shin, W.C.; Jung, S.J. Effects of Teriparatide on Treatment Outcomes in Osteoporotic Hip and Pelvic Bone Fractures: Meta-analysis and Systematic Review of Randomized Controlled Trials. Hip Pelvis 2020, 32, 182–191. [Google Scholar] [CrossRef] [PubMed]
- Baghdadi, S.; Kiyani, M.; Kalantar, S.H.; Shiri, S.; Sohrabi, O.; Beheshti Fard, S.; Afzal, S.; Khabiri, S.S. Mortality following proximal femoral fractures in elderly patients: A large retrospective cohort study of incidence and risk factors. BMC Musculoskelet. Disord. 2023, 24, 693. [Google Scholar] [CrossRef] [PubMed]
- Hoch, A.; Ozkurtul, O.; Pieroh, P.; Josten, C.; Bohme, J. Outcome and 2-Year Survival Rate in Elderly Patients With Lateral Compression Fractures of the Pelvis. Geriatr. Orthop. Surg. Rehabil. 2017, 8, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Zong, Y.; Li, J.; Li, Z.; Wang, W. Minimally invasive surgery and conservative treatment achieve similar clinical outcomes in patients with type II fragility fractures of the pelvis. J. Orthop. Surg. Res. 2025, 20, 210. [Google Scholar] [CrossRef] [PubMed]
- Nuber, S.; Ritter, B.; Fenwick, A.; Forch, S.; Wanzl, M.; Nuber, M.; Mayr, E. Midterm follow-up of elderly patients with fragility fractures of the pelvis: A prospective cohort-study comparing operative and non-operative treatment according to a therapeutic algorithm. Injury 2022, 53, 496–505. [Google Scholar] [CrossRef] [PubMed]
- Wilson, D.G.G.; Kelly, J.; Rickman, M. Operative management of fragility fractures of the pelvis—A systematic review. BMC Musculoskelet. Disord. 2021, 22, 717. [Google Scholar] [CrossRef] [PubMed]
- Walker, J.B.; Mitchell, S.M.; Karr, S.D.; Lowe, J.A.; Jones, C.B. Percutaneous Transiliac-Transsacral Screw Fixation of Sacral Fragility Fractures Improves Pain, Ambulation, and Rate of Disposition to Home. J. Orthop. Trauma 2018, 32, 452–456. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, M.; Takahashi, N. Surgical treatment of fragility fractures of the pelvis: Short-term outcomes of 42 patients. Eur. J. Orthop. Surg. Traumatol. 2024, 34, 3349–3354. [Google Scholar] [CrossRef] [PubMed]
- Arduini, M.; Saturnino, L.; Piperno, A.; Iundusi, R.; Tarantino, U. Fragility fractures of the pelvis: Treatment and preliminary results. Aging Clin. Exp. Res. 2015, 27 (Suppl. 1), S61–S67. [Google Scholar] [CrossRef] [PubMed]
- Keene, D.J.; Forde, C.; Sugavanam, T.; Williams, M.A.; Lamb, S.E. Exercise for people with a fragility fracture of the pelvis or lower limb: A systematic review of interventions evaluated in clinical trials and reporting quality. BMC Musculoskelet. Disord. 2020, 21, 435. [Google Scholar] [CrossRef] [PubMed]
- Piccione, F.; Maccarone, M.C.; Cortese, A.M.; Rocca, G.; Sansubrino, U.; Piran, G.; Masiero, S. Rehabilitative management of pelvic fractures: A literature-based update. Eur. J. Transl. Myol. 2021, 31, 9933. [Google Scholar] [CrossRef] [PubMed]
- Kjaervik, C.; Gjertsen, J.E.; Stensland, E.; Dybvik, E.H.; Soereide, O. Patient-reported outcome measures in hip fracture patients. Bone Jt. J. 2024, 106, 394–400. [Google Scholar] [CrossRef] [PubMed]
- Marten, O.; Brand, L.; Greiner, W. Feasibility of the EQ-5D in the elderly population: A systematic review of the literature. Qual. Life Res. 2022, 31, 1621–1637. [Google Scholar] [CrossRef] [PubMed]


| Theme | Open Questions | Suggestions and Existing Literature References |
|---|---|---|
| DIAGNOSTICS |
|
|
| ADMISSION MODEL |
|
|
| SURGICAL INDICATION |
|
|
| MODE of FIXATION |
|
|
| AUGMENTATION STRATEGIES |
|
|
| REHABILITATION TIMELINE & ENDPOINTS |
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gordon, A.; Saracco, M.; Giannoudis, P.V.; Kanakaris, N.K. Fragility Fractures of the Pelvis—Current Understanding and Open Questions. J. Clin. Med. 2025, 14, 5122. https://doi.org/10.3390/jcm14145122
Gordon A, Saracco M, Giannoudis PV, Kanakaris NK. Fragility Fractures of the Pelvis—Current Understanding and Open Questions. Journal of Clinical Medicine. 2025; 14(14):5122. https://doi.org/10.3390/jcm14145122
Chicago/Turabian StyleGordon, Amber, Michela Saracco, Peter V. Giannoudis, and Nikolaos K. Kanakaris. 2025. "Fragility Fractures of the Pelvis—Current Understanding and Open Questions" Journal of Clinical Medicine 14, no. 14: 5122. https://doi.org/10.3390/jcm14145122
APA StyleGordon, A., Saracco, M., Giannoudis, P. V., & Kanakaris, N. K. (2025). Fragility Fractures of the Pelvis—Current Understanding and Open Questions. Journal of Clinical Medicine, 14(14), 5122. https://doi.org/10.3390/jcm14145122







