Management of Myeloproliferative Neoplasms: An Integrative Approach
Abstract
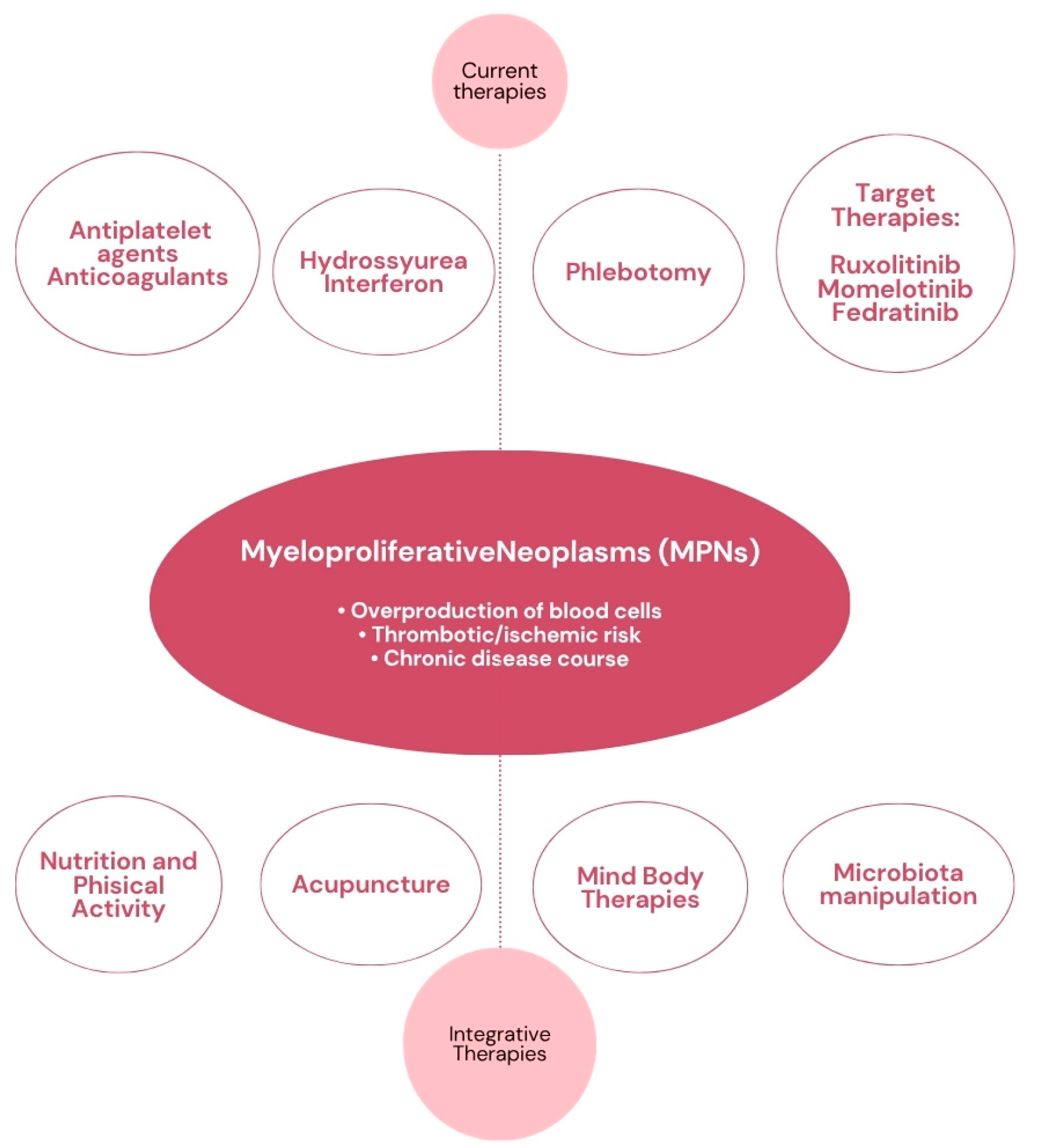
1. Introduction
2. Nutrition, Microbiota, and MPNs
2.1. Inflammation
2.2. IGF1 and Insulin
2.3. Obesity
2.4. Malnutrition
2.5. The Role of Microbiota
3. The Role of Supplements in MPNs
3.1. Curcumin
3.1.1. Preclinical Studies
3.1.2. Clinical Evidence
3.2. Vitamin D
3.2.1. Preclinical Studies
3.2.2. Clinical Evidence
3.3. Omega-3 Fatty Acids
3.3.1. Preclinical Studies
3.3.2. Clinical Evidence
3.4. N-Acetylcysteine (NAC)
3.4.1. Preclinical Studies
3.4.2. Clinical Evidence
3.5. Artemisinin
3.5.1. Preclinical Studies
3.5.2. Clinical Evidence
3.6. Vitamin C
Clinical Evidence
3.7. Quercetin
3.7.1. Preclinical Studies
3.7.2. Clinical Evidence
4. Acupuncture in MPNs
5. Physical Activity in MPNs
6. Mind–Body Therapies in MPNs
7. Other Integrative Approaches for MPNs
8. Discussion
9. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| BET | Bromodomain and Extra-Terminal Motif |
| BCL2 | B-Cell Lymphoma |
| BMI | Body Mass Index |
| CIM | Complementary and Integrative Medicine |
| CRP | C-Reactive Protein |
| DHA | Docosahexaenoic Acid |
| DIPSS | Dynamic International Prognostic Scoring System |
| EPA | Eicosapentaenoic Acid |
| Epo | Erythropoietin |
| ET | Essential Thrombocythemia |
| GLUT1 | Glucose Transporter 1 |
| IGF1 | Insulin-Like Growth Factor 1 |
| IH | Integrative Hematology |
| IL-1β | Interleukin-1β |
| IL6 | Interleukin 6 |
| IL8 | Interleukin 8 |
| IO | Integrative Oncology |
| JAK | Janus Chinasi |
| MAPK | Mitogen-Activated Protein Kinase |
| MGUS | Monoclonal Gammopathy of Undetermined Significance |
| MM | Multiple Myeloma |
| MPNs | Myeloproliferative Neoplasms |
| NAC | N-Acetyl Cysteine |
| OS | Overall Survival |
| PFKFB3 | 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase 3 |
| PFS | Progression-Free Survival |
| PMF | Primary Myelofibrosis |
| PNI | Prognostic Nutritional Index |
| PTEN | Phosphatase and Tensin Homolog |
| PV | Policytemia Vera |
| QoL | Quality of Life |
| SIMM | Survey of Integrative Medicine in Myeloproliferative Neoplasms |
| STAT | Signal Transducer and Activator of Transcription |
| TET2 | Tet Methylcytosine Dioxygenase 2 |
| TGF | Transforming Growth Factor |
| TNF α | Tumor Necrosis Factor-α |
| USDA | US Dietary Guidelines for Americans |
| VEGF | Vascular Endothelium Growth Factor |
References
- Tefferi, A.; Pardanani, A. Myeloproliferative Neoplasms: A Contemporary Review. JAMA Oncol. 2015, 1, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Arber, D.A.; Orazi, A.; Hasserjian, R.; Thiele, J.; Borowitz, M.J.; Le Beau, M.M.; Bloomfield, C.D.; Cazzola, M.; Vardiman, J.W. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood 2016, 127, 2391–2405. [Google Scholar] [CrossRef] [PubMed]
- Verstovsek, S.; Mesa, R.A.; Gotlib, J.; Levy, R.S.; Gupta, V.; DiPersio, J.F.; Catalano, J.V.; Deininger, M.; Miller, C.; Silver, R.T.; et al. A double-blind, placebo-controlled trial of ruxolitinib for myelofibrosis. N. Engl. J. Med. 2012, 366, 799–807. [Google Scholar] [CrossRef] [PubMed]
- Scherber, R.; Dueck, A.C.; Johansson, P.; Barbui, T.; Barosi, G.; Vannucchi, A.M.; Passamonti, F.; Andreasson, B.; Ferarri, M.L.; Rambaldi, A.; et al. The Myeloproliferative Neoplasm Symptom Assessment Form (MPN-SAF): International prospective validation and reliability trial. Blood 2011, 118, 401–408. [Google Scholar] [CrossRef] [PubMed]
- Mesa, R.A.; Niblack, J.; Wadleigh, M.; Verstovsek, S.; Camoriano, J.; Barnes, S.; Tan, A.D.; Atherton, P.J.; Sloan, J.A.; Tefferi, A. The burden of fatigue and quality of life in myeloproliferative disorders: An International Internet-Based Survey. Cancer 2007, 109, 68–76. [Google Scholar] [CrossRef] [PubMed]
- Geyer, H.L.; Scherber, R.M.; Dueck, A.C.; Kiladjian, J.J.; Xiao, Z.; Slot, S.; Zweegman, S.; Sackmann, F.; Fuentes, A.K.; Hernández-Maraver, D.; et al. Distinct clustering of symptom burden among myeloproliferative neoplasm patients: Retrospective assessment in 1470 patients. Blood 2014, 123, 3803–3810. [Google Scholar] [CrossRef] [PubMed]
- Mesa, R.A.; Scherber, R.M.; Geyer, H.L. Reducing symptom burden in patients with myeloproliferative neoplasms in the era of Janus kinase inhibitors. Leuk. Lymphoma 2015, 56, 1989–1999. [Google Scholar] [CrossRef] [PubMed]
- Connor, K.M.; Davidson, J.R. Development of a new resilience scale: The Connor-Davidson Resilience Scale (CD-RISC). Depress. Anxiety 2003, 18, 76–82. [Google Scholar] [CrossRef] [PubMed]
- Gowin, K.; Langlais, B.T.; Kosiorek, H.E.; Dueck, A.; Millstine, D.; Huberty, J.; Eckert, R.; Mesa, R.A. The SIMM study: Survey of integrative medicine in myeloproliferative neoplasms. Cancer Med. 2020, 9, 9445–9453. [Google Scholar] [CrossRef] [PubMed]
- Yun, H.; Sun, L.; Mao, J.J. Growth of integrative medicine at leading cancer centers between 2009 and 2016: A systematic analysis of NCI-designated comprehensive cancer center websites. J. Natl. Cancer Inst. Monogr. 2017, 2017, lgx004. [Google Scholar] [CrossRef] [PubMed]
- Witt, C.M.; Balneaves, L.G.; Cardoso, M.J.; Cohen, L.; Greenlee, H.; Johnstone, P.; Kücük, Ö.; Mailman, J.; Mao, J.J. A comprehensive definition for integrative oncology. J. Natl. Cancer Inst. Monogr. 2017, 2017, lgx012. [Google Scholar] [CrossRef] [PubMed]
- Andreazzoli, F.; Bonucci, M. Integrative Hematology: State of the Art. Int. J. Mol. Sci. 2023, 24, 1732. [Google Scholar] [CrossRef] [PubMed]
- Surapaneni, P.; Scherber, R.M. Integrative Approaches to Managing Myeloproliferative Neoplasms: The Role of Nutrition, Exercise, and Psychological Interventions. Curr. Hematol. Malig. Rep. 2019, 14, 164–170. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Peshin, S.; Larsen, A.; Gowin, K. Optimizing care: Integrative oncology in myeloproliferative neoplasm. Curr. Oncol. Rep. 2024, 26, 1135–1145. [Google Scholar] [CrossRef] [PubMed]
- WCRF Third Report. Available online: https://www.wcrf.org/wp-content/uploads/2024/11/Summary-of-Third-Expert-Report-2018.pdf (accessed on 30 April 2025).
- Shah, U.A.; Parikh, R.; Castro, F.; Bellone, M.; Lesokhin, A.M. Dietary and microbiome evidence in multiple myeloma and other plasma cell disorders. Leukemia 2023, 37, 964–980. [Google Scholar] [CrossRef] [PubMed]
- Malik, M.A.; Sweeney, N.W.; Jafri, M.; Derkach, A.; Chmielewski, C.; Adintori, P.A.; Mailankody, S.; Korde, N.; Tan, C.R.; Hassoun, H.; et al. Nutrition perceptions, needs and practices among patients with plasma cell disorders. Blood Cancer J. 2022, 12, 70. [Google Scholar] [CrossRef] [PubMed]
- Geyer, H.L.; Dueck, A.C.; Scherber, R.M.; Mesa, R.A. Impact of Inflammation on Myeloproliferative Neoplasm Symptom Development. Mediat. Inflamm. 2015, 2015, 284706. [Google Scholar] [CrossRef] [PubMed]
- Fleischman, A.G. Inflammation as a Driver of Clonal Evolution in Myeloproliferative Neoplasm. Mediat. Inflamm. 2015, 2015, 606819. [Google Scholar] [CrossRef] [PubMed]
- Longhitano, L.; Li Volti, G.; Giallongo, C.; Spampinato, M.; Barbagallo, I.; Di Rosa, M.; Romano, A.; Avola, R.; Tibullo, D.; Palumbo, G.A. The Role of Inflammation and Inflammasome in Myeloproliferative Disease. J. Clin. Med. 2020, 9, 2334. [Google Scholar] [CrossRef] [PubMed]
- Mendez Luque, L.F.; Avelar-Barragan, J.; Nguyen, H.; Nguyen, J.; Soyfer, E.M.; Liu, J.; Chen, J.H.; Mehrotra, N.; Kosiorek, H.E.; Dueck, A.; et al. The NUTRIENT Trial (NUTRitional Intervention among myEloproliferative Neoplasms): Feasibility Phase. medRxiv 2023, 2023.05.09.23289740. [Google Scholar] [CrossRef] [PubMed]
- Chen, E.C.; Johnston, H.; Patel, A.A. Targeted Therapy for MPNs: Going Beyond JAK Inhibitors. Curr. Hematol. Malig. Rep. 2023, 18, 41–55. [Google Scholar] [CrossRef] [PubMed]
- Tosti, V.; Bertozzi, B.; Fontana, L. Health Benefits of the Mediterranean Diet: Metabolic and Molecular Mechanisms. J. Gerontol. A Biol. Sci. Med. Sci. 2018, 73, 318–326. [Google Scholar] [CrossRef] [PubMed]
- Estruch, R.; Ros, E.; Salas-Salvadó, J.; Covas, M.I.; Corella, D.; Arós, F.; Gómez-Gracia, E.; Ruiz-Gutiérrez, V.; Fiol, M.; Lapetra, J.; et al. Primary Prevention of Cardiovascular Disease with a Mediterranean Diet Supplemented with Extra-Virgin Olive Oil or Nuts. N. Engl. J. Med. 2018, 378, e34. [Google Scholar] [CrossRef] [PubMed]
- Andreazzoli, F.; Fiorito, A.; Bonucci, M. ARTOI Nutritional Approach in the Hematological Patient: Is there a Rationale? J. Biomed. Res. Rev. 2019, 2, 50–55. [Google Scholar]
- Hsieh, H.H.; Yao, H.; Ma, Y.; Zhang, Y.; Xiao, X.; Stephens, H.; Wajahat, N.; Chung, S.S.; Xu, L.; Xu, J.; et al. Epo-IGF1R cross talk expands stress-specific progenitors in regenerative erythropoiesis and myeloproliferative neoplasm. Blood 2022, 140, 2371–2384. [Google Scholar] [CrossRef] [PubMed]
- Staerk, J.; Kallin, A.; Royer, Y.; Diaconu, C.C.; Dusa, A.; Demoulin, J.B.; Vainchenker, W.; Constantinescu, S.N. JAK2, the JAK2 V617F mutant and cytokine receptors. Pathol. Biol. 2007, 55, 88–91. [Google Scholar] [CrossRef] [PubMed]
- Melnik, B.C.; John, S.M.; Schmitz, G. Over-stimulation of insulin/IGF-1 signaling by Western diet may promote diseases of civilization: Lessons learnt from Laron syndrome. Nutr. Metab. 2011, 8, 41. [Google Scholar] [CrossRef] [PubMed]
- Yeh, H.C.; Maruthur, N.M.; Wang, N.Y.; Jerome, G.J.; Dalcin, A.T.; Tseng, E.; White, K.; Miller, E.R.; Juraschek, S.P.; Mueller, N.T.; et al. Effects of Behavioral Weight Loss and Metformin on IGFs in Cancer Survivors: A Randomized Trial. J. Clin. Endocrinol. Metab. 2021, 106, e4179–e4191. [Google Scholar] [CrossRef] [PubMed]
- Rahmani, J.; Montesanto, A.; Giovannucci, E.; Zand, H.; Barati, M.; Kopchick, J.J.; Mirisola, M.G.; Lagani, V.; Bawadi, H.; Vardavas, R.; et al. Association between IGF-1 levels ranges and all-cause mortality: A meta-analysis. Aging Cell 2022, 21, e13540. [Google Scholar] [CrossRef] [PubMed]
- Hosseinzadeh, Z.; Bhavsar, S.K.; Shojaiefard, M.; Saxena, A.; Merches, K.; Sopjani, M.; Alesutan, I.; Lang, F. Stimulation of the glucose carrier SGLT1 by JAK2. Biochem. Biophys. Res. Commun. 2011, 408, 208–213. [Google Scholar] [CrossRef] [PubMed]
- Rao, T.N.; Hansen, N.; Hilfiker, J.; Rai, S.; Majewska, J.M.; Leković, D.; Gezer, D.; Andina, N.; Galli, S.; Cassel, T.; et al. JAK2-mutant hematopoietic cells display metabolic alterations that can be targeted to treat myeloproliferative neoplasms. Blood 2019, 134, 1832–1846. [Google Scholar] [PubMed]
- Reddy, M.M.; Fernandes, M.S.; Deshpande, A.; Weisberg, E.; Inguilizian, H.V.; Abdel-Wahab, O.; Kung, A.L.; Levine, R.L.; Griffin, J.D.; Sattler, M. The JAK2V617F oncogene requires expression of inducible phosphofructokinase/fructose-bisphosphatase 3 for cell growth and increased metabolic activity. Leukemia 2012, 26, 481–489. [Google Scholar] [CrossRef] [PubMed]
- Mendez Luque, L.F.; Blackmon, A.L.; Ramanathan, G.; Fleischman, A.G. Key Role of Inflammation in Myeloproliferative Neoplasms: Instigator of Disease Initiation, Progression. and Symptoms. Curr. Hematol. Malig. Rep. 2019, 14, 145–153. [Google Scholar] [CrossRef] [PubMed]
- Duncombe, A.S.; Anderson, L.A.; James, G.; de Vocht, F.; Fritschi, L.; Mesa, R.; Clarke, M.; McMullin, M.F. Modifiable Lifestyle and Medical Risk Factors Associated With Myeloproliferative Neoplasms. Hemasphere 2020, 4, e327. [Google Scholar] [CrossRef] [PubMed]
- Leiba, A.; Duek, A.; Afek, A.; Derazne, E.; Leiba, M. Obesity and related risk of myeloproliferative neoplasms among israeli adolescents. Obesity 2017, 25, 1187–1190. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Ji, Y.; Kersten, S.; Qi, L. Mechanisms of Inflammatory Responses in Obese Adipose Tissue. Annu. Rev. Nutr. 2012, 32, 261–286. [Google Scholar] [CrossRef] [PubMed]
- Tefferi, A.; Vaidya, R.; Caramazza, D.; Finke, C.; Lasho, T.; Pardanani, A. Circulating interleukin (IL)-8, IL-2R, IL-12, and IL-15 levels are independently prognostic in primary myelofibrosis: A comprehensive cytokine profiling study. J. Clin. Oncol. 2011, 29, 1356–1363. [Google Scholar] [CrossRef] [PubMed]
- Christensen, S.F.; Scherber, R.M.; Brochmann, N.; Goros, M.; Gelfond, J.; Andersen, C.L.; Flachs, E.M.; Mesa, R. Body Mass Index and Total Symptom Burden in Myeloproliferative Neoplasms Discovery of a U-shaped Association. Cancers 2020, 12, 2202. [Google Scholar] [CrossRef] [PubMed]
- Moon, Y.S.; Kim, D.H.; Song, D.K. Serum tumor necrosis factor-α levels and components of the metabolic syndrome in obese adolescents. Metabolism 2004, 53, 863–867. [Google Scholar] [CrossRef] [PubMed]
- Pourcelot, E.; Trocme, C.; Mondet, J. Cytokine profiles in polycythemia vera and essential thrombocythemia patients: Clinical implications. Exp. Hematol. 2019, 42, 360–368. [Google Scholar] [CrossRef] [PubMed]
- Bowers, E.; Singer, K. Obesity-induced inflammation: The impact of the hematopoietic stem cell niche. JCI Insight 2021, 6, e145295. [Google Scholar] [CrossRef] [PubMed]
- Nam, S.Y.; Lee, E.J.; Kim, K.R.; Cha, B.S.; Song, Y.D.; Lim, S.K.; Lee, H.C.; Huh, K.B. Effect of obesity on total and free insulin-like growth factor (IGF)-1, and their relationship to IGF-binding protein (BP)-1, IGFBP-2, IGFBP-3, insulin, and growth hormone. Int. J. Obes. Relat. Metab. Disord. 1997, 21, 355–359. [Google Scholar] [CrossRef] [PubMed]
- Gallo, G.; Desideri, G.; Savoia, C. Update on Obesity and Cardiovascular Risk: From Pathophysiology to Clinical Management. Nutrients 2024, 16, 2781. [Google Scholar] [CrossRef] [PubMed]
- Mollé, N.; Krichevsky, S.; Kermani, P.; Silver, R.T.; Ritchie, E.; Scandura, J.M. Ruxolitinib can cause weight gain by blocking leptin signaling in the brain via JAK2/STAT3. Blood 2020, 135, 1062–1066. [Google Scholar] [CrossRef] [PubMed]
- Jang, A.; Hamad, H.; Yellapragada, S.V.; Sosa, I.R.; Rivero, G.A. Weight Loss Predicts Inferior Outcome in Polycythemia Vera Patients. Blood 2020, 136 (Suppl. 1), 22–23. [Google Scholar] [CrossRef]
- Tefferi, A.; Nicolosi, M.; Penna, D.; Mudireddy, M.; Szuber, N.; Lasho, T.L.; Hanson, C.A.; Ketterling, R.P.; Gangat, N.; Pardanani, A.D. Development of a prognostically relevant cachexia index in primary myelofibrosis using serum albumin and cholesterol levels. Blood Adv. 2018, 2, 1980–1984. [Google Scholar] [CrossRef] [PubMed]
- Fearon, K.C.H.; Glass, D.J.; Guttridge, D.C. Cancer cachexia: Mediators, signaling and metabolic pathways. Cell Metab. 2012, 16, 153–166. [Google Scholar] [CrossRef] [PubMed]
- Lucijanic, M.; Veletic, I.; Rahelic, D.; Pejsa, V.; Cicic, D.; Skelin, M.; Livun, A.; Tupek, K.M.; Stoos-Veic, T.; Lucijanic, T.; et al. Assessing serum albumin concentration, lymphocyte count and prognostic nutritional index might improve prognostication in patients with myelofibrosis. Wien. Klin. Wochenschr. 2018, 130, 126–133. [Google Scholar] [CrossRef] [PubMed]
- Greenfield, G.; McMullin, M.F. Ruxolitinib: Gaining more than intended. Blood 2020, 135, 983–984. [Google Scholar] [CrossRef] [PubMed]
- Oliver, A.; El Alaoui, K.; Haunschild, C.; Avelar-Barragan, J.; Mendez Luque, L.F.; Whiteson, K.; Fleischman, A.G. Fecal Microbial Community Composition in Myeloproliferative Neoplasm Patients Is Associated with an Inflammatory State. Microbiol. Spectr. 2022, 10, e0003222. [Google Scholar] [CrossRef] [PubMed]
- Elalaoui, K.; Weihe, C.; Oliver, A.; Craver, B.; Lai, H.Y.; Brooks, S.B.; Kim, D.; Martiny, J.; Whiteson, K.; Fleischman, A. Investigating the Role of the Gut Microbiome in the Inflammatory State of Myeloproliferative Neoplasms. Blood 2018, 132 (Suppl. 1), 3051. [Google Scholar] [CrossRef]
- Eickhardt-Dalbøge, C.S.; Ingham, A.C.; Andersen, L.O.; Nielsen, H.V.; Fuursted, K.; Stensvold, C.R.; Larsen, M.K.; Kjær, L.; Christensen, S.F.; Knudsen, T.A.; et al. The gut microbiota in patients with polycythemia vera is distinct from that of healthy controls and varies by treatment. Blood Adv. 2023, 7, 3326–3337. [Google Scholar] [CrossRef] [PubMed]
- Kotredes, K.P.; Thomas, B.; Gamero, A.M. The Protective Role of Type I Interferons in the Gastrointestinal Tract. Front Immunol. 2017, 8, 410. [Google Scholar] [CrossRef] [PubMed]
- Barone, M.; Barone, M.; Ricci, F.; Auteri, G.; Corradi, G.; Fabbri, F.; Papa, V.; Bandini, E.; Cenacchi, G.; Tazzari, P.L.; et al. An Abnormal Host/Microbiomes Signature of Plasma-Derived Extracellular Vesicles Is Associated to Polycythemia Vera. Front. Oncol. 2021, 11, 715217. [Google Scholar] [CrossRef] [PubMed]
- Scherber, R.M.; Langlais, B.T.; Geyer, H.; Dueck, A.; Kosoriek, H.; Johnston, C.; Padrnos, L.; Palmer, J.; Fleischman, A.G.; Mesa, R.A. Nutrition and supplement use characteristics in the myeloproliferative neoplasms: Results from the Nutrient Survey. Blood 2017, 8, 2193. [Google Scholar]
- He, Y.; Yue, Y.; Zheng, X.; Zhang, K.; Chen, S.; Du, Z. Curcumin, inflammation, and chronic diseases: How are they linked? Molecules 2015, 20, 9183–9213. [Google Scholar] [CrossRef] [PubMed]
- Giordano, A.; Tommonaro, G. Curcumin and Cancer. Nutrients 2019, 11, 2376. [Google Scholar] [CrossRef] [PubMed]
- Taverna, S.; Giallombardo, M.; Pucci, M.; Flugy, A.; Manno, M.; Raccosta, S.; Rolfo, C.; De Leo, G.; Alessandro, R. Curcumin inhibits in vitro and in vivo chronic myelogenous leukemia cells growth: A possible role for exosomal disposal of miR-21. Oncotarget 2015, 6, 21918–21933. [Google Scholar] [CrossRef] [PubMed]
- Petiti, J.; Rosso, V.; Lo Iacono, M.; Panuzzo, C.; Calabrese, C.; Signorino, E.; Pironi, L.; Cartellà, A.; Bracco, E.; Pergolizzi, B.; et al. Curcumin induces apoptosis in JAK2-mutated cells by the inhibition of JAK2/STAT and mTORC1 pathways. J. Cell. Mol. Med. 2019, 23, 4349–4357. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Zhou, N.; Zhu, Q. Curcumin inhibits proliferation and invasion of papillary thyroid carcinoma cells by inhibiting the JAK2/STAT3 pathway. J. BUON 2021, 26, 1635–1641. [Google Scholar] [PubMed]
- Duran, C. Available online: https://clinicaltrials.gov/study/NCT06063486 (accessed on 1 May 2025).
- Pardanani, A.; Drake, M.T.; Finke, C.; Lasho, T.L.; Rozell, S.A.; Jimma, T.; Tefferi, A. Vitamin D insufficiency in myeloproliferative neoplasms and myelodysplastic syndromes: Clinical correlates and prognostic studies. Am. J. Hematol. 2011, 86, 1013–1016. [Google Scholar] [CrossRef] [PubMed]
- Effat, H.; Abohashem, R.S.; Sharaky, M.; Mohammed, M.A. Vitamin D promotes anticancer effects of low-concentration cisplatin-treated non-small cell lung cancer cells via inhibiting the JAK2/STAT3 and TGF-β/SMAD4 pathways. Arch Pharm. 2025, 358, e2400933. [Google Scholar] [CrossRef] [PubMed]
- Wakahashi, K.; Minagawa, K.; Kawano, Y.; Kawano, H.; Suzuki, T.; Ishii, S.; Sada, A.; Asada, N.; Sato, M.; Kato, S.; et al. Vitamin D receptor-mediated skewed differentiation of macrophages initiates myelofibrosis and subsequent osteosclerosis. Blood 2019, 133, 1619–1629. [Google Scholar] [CrossRef] [PubMed]
- Yetgin, S.; Ozsoylu, S.; Ruacan, S.; Tekinalp, G.; Sarialioğlu, F. Vitamin D-deficiency rickets and myelofibrosis. J. Pediatr. 1989, 114, 213–217. [Google Scholar] [CrossRef] [PubMed]
- Irani, M.; Seifer, D.B.; Grazi, R.V.; Julka, N.; Bhatt, D.; Kalgi, B.; Irani, S.; Tal, O.; Lambert-Messerlian, G.; Tal, R. Vitamin D Supplementation Decreases TGF-β1 Bioavailability in PCOS: A Randomized Placebo-Controlled Trial. J. Clin. Endocrinol. Metab. 2015, 100, 4307–4314. [Google Scholar] [CrossRef] [PubMed]
- Mohammad, S.; Mishra, A.; Ashraf, M.Z. Emerging Role of Vitamin D and its Associated Molecules in Pathways Related to Pathogenesis of Thrombosis. Biomolecules 2019, 9, 649. [Google Scholar] [CrossRef] [PubMed]
- Kucukay, M.B.; Alanli, R. Vitamin D Replacement Effect on Platelet Counts. J. Coll. Physicians Surg. Pak. 2021, 31, 1064–1068. [Google Scholar] [CrossRef] [PubMed]
- Ishihara, T.; Yoshida, M.; Arita, M. Omega-3 fatty acid-derived mediators that control inflammation and tissue homeostasis. Int. Immunol. 2019, 31, 559–567. [Google Scholar] [CrossRef] [PubMed]
- Dyall, S.C.; Balas, L.; Bazan, N.G.; Brenna, J.T.; Chiang, N.; da Costa Souza, F.; Dalli, J.; Durand, T.; Galano, J.M.; Lein, P.J.; et al. Polyunsaturated fatty acids and fatty acid-derived lipid mediators: Recent advances in the understanding of their biosynthesis, structures, and functions. Prog. Lipid Res. 2022, 86, 101165. [Google Scholar] [CrossRef] [PubMed]
- Varney, M.E.; Hardman, W.E.; Sollars, V.E. Omega 3 fatty acids reduce myeloid progenitor cell frequency in the bone marrow of mice and promote progenitor cell differentiation. Lipids Health Dis. 2009, 8, 9. [Google Scholar] [CrossRef] [PubMed]
- Javaid, M.; Kadhim, K.; Bawamia, B.; Cartlidge, T.; Farag, M.; Alkhalil, M. Bleeding Risk in Patients Receiving Omega-3 Polyunsaturated Fatty Acids: A Systematic Review and Meta-Analysis of Randomized Clinical Trials. J. Am. Heart Assoc. 2024, 13, e032390. [Google Scholar] [CrossRef] [PubMed]
- Marty, C.; Lacout, C.; Droin, N.; Le Couédic, J.P.; Ribrag, V.; Solary, E.; Vainchenker, W.; Villeval, J.L.; Plo, I. A role for reactive oxygen species in JAK2 V617F myeloproliferative neoplasm progression. Leukemia 2013, 27, 2187–2195. [Google Scholar] [CrossRef] [PubMed]
- Saddadi, F.; Alatab, S.; Pasha, F.; Ganji, M.R.; Soleimanian, T. The effect of treatment with N-acetylcysteine on the serum levels of C-reactive protein and interleukin-6 in patients on hemodialysis. Saudi J. Kidney Dis. Transpl. 2014, 25, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Paterson, R.L.; Galley, H.F.; Webster, N.R. The effect of N-acetylcysteine on nuclear factor-κB activation, interleukin-6, interleukin-8, and intercellular adhesion molecule-1 expression in patients with sepsis. Crit. Care Med. 2003, 31, 2574–2578. [Google Scholar] [CrossRef] [PubMed]
- Mokra, D.; Mokry, J.; Barosova, R.; Hanusrichterova, J. Advances in the Use of N-Acetylcysteine in Chronic Respiratory Diseases. Antioxidants 2023, 12, 1713. [Google Scholar] [CrossRef] [PubMed]
- Jia, L.; Song, Q.; Zhou, C.; Li, X.; Pi, L.; Ma, X.; Li, H.; Lu, X.; Shen, Y. Dihydroartemisinin as a Putative STAT3 Inhibitor, Suppresses the Growth of Head and Neck Squamous Cell Carcinoma by Targeting Jak2/STAT3 Signaling. PLoS ONE 2016, 11, e0147157. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Xu, W.; Wang, H.; Tang, T.; Ma, J.; Cui, Z.; Shi, H.; Qin, T.; Zhou, H.; Li, L.; et al. Ferroptosis plays an essential role in the antimalarial mechanism of low-dose dihydroartemisinin. Biomed. Pharmacother. 2022, 148, 112742. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.; Yan, S.; Zhu, X.; Li, C.Y.; Liu, Z.; Xiong, J.W. Antimalarial drug artemisinin depletes erythrocytes by activating apoptotic pathways in zebrafish. Exp. Hematol. 2015, 43, 331–341.e8. [Google Scholar] [CrossRef] [PubMed]
- Dolivo, D.; Weathers, P.; Dominko, T. Artemisinin and artemisinin derivatives as anti-fibrotic therapeutics. Acta Pharm. Sin. B 2021, 11, 322–339. [Google Scholar] [CrossRef] [PubMed]
- Premnath, N.; Chung, S.S.; Weinberg, O.K.; Ikpefan, R.; Pandey, M.; Kaur, G.; Geethakumari, P.R.; Afrough, A.; Awan, F.T.; Anderson, L.D., Jr.; et al. Clinical and molecular characteristics associated with Vitamin C deficiency in myeloid malignancies; real world data from a prospective cohort. Leuk. Res. 2023, 125, 107001. [Google Scholar] [CrossRef] [PubMed]
- Xie, Z.; Fernandez, J.; Lasho, T.; Finke, C.; Amundson, M.; McCullough, K.B.; LaPlant, B.R.; Mangaonkar, A.A.; Gangat, N.; Reichard, K.K.; et al. High-dose IV ascorbic acid therapy for patients with CCUS with TET2 mutations. Blood 2024, 144, 2456–2461. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Zhu, H.; Huang, J.; Zhu, Y.; Hong, M.; Zhu, H.; Zhang, J.; Li, S.; Yang, L.; Lian, Y.; et al. The synergy of Vitamin C with decitabine activates TET2 in leukemic cells and significantly improves overall survival in elderly patients with acute myeloid leukemia. Leuk. Res. 2018, 66, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Gillberg, L.; Ørskov, A.D.; Nasif, A.; Ohtani, H.; Madaj, Z.; Hansen, J.W.; Rapin, N.; Mogensen, J.B.; Liu, M.; Dufva, I.H.; et al. Oral vitamin C supplementation to patients with myeloid cancer on azacitidine treatment: Normalization of plasma vitamin C induces epigenetic changes. Clin. Epigenetics 2019, 11, 143. [Google Scholar] [CrossRef] [PubMed]
- Tefferi, A.; Pardanani, A.; Lim, K.H.; Abdel-Wahab, O.; Lasho, T.L.; Patel, J.; Gangat, N.; Finke, C.M.; Schwager, S.; Mullally, A.; et al. TET2 mutations and their clinical correlates in polycythemia vera, essential thrombocythemia and myelofibrosis. Leukemia 2009, 23, 905–911. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, B.M.; Sanford, K.W.; Fisher, B.J.; Martin, E.J.; Contaifer, D., Jr.; Warncke, U.O.; Wijesinghe, D.S.; Chalfant, C.E.; Brophy, D.F.; Fowler Iii, A.A.; et al. Impact of high dose vitamin C on platelet function. World J. Crit. Care Med. 2017, 6, 37–47. [Google Scholar] [CrossRef] [PubMed]
- Pham, T.N.D.; Stempel, S.; Shields, M.A.; Spaulding, C.; Kumar, K.; Bentrem, D.J.; Matsangou, M.; Munshi, H.G. Quercetin Enhances the Anti-Tumor Effects of BET Inhibitors by Suppressing hnRNPA1. Int. J. Mol. Sci. 2019, 20, 4293. [Google Scholar] [CrossRef] [PubMed]
- Reyes-Farias, M.; Carrasco-Pozo, C. The Anti-Cancer Effect of Quercetin: Molecular Implications in Cancer Metabolism. Int. J. Mol. Sci. 2019, 20, 3177. [Google Scholar] [CrossRef] [PubMed]
- Aghababaei, F.; Hadidi, M. Recent Advances in Potential Health Benefits of Quercetin. Pharmaceuticals 2023, 16, 1020. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Li, Y.; Wang, W.; Wang, L.; Hu, S.; Xiao, X.; Hu, C.; Dai, Y.; Zhang, Y.; Li, Z.; et al. The construction of preclinical evidence for the treatment of liver fibrosis with quercetin: A systematic review and meta-analysis. Phytother. Res. 2022, 36, 3774–3791. [Google Scholar] [CrossRef] [PubMed]
- Nambiar, A.; Kellogg, D., III; Justice, J.; Goros, M.; Gelfond, J.; Pascual, R.; Hashmi, S.; Masternak, M.; Prata, L.; LeBrasseur, N.; et al. Senolytics dasatinib and quercetin in idiopathic pulmonary fibrosis: Results of a phase I, single-blind, single-center, randomized, placebo-controlled pilot trial on feasibility and tolerability. EbioMedicine 2023, 90, 104481. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Cheng, S.; Wang, J.; Jin, Y.; Yang, H.; Lin, Q.; Xu, S.; Hui, L.; Yin, Q.; Yang, Y.; et al. Acupuncture for the Treatment of Itch: Peripheral and Central Mechanisms. Front Neurosci. 2022, 15, 786892. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.; Hur, S.; Hwang, C.; Jang, E.; Lee, J.; Kim, Y. A Case Report on Symptom Improvement in a Polycythemia Vera Patient Treated with Acupuncture. J. Int. Korean Med. 2021, 42, 976–981. [Google Scholar] [CrossRef]
- Kumada, K.; Matsumoto-Miyazaki, J.; Okada, H.; Okura, H.; Sato, Y. Successful Administration of Kampo Medicine and Acupuncture Treatment to Improve Erythromelalgia: A Case Report. Cureus 2024, 16, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Lin, L.; Li, H.; Hu, Y.; Tian, L. Effects of acupuncture on cancer-related fatigue: A meta-analysis. Support. Care Cancer 2018, 26, 415–425. [Google Scholar] [CrossRef] [PubMed]
- Levy Yurkovski, I.; Andreazzoli, F.; Ben-Arye, E.; Attias, S.; Tadmor, T. Integrative Approaches in the Treatment of Patients Affected by Lymphoma. Curr. Oncol. Rep. 2023, 25, 1523–1534. [Google Scholar] [CrossRef] [PubMed]
- El Iskandarani, S.; Deng, G. Acupuncture in hematologic malignancies and hematopoietic cell transplantation. Blood Rev. 2022, 56, 100985. [Google Scholar] [CrossRef] [PubMed]
- Frenkel, M.; Sapire, K. Complementary and Integrative Medicine in Hematologic Malignancies: Questions and Challenges. Curr. Oncol. Rep. 2017, 19, 79. [Google Scholar] [CrossRef] [PubMed]
- Acupuncture Association of Chartered Physiotherapists (AACP). AACP Guidelines for Safe Practice of Acupuncture. 2017. Available online: https://www.aacp.org.uk/assets/ckfinder_library/files/AACP%20Safety%20Guidelines%202017%20online.pdf (accessed on 27 March 2025).
- Health Centre. Infection Risk with Acupuncture. Available online: https://www.healthcentre.org.uk/acupuncture/infection-risk-with-acupuncture.html (accessed on 27 March 2025).
- Kayo, T.; Suzuki, M.; Mitsuma, T.; Suzuki, M.; Ikeda, S.; Sukegawa, M.; Tsunoda, S.; Ohta, M. Bleeding Risk of Acupuncture for Patients with Hematological Malignancies Accompanying Thrombocytopenia: A Retrospective Chart Review. J. Integr. Complement. Med. 2024, 30, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Eckert, R.; Huberty, J.; Gowin, K.; Mesa, R.; Marks, L. Physical activity as a nonpharmacological symptom management approach in myeloproliferative neoplasms: Recommendations for future research. Integr. Cancer Ther. 2017, 16, 439–450. [Google Scholar] [CrossRef] [PubMed]
- Cormie, P.; Zopf, E.M.; Zhang, X.; Schmitz, K.H. The impact of exercise on cancer mortality, recurrence, and treatment-related adverse effects. Epidemiol. Rev. 2017, 39, 71–92. [Google Scholar] [CrossRef] [PubMed]
- Moore, S.C.; Lee, I.M.; Weiderpass, E.; Campbell, P.T.; Sampson, J.N.; Kitahara, C.M.; Keadle, S.K.; Arem, H.; De Gonzalez, A.B.; Hartge, P.; et al. Association of leisure-time physical activity with risk of 26 types of cancer in 1.44 million adults. JAMA Intern. Med. 2016, 176, 816–825. [Google Scholar] [CrossRef] [PubMed]
- Ballard-Barbash, R.; Friedenreich, C.M.; Courneya, K.S.; Siddiqi, S.M.; McTiernan, A.; Alfano, C.M. Physical activity, biomarkers, and disease outcomes in cancer survivors: A systematic review. J. Natl. Cancer Inst. 2012, 104, 815–840. [Google Scholar] [CrossRef] [PubMed]
- Shephard, R.J.; Shek, P.N. Effects of exercise and training on natural killer cell counts and cytolytic activity: A meta-analysis. Sports Med. 1999, 28, 177–195. [Google Scholar] [CrossRef] [PubMed]
- Idorn, M.; thor Straten, P. Exercise and cancer: From “healthy” to “therapeutic”? Cancer Immunol. Immunother. 2017, 66, 667–671. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, L.; Christensen, J.F.; Hojman, P. Effects of exercise on tumor physiology and metabolism. Cancer J. 2015, 21, 111–116. [Google Scholar] [CrossRef] [PubMed]
- Löf, M.; Bergström, K.; Weiderpass, E. Physical activity and biomarkers in breast cancer survivors: A systematic review. Maturitas 2012, 73, 134–142. [Google Scholar] [CrossRef] [PubMed]
- Courneya, K.S.; Segal, R.J.; Mackey, J.R.; Gelmon, K.; Reid, R.D.; Friedenreich, C.M.; Ladha, A.B.; Proulx, C.; Vallance, J.K.; Lane, K.; et al. Effects of aerobic and resistance exercise in breast cancer patients receiving adjuvant chemotherapy: A multicenter randomized controlled trial. J. Clin. Oncol. 2007, 25, 4396–4404. [Google Scholar] [CrossRef] [PubMed]
- Denham, J.; Marques, F.Z.; O’Brien, B.J.; Charchar, F.J. Exercise: Putting action into our epigenome. Sports Med. 2014, 44, 189–209. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.W.; Apostolakis, S.; Lip, G.Y. Exercise-induced changes in inflammatory processes: Implications for thrombogenesis in cardiovascular disease. Ann. Med. 2014, 46, 439–455. [Google Scholar] [CrossRef] [PubMed]
- Tolstrup Larsen, R.; Tang, L.H.; Brochmann, N.; Meulengracht Flachs, E.; Illemann Christensen, A.; Hasselbalch, H.C.; Zwisler, A.D. Associations between fatigue, physical activity, and QoL in patients with myeloproliferative neoplasms. Eur. J. Haematol. 2018, 100, 550–559. [Google Scholar] [CrossRef] [PubMed]
- Scherber, R.M.; Kosiorek, H.E.; Senyak, Z.; Dueck, A.C.; Clark, M.M.; Boxer, M.A.; Geyer, H.L.; McCallister, A.; Cotter, M.; Van Husen, B.; et al. Comprehensively understanding fatigue in patients with myeloproliferative neoplasms. Cancer 2016, 122, 477–485. [Google Scholar] [CrossRef] [PubMed]
- Gowin, K.L.; Langlais, B.T.; Millstine, D.; Kosiorek, H.E.; Huberty, J.; Eckert, R.; Mesa, R.A. Survey of Integrative Medicine in Myeloproliferative Neoplasms (The SIMM Study-2). Blood 2018, 132, 3047. [Google Scholar] [CrossRef]
- Pedersen, K.M.; Zangger, G.; Brochmann, N.; Grønfeldt, B.M.; Zwisler, A.D.; Hasselbalch, H.C.; Tang, L.H. The effectiveness of exercise-based rehabilitation to patients with myeloproliferative neoplasms—An explorative study. Eur. J. Cancer Care 2018, 27, e12865. [Google Scholar] [CrossRef] [PubMed]
- Ligibel, J.A.; Denlinger, C.S. New NCCN guidelines® for survivorship care. J. Natl. Compr. Cancer Netw. 2013, 11, 640–644. [Google Scholar] [CrossRef] [PubMed]
- Felser, S.; Rogahn, J.; Hollenbach, L.; Gruen, J.; le Coutre, P.; Al-Ali, H.K.; Schulze, S.; Muegge, L.O.; Kraze-Kliebhahn, V.; Junghanss, C. Physical exercise recommendations for patients with polycythemia vera based on preferences identified in a large international patient survey study of the East German Study Group for Hematology and Oncology (OSHO# 97). Cancer Med. 2023, 12, 18235–18245. [Google Scholar] [CrossRef] [PubMed]
- Felser, S.; Rogahn, J.; le Coutre, P.; Al-Ali, H.K.; Schulze, S.; Muegge, L.O.; Gruen, J.; Geissler, J.; Kraze-Kliebhahn, V.; Junghanss, C. Anxieties, age and motivation influence physical activity in patients with myeloproliferative neoplasms-a multicenter survey from the East German study group for hematology and oncology (OSHO# 97). Front. Oncol. 2023, 12, 1056786. [Google Scholar]
- Mind-Body Practice. Available online: https://www.cancer.gov/publications/dictionaries/cancer-terms/def/mind-body-practice (accessed on 1 May 2025).
- Deleemans, J.M.; Mather, H.; Spiropoulos, A.; Toivonen, K.; Baydoun, M.; Carlson, L.E. Recent progress in mind–body therapies in Cancer Care. Curr. Oncol. Rep. 2023, 25, 293–307. [Google Scholar] [CrossRef] [PubMed]
- Bower, J.E.; Irwin, M.R. Mind–body therapies and control of inflammatory biology: A descriptive review. Brain Behav. Immun. 2016, 51, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Carney, L.M.; Park, C.L.; Hingorany, P. The mechanisms of mindfulness-based stress reduction and mindfulness-based cognitive therapy for cancer patients and survivors: A systematic review. Psychol. Conscious. Theory Res. Pract. 2023. [Google Scholar] [CrossRef]
- Buffart, L.M.; van Uffelen, J.G.; Riphagen, I.I.; Brug, J.; van Mechelen, W.; Brown, W.J.; Chinapaw, M.J. Physical and psychosocial benefits of yoga in cancer patients and survivors, a systematic review and meta-analysis of randomized controlled trials. BMC Cancer 2012, 12, 559. [Google Scholar] [CrossRef] [PubMed]
- Huberty, J.; Eckert, R.; Dueck, A.; Kosiorek, H.; Larkey, L.; Gowin, K.; Mesa, R. Online yoga in myeloproliferative neoplasm patients: Results of a randomized pilot trial to inform future research. BMC Complement. Altern. Med. 2019, 19, 121. [Google Scholar] [CrossRef] [PubMed]
- Huberty, J.; Eckert, R.; Larkey, L.; Gowin, K.; Mitchell, J.; Mesa, R. Perceptions of myeloproliferative neoplasm patients participating in an online yoga intervention: A qualitative study. Integr. Cancer Ther. 2018, 17, 1150–1162. [Google Scholar] [CrossRef] [PubMed]
- Huberty, J.; Eckert, R.; Gowin, K.; Mitchell, J.; Dueck, A.C.; Ginos, B.F.; Larkey, L.; Mesa, R. Feasibility study of online yoga for symptom management in patients with myeloproliferative neoplasms. Haematologica 2017, 102, e384. [Google Scholar] [CrossRef] [PubMed]
- Eckert, R.; Huberty, J.; Dueck, A.; Kosiorek, H.; Larkey, L.; Mesa, R.A. A pilot study of online yoga to improve fatigue and quality of life in myeloproliferative neoplasm patients. Blood 2017, 130, 3443. [Google Scholar]
- Eckert, R.; Huberty, J.; Gowin, K.L.; Ginos, B.; Kosiorek, H.E.; Dueck, A.C.; Mesa, R.A. Impact of weight on symptom burden outcomes in myeloproliferative neoplasm patients participating in an online yoga intervention. Blood 2016, 128, 5481. [Google Scholar] [CrossRef]
- van Waart, H.; van Harten, W.H.; Buffart, L.M.; Sonke, G.S.; Stuiver, M.M.; Aaronson, N.K. Why do patients choose (not) to participate in an exercise trial during adjuvant chemotherapy for breast cancer? Psycho-Oncology 2016, 25, 964–970. [Google Scholar] [CrossRef] [PubMed]
- Kabat-Zinn, J. Living FC. Using the Wisdom of Your Body and Mind to Face Stress, Pain, and Illness; Bantam Doubleday Dell: New York, NY, USA, 1990; p. 2383411. [Google Scholar]
- Huberty, J.; Eckert, R.; Larkey, L.; Kurka, J.; De Jesús, S.A.; Yoo, W.; Mesa, R. Smartphone-based meditation for myeloproliferative neoplasm patients: Feasibility study to inform future trials. JMIR Form. Res. 2019, 3, e12662. [Google Scholar] [CrossRef] [PubMed]
- Win, H.; Russell, S.; Wertheim, B.C.; Maizes, V.; Crocker, R.; Brooks, A.J.; Mesa, R.; Huberty, J.; Geyer, H.; Eckert, R.; et al. Mobile app intervention on reducing the myeloproliferative neoplasm symptom burden: Pilot feasibility and acceptability study. JMIR Form. Res. 2022, 6, e33581. [Google Scholar] [CrossRef] [PubMed]
- Puzia, M.E.; Huberty, J.; Eckert, R.; Larkey, L.; Mesa, R. Associations between global mental health and response to an app-based meditation intervention in myeloproliferative neoplasm patients. Integr. Cancer Ther. 2020, 19, 1534735420927780. [Google Scholar] [CrossRef] [PubMed]
- Huberty, J.; Eckert, R.; Larkey, L.; Joeman, L.; Mesa, R. Experiences of using a consumer-based mobile meditation app to improve fatigue in myeloproliferative patients: Qualitative study. JMIR Cancer 2019, 5, e14292. [Google Scholar] [CrossRef] [PubMed]
- Fincham, G.W.; Mavor, K.; Dritschel, B. Effects of mindfulness meditation duration and type on well-being: An online dose-ranging randomized controlled trial. Mindfulness 2023, 14, 1171–1182. [Google Scholar] [CrossRef] [PubMed]
| Modality | Mechanism | Contraindications | Research Priorities |
|---|---|---|---|
| Disease Management | |||
| Mediterranean diet | Anti-inflammatory, ↓CV risk [23,24] | Safe | RCT: inflammation markers, BMI, QOL [5,39] |
| Vitamin D | ↓Thrombosis risk [68]; JAK/STAT interference [64] | BM fibrosis interaction [65,66,67] | Dosing studies, serum monitoring |
| Curcumin | Curcumin JAK/STAT inhibitor [57,58,59,60,61] | Anticoagulant interaction | Safety/efficacy trials, drug interactions |
| Omega-3 | Anti-inflammatory [71]; ↓hypercoagulability [71] | Safe | Optimal dosing, bleeding risk assessment |
| NAC | Antioxidant [74]; ↓ROS, NFκB [75,76] | Safe | IV vs. oral trials, dose-response |
| Artemisinin | JAK/STAT inhibitor [78,79] | Low toxicity | Phase I/II trials in MPN |
| Quercetin | JAK2/BET modulator [84,85,86] | Unknown | Safety/efficacy trials, drug interactions |
| Vitamin C | Unclear in MPN | May worsen PV [87] | Safety/efficacy trials |
| Symptom Management | |||
| Mindfulness | ↑QOL, symptom relief [133,134] | Technical limitations [135,137] | RCT: standardized protocols, QOL |
| Yoga | ↓TNF [130] | Spleen irritation [131] | Protocols for MPN |
| Physical Activity | ↓Fatigue, ↑strength [116,117] | Safe | Exercise prescription trials |
| Acupuncture | Pruritus, fatigue, pain relief [93,94,95,96,97,98,99] | Bleeding/infection risk [100,101] | RCT: inflammation markers, QOL |
| Touch/Music/Art | Symptom relief, mood, QOL | Safe | Comparative effectiveness research |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Andreazzoli, F.; Levy Yurkovski, I.; Gowin, K.; Bonucci, M. Management of Myeloproliferative Neoplasms: An Integrative Approach. J. Clin. Med. 2025, 14, 5080. https://doi.org/10.3390/jcm14145080
Andreazzoli F, Levy Yurkovski I, Gowin K, Bonucci M. Management of Myeloproliferative Neoplasms: An Integrative Approach. Journal of Clinical Medicine. 2025; 14(14):5080. https://doi.org/10.3390/jcm14145080
Chicago/Turabian StyleAndreazzoli, Francesca, Ilana Levy Yurkovski, Krisstina Gowin, and Massimo Bonucci. 2025. "Management of Myeloproliferative Neoplasms: An Integrative Approach" Journal of Clinical Medicine 14, no. 14: 5080. https://doi.org/10.3390/jcm14145080
APA StyleAndreazzoli, F., Levy Yurkovski, I., Gowin, K., & Bonucci, M. (2025). Management of Myeloproliferative Neoplasms: An Integrative Approach. Journal of Clinical Medicine, 14(14), 5080. https://doi.org/10.3390/jcm14145080






