Heart Failure Syndromes: Different Definitions of Different Diseases—Do We Need Separate Guidelines? A Narrative Review
Abstract
1. Introduction
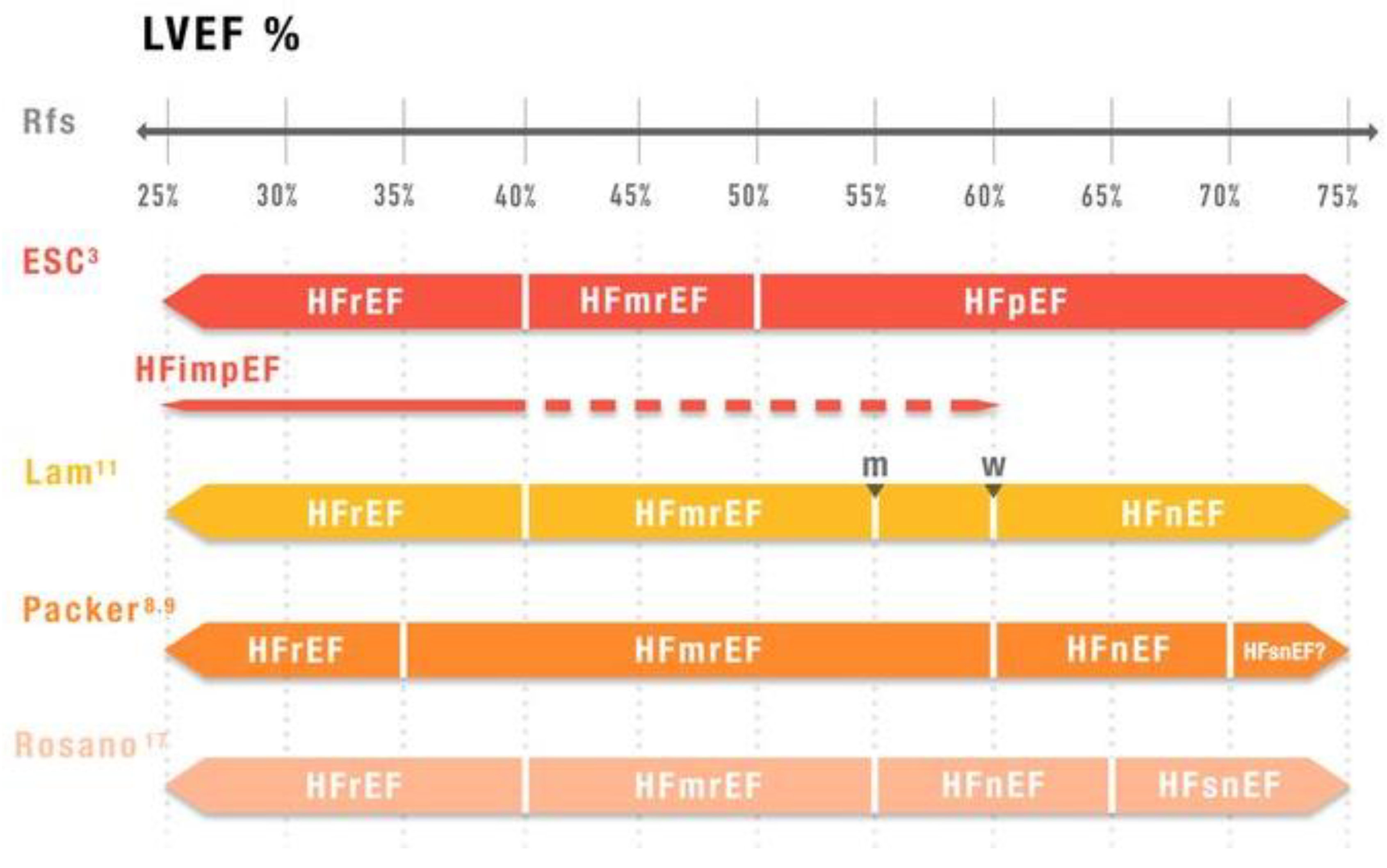
2. Limitations of LVEF-Based Classification
3. Phenotype-Specific Evidence and Challenges
- 1.
- HFrEF
- The Multicenter Postinfarction Research Group analyzed 886 patients post myocardial infarction (MI) and identified LVEF < 40% as one of the four independent risk factors for mortality [29].
- The Survival And Ventricular Enlargement (SAVE) trial demonstrated significant reductions in total and cardiovascular mortality and morbidity in patients with recent MI and LVEF ≤ 40% who were treated with captopril [30]. In this trial, the 40% cutoff was based on earlier studies showing increased mortality and sudden cardiac death in patients with LVEF ≤ 40% [31].
- 2.
- HFmrEF
- HFmrEF may be defined as an “HF gray zone” [39], due to its heterogeneity. These patients tend to have a similar prevalence of certain comorbidities (e.g., ischemic heart disease) as seen in HFrEF but lower rates of others (e.g., older age, arterial hypertension, atrial fibrillation, chronic kidney disease) compared to HFpEF.
- Cardiovascular outcomes are generally better than in HFrEF, but the risk of non-cardiovascular adverse events is higher than in HFrEF and comparable to HFpEF [38].
- RCTs exclusively targeting patients with HFmrEF are lacking. Most studies have focused on patients with HFmrEF/HFpEF, therefore with LVEF > 40%.
- Recent focused update of ESC HF Guidelines [3] recommend SGLT2i with class 1A indication, while the ACC/AHA/HFSA Guidelines assign a class 2a/B-R rating [4]. These agents have demonstrated a significant reduction in composite end-points of cardiovascular death or HF hospitalization in RCTs. However, the benefits were primarily driven by reduced HF hospitalizations. Regarding other drug classes, the HF Guidelines provide a class 2b, level C recommendation for ACEI/ARB/ARNI, MRA, and betablockers [3,4].
- More recently the FINEARTS-HF trial demonstrated that the nonsteroidal MRA finerenone significantly reduced worsening HF or cardiovascular death in patients with HFmrEF/HFpEF, with the benefits fully attributable to reduced worsening HF [41].
- 3.
- HFpEF
- 4.
- HFimpEF
- 5.
- HFsnHF
4. Emerging Tools to Improve Phenotyping Hf Patients
- a.
- Three-dimensional speckle-tracking echocardiography allows for a better evaluation of global and regional function than 2D-TTE, particularly in patients with HF [55].
- b.
- Cardiac magnetic resonance imaging (CMRI) is considered the gold standard for the assessment of cardiac volumes and LVEF, for tissue characterization and mechanical dyssynchrony in patients with HFrEF [56]. This is essential information in order to provide a correct indication regarding device implantation.
- c.
- In selected patients with coronary artery disease, nuclear imaging techniques, such as equilibrium blood pool ventriculography or gated single-photon emission CT (SPECT), may be useful, particularly when contemporary information on myocardial perfusion is needed [56].
- d.
- Artificial intelligence (AI) and machine learning is transforming the cardiovascular imaging, dramatically improving the image acquisition process. AI may be useful in echocardiography, CMI, cardiac and coronary computer tomography, and nuclear cardiology [58].
- e.
- Moreover, omics technologies may further elucidate the underlying genetic mechanisms of HF.
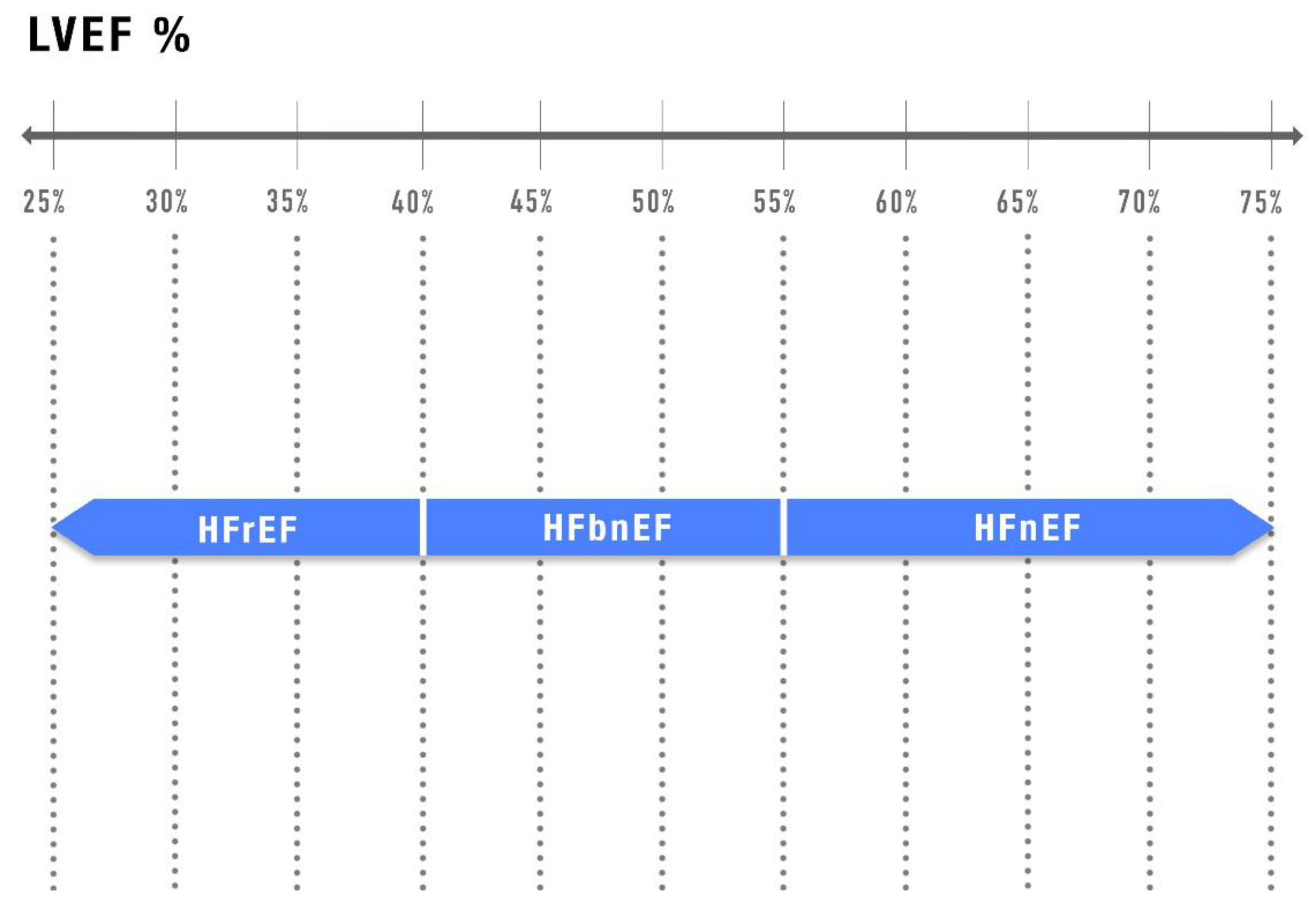
5. Alternative Classifications and Future Directions
6. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Savarese, G.; Becher, P.M.; Lund, L.H.; Seferovic, P.; Rosano, G.M.C.; Coats, A.J.S. Global burden of heart failure: A comprehensive and updated review of epidemiology. Cardiovasc. Res. 2023, 118, 3272–3287. [Google Scholar] [CrossRef]
- Seferovic, P.M.; Vardas, P.; Jankowska, E.A.; Maggioni, A.P.; Timmis, A.; Milinković, I.; Polovina, M.; Lainščak, M.; Timmis, A.; Huculeci, R.; et al. The Heart Failure Association atlas: Heart failure epidemiology and management statistics 2019. Eur. J. Heart Fail. 2021, 23, 906–914. [Google Scholar] [CrossRef]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: Developed by the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur. Heart J. 2023, 44, 3627–3639. [Google Scholar] [PubMed]
- Heidenreich, P.A.; Bozkurt, B.; Aguilar, D.; Allen, L.A.; Byun, J.J.; Colvin, M.M.; Deswal, A.; Drazner, M.H.; Dunlay, S.M.; Evers, L.R.; et al. 2022 AHA/ACC/HFSA guideline for the management of heart failure: A report of the american college of Cardiology/american heart association joint committee on clinical practice guidelines. Circulation 2022, 145, e895–e1032. [Google Scholar] [CrossRef] [PubMed]
- Bozkurt, B.; Coats, A.J.; Tsutsui, H.; Abdelhamid, M.; Adamopoulos, S.; Albert, N.; Anker, S.D.; Atherton, J.; Böhm, M.; Butler, J.; et al. Universal definition and classification of heart failure: A report of the heart failure society of america, heart failure association of the european society of cardiology, Japanese heart failure society and writing committee of the universal definition of heart failure. Eur. J. Heart Fail. 2021, 23, 352–380. [Google Scholar] [PubMed]
- Kalogeropoulos, A.P.; Fonarow, G.C.; Georgiopoulou, V.; Burkman, G.; Siwamogsatham, S.; Patel, A.; Papadimitriou, L.; Butler, J. Characteristics and outcomes of adult outpatients with heart failure and improved or recovered ejection fraction. JAMA Cardiol. 2016, 1, 510–518. [Google Scholar] [CrossRef]
- Wilcox, J.E.; Fang, J.C.; Margulies, K.B.; Mann, D.L. Heart Failure with Recovered Left Ventricular Ejection Fraction: JACC Scientific Expert Panel. J. Am. Coll. Cardiol. 2020, 76, 719–734. [Google Scholar] [CrossRef]
- Packer, M. A reclassification of heart failure based on recognition of heart failure with normal to supernormal ejection fraction, a clinically common form of cardiac contracture, with distinctive pathophysiological and therapeutic features. Eur. J. Heart Fail. 2023, 25, 669–672. [Google Scholar] [CrossRef]
- Packer, M. Left Ventricular Ejection Fraction in Heart Failure: Crazy, Stupid Love-and Maybe, Redemption. J. Am. Heart Assoc. 2024, 13, e034642. [Google Scholar] [CrossRef]
- Christersson, M.; Gustafsson, S.; Lampa, E.; Almstedt, M.; Cars, T.; Bodegård, J.; Arefalk, G.; Sundström, J. Usefulness of Heart Failure Categories Based on Left Ventricular Ejection Fraction. J. Am. Heart Assoc. 2024, 13, e032257. [Google Scholar] [CrossRef]
- Lam, C.S.P.; Solomon, S.D. Classification of Heart Failure According to Ejection Fraction: JACC Review Topic of the Week. J. Am. Coll. Cardiol. 2021, 77, 3217–3225. [Google Scholar] [CrossRef] [PubMed]
- Ohte, N.; Kikuchi, S.; Iwahashi, N.; Kinugasa, Y.; Dohi, K.; Takase, H.; Masai, K.; Inoue, K.; Okumura, T.; Hachiya, K.; et al. Unfavourable outcomes in patients with heart failure with higher preserved left ventricular ejection fraction. Eur. Heart J. Cardiovasc. Imaging 2023, 24, 293–300. [Google Scholar] [CrossRef] [PubMed]
- Smiseth, O.A.; Fernandes, J.F.; Lamata, P. The challenge of understanting heart failure with supernormal left ventricular ejection fraction: Time for building the patient’s digital twin. Eur. Heart J. Cardiovasc. Imaging 2023, 24, 301–303. [Google Scholar] [CrossRef] [PubMed]
- Wehner, G.J.; Jing, L.; Haggerty, C.M.; Suever, J.D.; Leader, J.B.; Hartzel, D.N.; Kirchner, H.L.; Manus, J.N.A.; James, N.; Ayar, Z.; et al. Routinely reported ejection fraction and mortality in clinical practice: Where does the nadir of risk lie? Eur. Heart J. 2020, 41, 1249–1257. [Google Scholar] [CrossRef]
- Lund, L.H.; Pitt, B.; Metra, M. Left ventricular ejection fraction as the primary heart failure phenotyping parameter. Eur. J. Heart Fail. 2022, 24, 1158–1161. [Google Scholar] [CrossRef]
- Romanò, M. New Disease Trajectories of Heart Failure: Challenges in Determining the Ideal Timing of Palliative Care Implementation. J. Palliat. Med. 2024, 9, 1118–1124. [Google Scholar] [CrossRef]
- Rosano, G.M.C.; Teerlink, J.R.; Kinugawa, K.; Bayes-Genis, A.; Chioncel, O.; Fang, J.; Greenberg, B.; Ibrahim, N.E.; Imamura, T.; Inomata, T.; et al. The use of Left Ventricular Ejection Fraction in the Diagnosis and Management of Heart Failure. A Clinical Consensus Statement of the Heart Failure Association (HFA) of the ESC, the Heart Failure Society of America (HFSA), and the Japanese Heart Failure Society (JHFS). Eur. J. Card. Fail. 2025, Online ahead of print. [CrossRef]
- Tsutsui, H.; Ide, T.; Ito, H.; Kihara, Y.; Kinugawa, K.; Kinugawa, S.; Makaya, M.; Murohara, T.; Node, K.; Saito, Y.; et al. JCS/JHFS 2021 Guideline Focused Update on Diagnosis and Treatment of Acute and Chronic Heart Failure. Circ. J. 2021, 85, 2252–2291. [Google Scholar] [CrossRef]
- Chioncel, O.; Lainscak, M.; Seferovic, P.M.; Anker, S.D.; Crespo-Leiro, M.G.; Harjola, V.; Parissis, J.; Laroche, C.; Piepoli, M.F.; Fonseca, C.; et al. Epidemiology and one-year outcomes in patients with chronic heart failure and preserved, mid-range and reduced ejection fraction: An analysis of the ESC Heart Failure Long-Term Registry. Eur. J. Heart Fail. 2017, 19, 1574–1585. [Google Scholar] [CrossRef]
- Stolfo, D.; Lund, L.H.; Benson, L.; Hage, C.; Sinagra, G.; Dahlström, U.; Savarese, G. Persistent high burden of heart failure across the ejection fraction spectrum in a nationwide setting. J. Am. Heart Assoc. 2022, 11, e026708. [Google Scholar] [CrossRef]
- Savarese, G.; Gatti, P.; Benson, L.; Adamo, M.; Chioncel, O.; Crespo-Leiro, M.G.; Anker, S.D.; Coats, A.J.S.; Filippatos, G.; Lainscak, M.; et al. Left ventricular ejection fraction digit bias and reclassification of heart failure with mildly reduced vs reduced ejection fraction based on the 2021 definition and classification of heart failure. Am. Heart J. 2024, 267, 52–61. [Google Scholar] [CrossRef] [PubMed]
- Fonarow, G.C.; Stough, W.G.; Abraham, W.T.; Albert, N.M.; Gheorghiade, M.; Greenberg, B.H.; O’Connor, C.M.; Sun, J.L.; Yancy, C.W.; Young, J.B.; et al. Characteristics, treatments, and outcomes of patients with preserved systolic function hospitalized for heart failure: A report from the OPTIMIZE-HF registry. J. Am. Coll. Cardiol. 2007, 50, 768–777. [Google Scholar] [CrossRef] [PubMed]
- Kapoor, J.R.; Kapoor, R.; Ju, C.; Eapen, Z.J.; Hernandez, A.F.; Butler, J.; Yancy, C.W.; Fonarow, G.C. Precipitating clinical factors, heart failure characterization, and outcomes in patients hospitalized with heart failure with reduced, borderline, and preserved ejection fraction. JACC Heart Fail. 2016, 4, 464–472. [Google Scholar] [CrossRef] [PubMed]
- Joseph, P.; Dokainish, H.; McCready, T.; Budaj, A.; Roy, A.; Ertl, G.; Gomez-Mesa, J.E.; Leong, D.; Ezekowitz, J.; Hage, C.; et al. A multinational registry to study the characteristics and outcomes of heart failure patients: The global congestive heart failure (G-CHF) registry. Am. Heart J. 2020, 227, 56–63. [Google Scholar] [CrossRef]
- Wang, N.; Hales, S.; Barin, E.; Tofler, G. Characteristics and outcome for heart failure patients with mid-range ejection fraction. J. Cardiovasc. Med. 2018, 19, 297–303. [Google Scholar] [CrossRef]
- Horiuchi, Y.; Asami, M.; Ide, T.; Yahagi, K.; Komiyama, K.; Yuzawa, H.; Tanaka, J.; Aoki, J.; Matsushima, S.; Tohyama, T.; et al. Prevalence, characteristics and cardiovascular and non-cardiovascular outcomes in patients with heart failure with supra-normal ejection fraction: Insight from the JROADHF study. Eur. J. Heart Fail. 2023, 25, 989–998. [Google Scholar] [CrossRef]
- Vardeny, O.; Desai, A.S.; Jhund, P.S.; Fang, J.C.; Claggett, B.; de Boer, R.A.; Fernandez, A.F.; Inzucchi, S.E.; Kosiborod, M.N.; Lam, C.S.P.; et al. Dapagliflozin and Mode of Death in Heart Failure with Improved Ejection Fraction: A Post Hoc Analysis of the DELIVER Trial. JAMA Cardiol. 2024, 9, 283–289. [Google Scholar] [CrossRef]
- Borlaug, B.A.; Sharma, K.; Shah, S.J.; Ho, J.E. Heart Failure with Preserved Ejection Fraction: JACC Scientific Statement. J. Am. Coll. Cardiol. 2023, 81, 1810–1834. [Google Scholar] [CrossRef]
- The Multicenter Postinfarction Research Group. Risk Stratification and Survival after Myocardial Infarction. N. Engl. J. Med. 1983, 309, 331–336. [Google Scholar] [CrossRef]
- Pfeffer, M.A.; Braunwald, E.; Moye, L.A.; Basta, L.; Brown, E.J.; Cuddy, T.E.; Davis, B.R.; Geltman, E.M.; Goldman, S.; Flaker, G.C.; et al. Effect of captopril on mortality and morbidity in patients with left ventricular dysfunction after myocardial infarction: Results of the Survival and Ventricular Enlargement Trial. N. Engl. J. Med. 1992, 327, 669–677. [Google Scholar] [CrossRef]
- Norris, R.M.; Barnaby, P.F.; Brandt, P.W.; Geary, G.G.; Whitlock, R.M.; Wild, C.J.; Barratt-Boyes, B.G. Prognosis after recovery from first acute myocardial infarction: Determinants of reinfarction and sudden death. Am. J. Cardiol. 1984, 53, 408–413. [Google Scholar] [CrossRef] [PubMed]
- Zeppenfeld, K.; Tfelt-Hansen, J.; de Riva, M.; Winkel, B.G.; Behr, E.R.; Blom, N.A.; Charron, P.; Corrado, D.; Dagres, N.; de Chillou, C.; et al. ESC Scientific Document Group. 2022 ESC Guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death. Eur. Heart J. 2022, 43, 3997–4126. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, P.W.; Pieske, B.; Anstrom, K.J.; Ezekowitz, J.; Hernandez, A.F.; Butler, J.; Lam, C.S.P.; Ponikowski, P.; Voors, A.A.; Jia, G.; et al. Vericiguat in Patients with Heart Failure and Reduced Ejection Fraction. N. Engl. J. Med. 2020, 382, 1883–1893. [Google Scholar] [CrossRef] [PubMed]
- McMurray, J.J.; Packer, M.; Desai, A.S.; Gong, J.; Lefkowitz, M.P.; Rizkala, A.R.; Rouleau, J.L.; Shi, V.C.; Solomon, S.D.; Swedberg, K.; et al. PARADIGM-HF Investigators and Committees. Angiotensin-neprilysin inhibition versus enalapril in heart failure. N. Engl. J. Med. 2014, 371, 993–1004. [Google Scholar] [CrossRef]
- Teerlink, J.R.; Diaz, R.; Felker, G.M.; McMurray, J.J.V.; Metra, M.; Solomon, S.D.; Adams, K.F.; Anand, I.; Arias-Mendoza, A.; Biering-Sørensen, T.; et al. Cardiac Myosin Activation with Omecamtiv Mecarbil in Systolic Heart Failure. N. Engl. J. Med. 2021, 384, 105–116. [Google Scholar] [CrossRef]
- Lavalle, C.; Mariani, M.V.; Severino, P.; Palombi, M.; Trivigno, S.; D’Amato, A.; Silvetti, G.; Pierucci, N.; Di Lullo, L.; Chimenti, C.; et al. Efficacy of Modern Therapies for Heart Failure with Reduced Ejection Fraction in Specific Population Subgroups: A Systematic Review and Network Meta-Analysis. Cardiorenal Med. 2024, 14, 570–580. [Google Scholar] [CrossRef]
- Yancy, C.W.; Jessup, M.; Bozkurt, B.; Butler, J.; Casey, D.E., Jr.; Drazner, M.H.; Fonarow, G.C.; Geraci, S.A.; Horwich, T.; Januzzi, J.L.; et al. 2013 ACCF/AHA guideline for the management of heart failure: A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J. Am. Coll. Cardiol. 2013, 62, e147–e239. [Google Scholar] [CrossRef]
- Savarese, G.; Stolfo, D.; Sinagra, G.; Lund, L.H. Heart failure with mid-range or mildly reduced ejection fraction. Nat. Rev. Cardiol. 2022, 19, 100–116. [Google Scholar] [CrossRef]
- Hamdani, N.; El-Battrawy, I. Between the Beats: Unraveling Diagnostic, Therapeutic Challenges, and Sex Differences in Heart Failure’s Gray Zone. J. Am. Heart Assoc. 2025, 14, e038364. [Google Scholar] [CrossRef]
- Meijs, C.; Brugts, J.J.; Lund, L.H.; Linssen, G.C.M.; La Rocca, H.B.; Dahlström, U.; Vaartjes, I.; Koudstaal, S.; Asselbergs, F.W.; Savarese, G.; et al. Identifying distinct clinical clusters in heart failure with mildly reduced ejection fraction. Int. J. Cardiol. 2023, 386, 83–90. [Google Scholar] [CrossRef]
- Solomon, S.D.; McMurray, J.J.V.; Vaduganathan, M.; Claggett, B.; Jhund, P.S.; Desai, A.S.; Henderson, A.D.; Lam, C.S.P.; Pitt, B.; Senni, M.; et al. Finerenone in Heart Failure with Mildly Reduced or Preserved Ejection Fraction. N. Engl. J. Med. 2024, 391, 1475–1485. [Google Scholar] [CrossRef]
- Stolfo, D.; Lund, L.H.; Sinagra, G.; Lindberg, F.; Dahlström, U.; Rosano, G.; Savarese, G. Heart failure pharmacological treatments and outcomes in heart failure with mildly reduced ejection fraction. Eur. Heart J. Cardiovasc. Pharmacother. 2023, 9, 526–535. [Google Scholar] [CrossRef] [PubMed]
- Kosiborod, M.N.; Deanfield, J.; Pratley, R.; Borlaug, B.A.; Butler, J.; Davies, M.; Emerson, S.S.; Kahn, S.E.; Kitzman, D.W.; Lingvay, I.; et al. Semaglutide versus placebo in patients with heart failure and mildly reduced or preserved ejection fraction: A pooled analysis of the SELECT, FLOW, STEP-HFpEF, and STEP-HFpEF DM randomised trials. Lancet 2024, 404, 949–961. [Google Scholar] [CrossRef] [PubMed]
- Anker, S.D.; Usman, M.S.; Anker, M.S.; Butler, J.; Böhm, M.; Abraham, W.T.; Adamo, M.; Chopra, V.K.; Cicoira, M.; Cosentino, F.; et al. Patient phenotype profiling in heart failure with preserved ejection fraction to guide therapeutic decision making. A scientific statement of the Heart Failure Association, the European Heart Rhythm Association of the European Society of Cardiology, and the European Society of Hypertension. Eur. J. Heart Fail. 2023, 25, 936–955. [Google Scholar] [PubMed]
- Balestrieri, G.; Limonta, R.; Ponti, E.; Merlo, A.; Sciatti, E.; D’Isa, S.; Gori, M.; Casu, G.; Giannattasio, C.; Senni, M.; et al. The Therapy and Management of Heart Failure with Preserved Ejection Fraction: New Insights on Treatment. Card. Fail. Rev. 2024, 10, e05. [Google Scholar] [CrossRef]
- Uijl, A.; Savarese, G.; Vaartjes, I.; Dahlström, U.; Brugts, J.J.; Linssen, G.C.M.; van Empel, V.; Brunner-La Rocca, H.P.; Asselbergs, F.W.; Lund, L.H.; et al. Identification of distinct phenotypic clusters in heart failure with preserved ejection fraction. Eur. J. Heart Fail. 2021, 23, 973–982. [Google Scholar] [CrossRef]
- Shahim, A.; Donal, E.; Hage, C.; Oger, E.; Savarese, G.; Persson, H.; Haugen-Löfman, I.; Ennezat, P.; Sportouch-Dukhan, C.; Drouet, E.; et al. Rates and predictors of cardiovascular and non-cardiovascular outcomes in heart failure with preserved ejection fraction. ESC Heart Fail. 2024, 11, 3572–3583. [Google Scholar] [CrossRef]
- Capone, F.; Vettor, R.; Schiattarella, G.G. Cardiometabolic HFpEF: NASH of the Heart. Circulation 2023, 147, 451–453. [Google Scholar] [CrossRef]
- Tini, G.; Cannatà, A.; Canepa, M.; Masci, P.G.; Pardini, M.; Giacca, M.; Sinagra, G.; Marchionni, N.; Del Monte, F.; Udelson, J.E.; et al. Is heart failure with preserved ejection fraction a ‘dementia’ of the heart? Heart Fail. Rev. 2022, 27, 587–594. [Google Scholar] [CrossRef]
- Teramoto, K.; Ouwerkerk, W.; Tay, W.; Tromp, J.; Katherine Teng, T.H.; Chandramouli, C.; Lawson, C.A.; Huang, W.; Hung, C.L.; Chopra, V.; et al. Clinical Features of Heart Failure with Normal Ejection Fraction: Insights from the ASIAN-HF Registry. JACC Asia 2023, 5, 739–751. [Google Scholar] [CrossRef]
- Cannata, A.; McDonagh, T.A. Heart Failure with Preserved Ejection Fraction. N. Engl. J. Med. 2025, 392, 173–184. [Google Scholar] [CrossRef]
- Agostoni, P.; Pluchinotta, F.R.; Salvioni, E.; Mapelli, M.; Galotta, A.; Bonomi, A.; Magrì, D.; Perna, E.; Paolillo, S.; Corrà, U.; et al. Heart failure patients with improved ejection fraction: Insights from the MECKI score database. Eur. J. Heart Fail. 2023, 25, 1976–1984. [Google Scholar] [CrossRef]
- Kodur, N.; Tang, W.H.W. Management of Heart Failure with Improved Ejection Fraction: Current Evidence and Controversies. JACC Heart Fail. 2025, 13, 537–553. [Google Scholar] [CrossRef]
- Landucci, L.; Faxén, U.L.; Benson, L.; Rosano, G.M.C.; Dahlström, U.; Lund, L.H.; Savarese, G. Characterizing Heart Failure Across the Spectrum of the Preserved Ejection Fraction: Does Heart Failure with Supranormal Ejection Fraction Exist? Data from the Swedish Heart Failure Registry. J. Am. Heart Assoc. 2025, 14, e037502. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Lin, Y.; Ji, M.; Wu, W.; Li, H.; Qian, M.; Zhang, L.; Xie, M.; Li, Y. Clinical Utility of Three-Dimensional Speckle-Tracking Echocardiography in Heart Failure. J. Clin. Med. 2022, 11, 6307. [Google Scholar] [CrossRef] [PubMed]
- Gosling, R.C.; Al-Mohammad, A. The Role of Cardiac Imaging in Heart Failure with Reduced Ejection Fraction. Card. Fail. Rev. 2022, 8, e22. [Google Scholar] [CrossRef]
- Wenzel, J.P.; Albrecht, J.N.; Toprak, B.; Petersen, E.; Nikorowitsch, J.; Cavus, E.; Jahnke, C.; Riedl, K.A.; Adam, G. Head-to-head comparison of cardiac magnetic resonance imaging and transthoracic echocardiography in the general population (MATCH). Clin. Res. Cardiol. 2025. [Google Scholar] [CrossRef]
- Khan, M.S.; Arshad, M.S.; Greene, S.J.; Van Spall, H.G.C.; Pandey, A.; Vemulapalli, S.; Perakslis, E.; Butler, J. Artificial intelligence and heart failure: A state-of-the-art review. Eur. J. Heart Fail. 2023, 25, 1507–1525. [Google Scholar] [CrossRef]
- Toma, M.; Mak, G.J.; Chen, V.; Hollander, Z.; Shannon, C.P.; Lam, K.K.Y.; Ng, R.T.; Tebbutt, S.J.; Wilson-McManus, J.E.; Ignaszewski, A.; et al. Differentiating heart failure phenotypes using sex-specific transcriptomic and proteomic biomarker panels. ESC Heart Fail. 2017, 4, 301–311. [Google Scholar] [CrossRef]
- Triposkiadis, F.; Xanthopoulos, A.; Drakos, S.G.; Boudoulas, K.D.; Briasoulis, A.; Skoularigis, J.; Tsioufis, K.; Boudoulas, H.; Starling, R.C. Back to the basics: The need for an etiological classification of chronic heart failure. Curr. Probl. Cardiol. 2024, 49, 102460. [Google Scholar] [CrossRef]
- Docherty, K.F.; Bayes-Genis, A.; Butler, J.; Coats, A.J.S.; Drazner, M.H.; Joyce, E.; Lam, C.S.P. The four pillars of HFrEF therapy: Is it time to treat heart failure regardless of ejection fraction? Eur. Heart J. Suppl. 2022, 24, L10–L19. [Google Scholar] [CrossRef]
- Forrest, I.S.; Rocheleau, G.; Bafna, S.; Argulian, E.; Narula, J.; Natarajan, P.; Do, R. Genetic and phenotypic profiling of supranormal ejection fraction reveals decreased survival and underdiagnosed heart failure. Eur. J. Heart Fail. 2022, 24, 2118–2127. [Google Scholar] [CrossRef] [PubMed]
- Butler, J.; Packer, M.; Filippatos, G.; Ferreira, J.P.; Zeller, C.; Schnee, J.; Brueckmann, M.; Pocock, S.J.; Zannad, F.; Anker, S.D. Effect of empagliflozin in patients with heart failure across the spectrum of left ventricular ejection fraction. Eur. Heart J. 2022, 43, 416–426. [Google Scholar] [CrossRef] [PubMed]
- Inciardi, R.M.; Riccardi, M.; Savarese, G.; Metra, M.; Vaduganathan, M.; Solomon, S.D. Tailoring medical therapy for heart failure with preserved ejection fraction. Eur. J. Heart Fail. 2025, 27, 190–193. [Google Scholar] [CrossRef] [PubMed]
- Lang, R.M.; Badano, L.P.; Mor-Avi, V.; Afilalo, J.; Armstrong, A.; Ernande, L.; Iachskampf, F.A.; Foster, E.; Goldstein, S.A.; Kuznetsova, T.; et al. Recommendations for cardiac chamber quantification by echocardiography in adults: An update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J. Am. Soc. Echocardiogr. 2015, 28, 1–39.e14. [Google Scholar] [CrossRef]
- Savarese, G.; Schiattarella, G.G.; Lindberg, F.; Anker, M.S.; Bayes-Genis, A.; Bäck, M.; Braunschweig, F.; Bucciarelli-Ducci, C.; Butler, J.; Cannata, A.; et al. Heart failure and obesity: Translational approaches and therapeutic perspectives. A scientific statement of the Heart Failure Association of the ESC. Eur. J. Heart Fail. 2025, Epub ahead of print. [CrossRef]
| Sources (pts) | HFrEF | HFmEF | HFpEF | HFimpEF | HFsnEF |
|---|---|---|---|---|---|
| ESC-HF-LT [19] (20896) | 62% | 14% | 24% | ||
| Swedish HF Registry [20] (76453) | 53% | 23% | 24% | ||
| OPTIMIZE-HF Registry [22] (41217) | 49% | 17% | 24% | ||
| GWTG-HF study [23] (99825) | 49% | 13% | 38% | ||
| G-CHF [24] (23047) | 54% | 21% | 24% | ||
| Management of Cardiac Failure program in Northern Sydney Australia (5236) [25] | 47.8% | 14.9% | 37.4% | ||
| Deliver Trial (1151) [27] | 18% | ||||
| Wehner (203135) [14] | 12% |
| Trials | Device | LVEF (%) |
|---|---|---|
| MADIT II | ICD | ≤30 |
| SCD-HeFT | ICD | ≤35 (median 25) |
| CARE HF | CRT | ≤35 (median 25) |
| Trials | Drug | LVEF (%) |
|---|---|---|
| MERIT HF | Metoprolol XL | ≤40 (mean 28 ± 7) |
| CIBIS II | Bisoprolol | ≤35 (mean 27 ± 6) |
| COPERNICUS | Carvedilol | <25 (mean 19.8 ± 4) |
| CONSENSUS | Enalapril | NYHA IV |
| SOLVD | Enalapril | ≤35 (mean 24.9) |
| Val-HeFT | Valsartan | <40 (mean 26.6 ± 7.3) |
| RALES | Spironolactone | ≤35 (mean 25.2 ± 6.8) |
| EMPHASIS-HF | Eplerenone | ≤35 (mean 26.2 ± 4.6) |
| PARADIGM-HF | Sacubitril/Valsartan | ≤40, amended to 35 (mean 29.6 ± 6.1) |
| DAPA-HF | Dapaglifozin | ≤40 (mean 31.2 ± 6.7) |
| EMPEROR-Reduced | Empaglifozin | ≤40 (mean 31.2 ± 6.7) |
| VICTORIA | Vericiguat | <45 (86% < 40) |
| GALACTIC-HF | Omecamtiv mecarbil | ≤35 (mean 26.6 ± 6.3) |
| Drug | CKD | CAD | Age > 65 Y | Age > 75 Y | NYHA III/IV | DM | Already ARNI | No ARNI | Women |
|---|---|---|---|---|---|---|---|---|---|
| Dapaglifozin | 0.72 | 0.77 | 0.72 | 0.68 | 0.90 | 0.75 | 0.75 | 0.74 | 0.79 |
| Empaglifozin | 0.83 | 0.82 | 0.78 | 0.86 | 0.83 | 0.72 | 0.64 | 0.77 | 0.59 |
| ARNI | 0.79 | 0.90 | 0.80 | 0.86 | |||||
| Placebo | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| Omecamtiv | 0.98 | 0.90 | 0.94 | 0.88 | 0.93 | 0.97 | 0.91 | 0.95 | |
| Vericiguat | 0.84 | 0.92 | 0.94 | 1.04 | 0.87 | 0.88 | 0.90 | 0.88 |
| Cluster | Young–Low Comorbidity | Ischemic | Atrial Fibrillation | Wide QRS-Device | Metabolic | Cardio-Renal |
|---|---|---|---|---|---|---|
| % | 17 | 13 | 20 | 9 | 19 | 22 |
| NYHA class (% III/IV) | 9.3 | 7.8 | 24.7 | 41.6 | 43.1 | 46.6 |
| IHD (%) | 37.7 | 82.9 | 21.7 | 70.1 | 75.2 | 64.7 |
| AF (%) | 26.5 | 15.3 | 87.4 | 85.7 | 61.3 | 74.7 |
| Hypertension (%) | 43.1 | 68.7 | 54.3 | 71.3 | 97.7 | 91.8 |
| Diabetes mellitus (%) | 14.0 | 10.0 | 3.8 | 26.8 | 84.1 | 22.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Romanò, M. Heart Failure Syndromes: Different Definitions of Different Diseases—Do We Need Separate Guidelines? A Narrative Review. J. Clin. Med. 2025, 14, 5090. https://doi.org/10.3390/jcm14145090
Romanò M. Heart Failure Syndromes: Different Definitions of Different Diseases—Do We Need Separate Guidelines? A Narrative Review. Journal of Clinical Medicine. 2025; 14(14):5090. https://doi.org/10.3390/jcm14145090
Chicago/Turabian StyleRomanò, Massimo. 2025. "Heart Failure Syndromes: Different Definitions of Different Diseases—Do We Need Separate Guidelines? A Narrative Review" Journal of Clinical Medicine 14, no. 14: 5090. https://doi.org/10.3390/jcm14145090
APA StyleRomanò, M. (2025). Heart Failure Syndromes: Different Definitions of Different Diseases—Do We Need Separate Guidelines? A Narrative Review. Journal of Clinical Medicine, 14(14), 5090. https://doi.org/10.3390/jcm14145090





