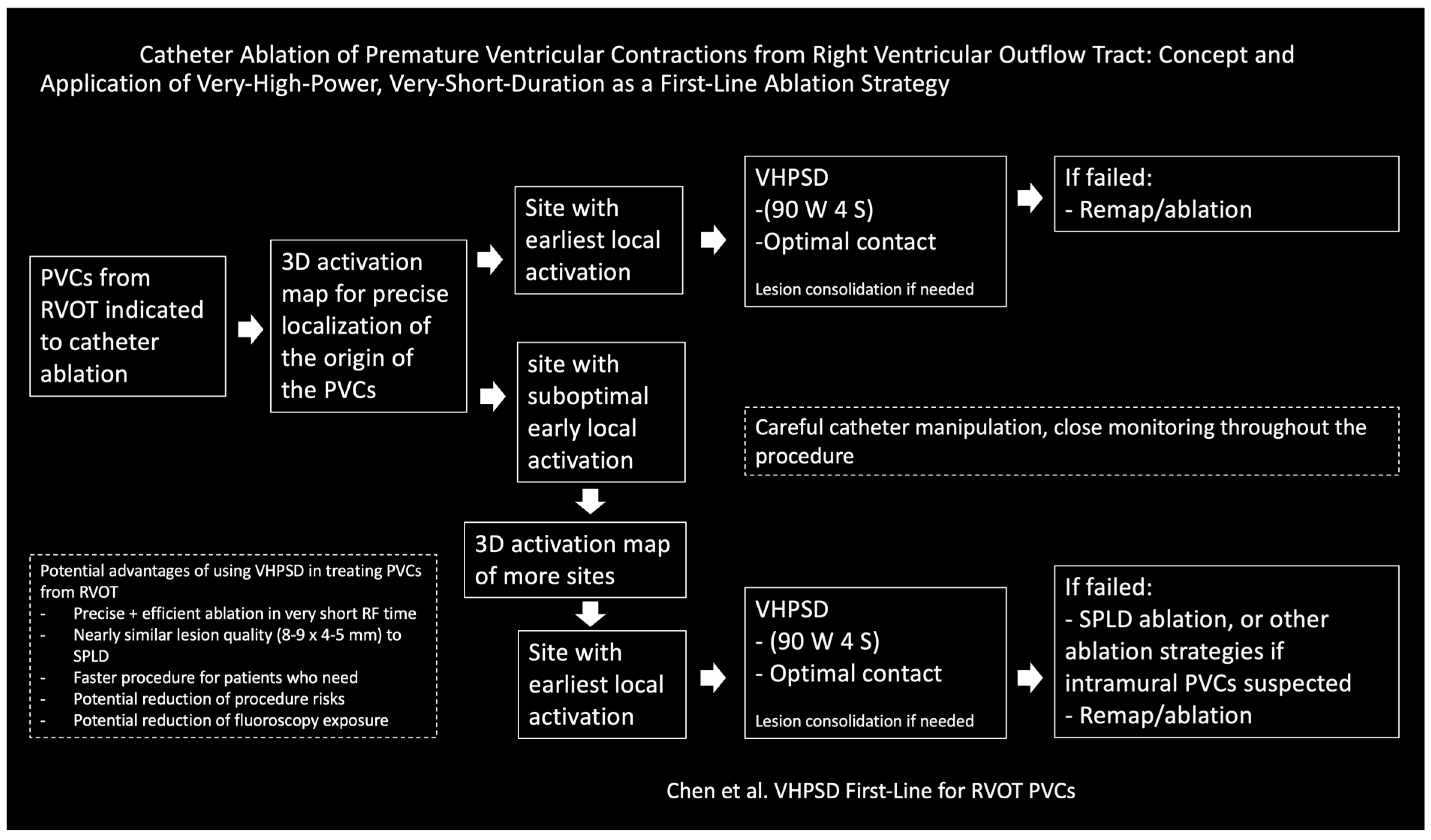
Catheter Ablation of Premature Ventricular Contractions from Right Ventricular Outflow Tract: Concept and Application of Very-High-Power, Very-Short-Duration as a First-Line Ablation Strategy
Abstract
1. Introduction
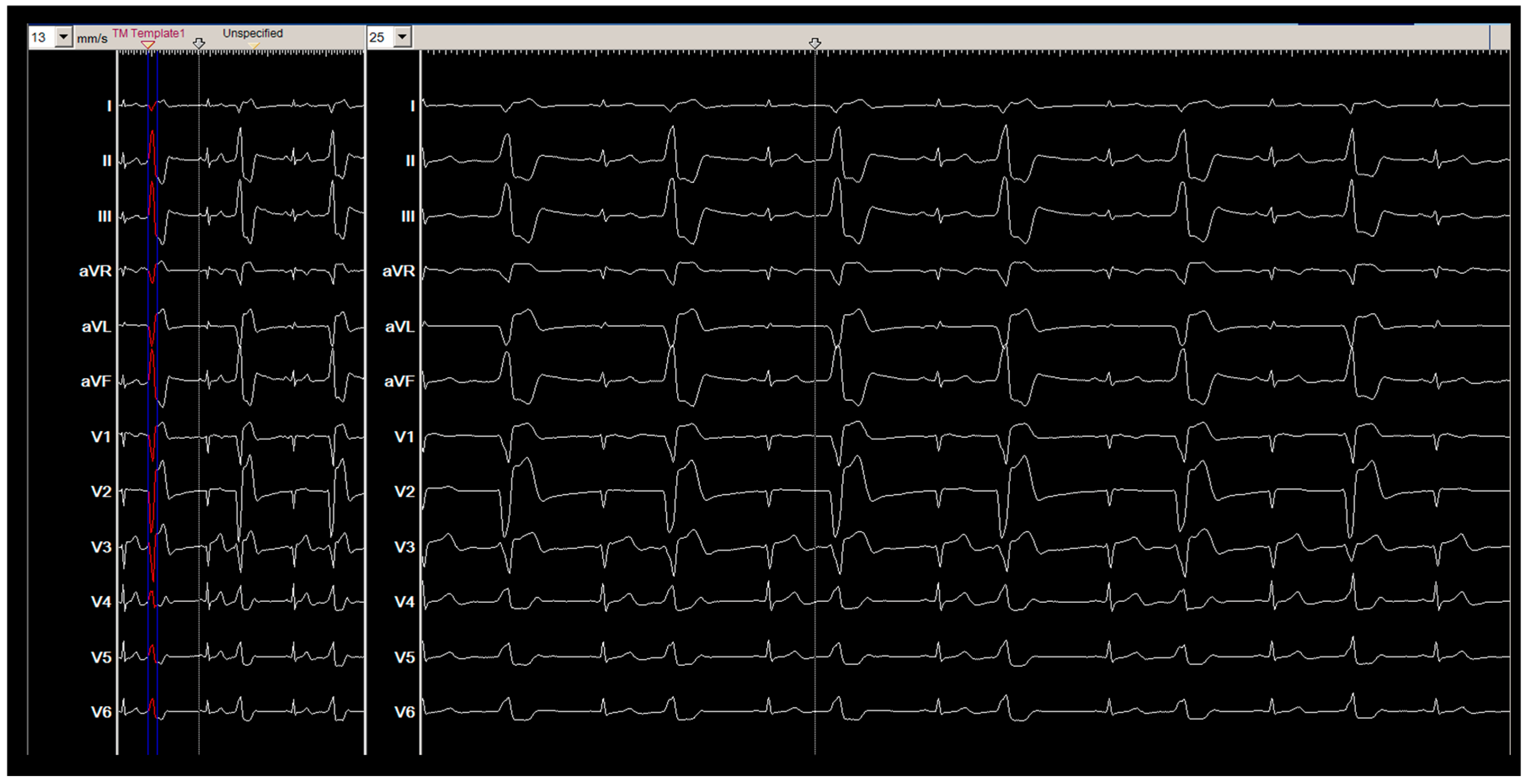
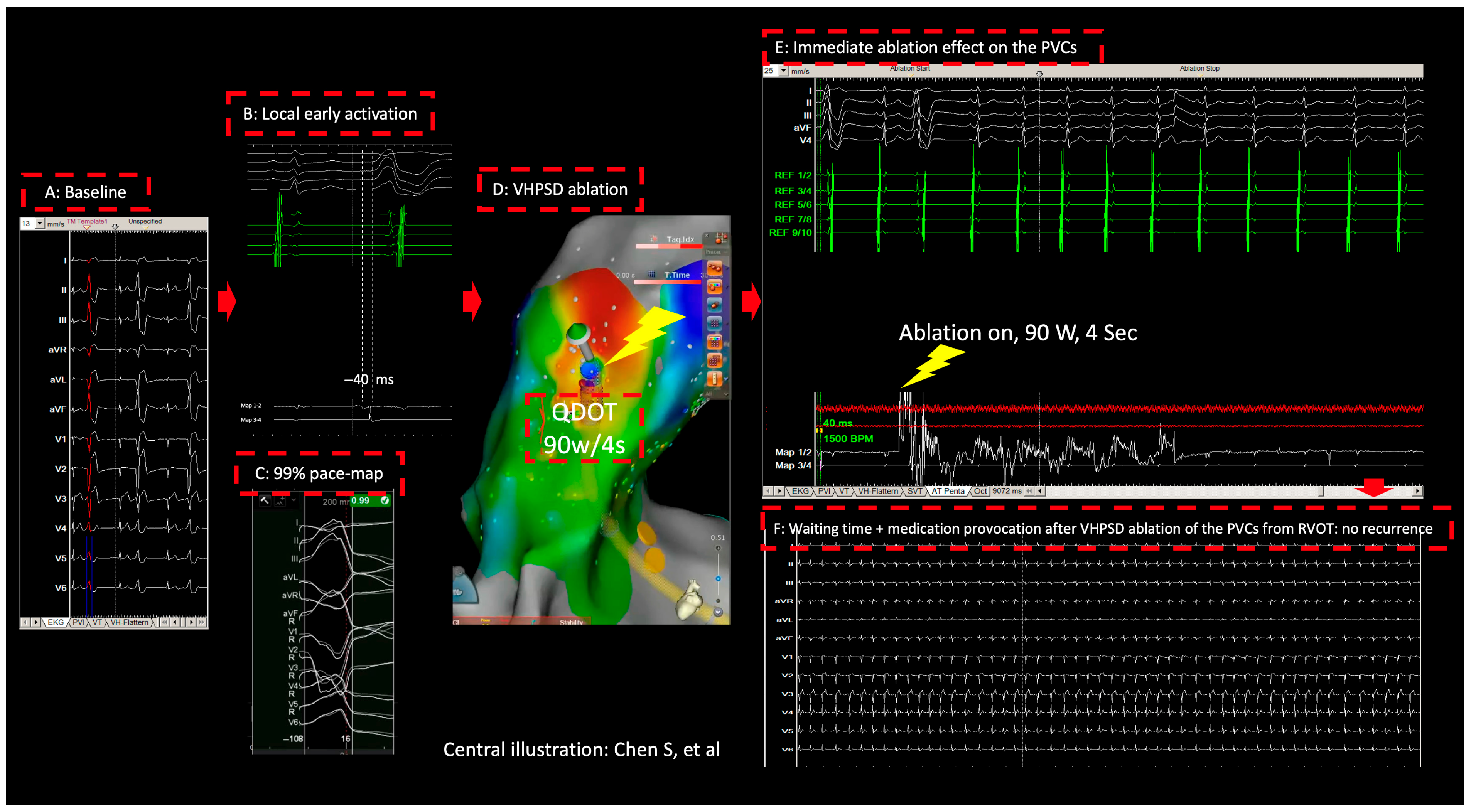
2. Method and Results
3. Discussion
4. Summary
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cronin, E.M.; Bogun, F.M.; Maury, P.; Peichl, P.; Chen, M.; Namboodiri, N.; Aguinaga, L.; Leite, L.R.; Al-Khatib, S.M.; Anter, E.; et al. 2019 HRS/EHRA/APHRS/LAHRS expert consensus statement on catheter ablation of ventricular arrhythmias: Executive summary. J. Interv. Card. Electrophysiol. 2020, 59, 81–133. [Google Scholar] [CrossRef] [PubMed]
- De Silva, K.; Haqqani, H.; Mahajan, R.; Qian, P.; Chik, W.; Voskoboinik, A.; Kistler, P.M.; Lee, G.; Jackson, N.; Kumar, S. Catheter Ablation vs Antiarrhythmic Drug Therapy for Treatment of Premature Ventricular Complexes: A Systematic Review. JACC Clin. Electrophysiol. 2023, 9, 873–885. [Google Scholar] [CrossRef] [PubMed]
- Ling, Z.; Liu, Z.; Su, L.; Zipunnikov, V.; Wu, J.; Du, H.; Woo, K.; Chen, S.; Zhong, B.; Lan, X.; et al. Radiofrequency ablation versus antiarrhythmic medication for treatment of ventricular premature beats from the right ventricular outflow tract: Prospective randomized study. Circ. Arrhythmia Electrophysiol. 2014, 7, 237–243. [Google Scholar] [CrossRef] [PubMed]
- Chen, S. Very high-power short duration 90 W/4 s (vHPSD) vs. vHPSD-combined ablation index-guided 50 W ablation (hybrid) approach for pulmonary vein isolation in treating atrial fibrillation: Have we found the best radiofrequency recipe? J. Interv. Card. Electrophysiol. 2024, 67, 1483–1485. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, H.; Ikeda, A.; Sharma, T.; Govari, A.; Ashton, J.; Maffre, J.; Lifshitz, A.; Fuimaono, K.; Yokoyama, K.; Wittkampf, F.H.; et al. Comparison of In Vivo Tissue Temperature Profile and Lesion Geometry for Radiofrequency Ablation With High Power-Short Duration and Moderate Power-Moderate Duration: Effects of Thermal Latency and Contact Force on Lesion Formation. Circ. Arrhythmia Electrophysiol. 2021, 14, 605–617. [Google Scholar] [CrossRef] [PubMed]
- Teumer, Y.; Ziemssen, H.; Katov, L.; Bothner, C.; Mayer, B.; Rottbauer, W.; Weinmann-Emhardt, K. Comparative lesion metrics analysis of very high power and high power short duration radiofrequency ablation in a Porcine ex vivo model. Sci. Rep. 2025, 15, 20215. [Google Scholar] [CrossRef] [PubMed]
- Saremi, F.; Ho, S.Y.; Cabrera, J.A.; Sánchez-Quintana, D. Right ventricular outflow tract imaging with CT and MRI: Part 1, Morphology. Am. J. Roentgenol. 2013, 200, W39–W50. [Google Scholar] [CrossRef] [PubMed]
- Labrada, L.; Vaidy, A.; Vaidya, A. Right ventricular assessment in pulmonary hypertension. Curr. Opin. Pulm. Med. 2023, 29, 348–354. [Google Scholar] [CrossRef] [PubMed]
- Futyma, P.; Chen, S.; Enriquez, A.; Pürerfellner, H.; Santangeli, P. Bipolar ablation of ventricular arrhythmias: Step-by-step. J. Cardiovasc. Electrophysiol. 2023, 34, 2599–2606. [Google Scholar] [CrossRef] [PubMed]
- Neira, V.; Santangeli, P.; Futyma, P.; Sapp, J.; Valderrabano, M.; Garcia, F.; Enriquez, A. Ablation strategies for intramural ventricular arrhythmias. Heart Rhythm 2020, 17, 1176–1184. [Google Scholar] [CrossRef] [PubMed]
| VHPSD | SPLD | |
|---|---|---|
| Targeted anatomy/structure | RVOT | RVOT |
| RVOT wall thickness | ~3–5 mm | ~3–5 mm |
| Ablation size/depth | Depth: ~4 mm, Width: ~7–8 mm | Depth: ~6 mm, Width: ~7–9 mm |
| Ablation effectiveness | Often effective | Often effective |
| Ablation thermal kinetics | More resistive heating, less conductive heating | More conductive heating, higher risk to deeper tissue or adjacent structures |
| Collateral risk | Lower risk of deeper injury (e.g., coronary arteries, phrenic nerve) | Greater potential for deeper tissue injury, possible risk to adjacent structures (e.g., coronary arteries, phrenic nerve) |
| Risk of steam-pops | Lower risk of steam pops due to very short ablation duration | Higher risk of steam pops due to prolonged ablation duration |
| Catheter stability considerations | Very short ablation duration (~4 s) requires less need to maintain stable contact, advantageous in the mobile RVOT (affected by respiratory and cardiac motion) | Very long ablation duration (~60 s or more) requires maintaining stable contact for extended periods, challenging in the mobile RVOT (affected by respiratory and cardiac motion) |
| Edema formation | Less acute edema, reducing risk of masking incomplete lesions | More acute edema, potentially masking incomplete lesions |
| Procedure efficiency | Much faster lesion creation, reduced overall RF time, less catheter dwell time | Slower lesion creation, increased overall RF time, more catheter dwell time |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, S.; Ebrahimi, R.; Futyma, P.; Graeger, S.; Mirzayeva, G.; Neumann, A.; Schneppe, D.; Sartori, L.V.; Janschel, S.; Kiuchi, M.G.; et al. Catheter Ablation of Premature Ventricular Contractions from Right Ventricular Outflow Tract: Concept and Application of Very-High-Power, Very-Short-Duration as a First-Line Ablation Strategy. J. Clin. Med. 2025, 14, 5118. https://doi.org/10.3390/jcm14145118
Chen S, Ebrahimi R, Futyma P, Graeger S, Mirzayeva G, Neumann A, Schneppe D, Sartori LV, Janschel S, Kiuchi MG, et al. Catheter Ablation of Premature Ventricular Contractions from Right Ventricular Outflow Tract: Concept and Application of Very-High-Power, Very-Short-Duration as a First-Line Ablation Strategy. Journal of Clinical Medicine. 2025; 14(14):5118. https://doi.org/10.3390/jcm14145118
Chicago/Turabian StyleChen, Shaojie, Ramin Ebrahimi, Piotr Futyma, Sebastian Graeger, Gozal Mirzayeva, Anna Neumann, Daniel Schneppe, Luiz Vinícius Sartori, Sarah Janschel, Márcio Galindo Kiuchi, and et al. 2025. "Catheter Ablation of Premature Ventricular Contractions from Right Ventricular Outflow Tract: Concept and Application of Very-High-Power, Very-Short-Duration as a First-Line Ablation Strategy" Journal of Clinical Medicine 14, no. 14: 5118. https://doi.org/10.3390/jcm14145118
APA StyleChen, S., Ebrahimi, R., Futyma, P., Graeger, S., Mirzayeva, G., Neumann, A., Schneppe, D., Sartori, L. V., Janschel, S., Kiuchi, M. G., Martinek, M., & Pürerfellner, H. (2025). Catheter Ablation of Premature Ventricular Contractions from Right Ventricular Outflow Tract: Concept and Application of Very-High-Power, Very-Short-Duration as a First-Line Ablation Strategy. Journal of Clinical Medicine, 14(14), 5118. https://doi.org/10.3390/jcm14145118