1. Introduction
Hypopharyngeal squamous cell carcinoma (HPSCC) is a particularly aggressive form of head and neck cancer (HNC) with a poor prognosis [
1]. HPSCC is among the most challenging HNCs in terms of prognosis, with a reported 5-year overall survival (OS) rate of only approximately 30–35% [
2]. The complex anatomical structures of the hypopharynx, coupled with the typically advanced stage at diagnosis, contribute to the challenges in managing this malignancy [
3]. Curative radiotherapy, often combined with chemotherapy, is the cornerstone treatment for patients who are not candidates for surgery [
4,
5,
6]. However, predicting the treatment outcomes and overall prognosis remains a significant challenge in clinical practice.
In the search for reliable prognostic markers, systemic inflammation has been identified as a crucial factor influencing cancer progression and patient survival [
7]. Numerous inflammatory markers have been recognized as prognostic predictors in patients with cancer. Among these, inflammatory cytokines are critical, playing key roles in cancer development, prognosis, and treatment [
8]. Interleukins and tumor necrosis factors are particularly noteworthy. The prognostic significance of serum C-reactive protein (CRP) levels has been extensively researched in head and neck squamous cell carcinoma [
9,
10]. HPSCC is characterized by submucosal tumor spread that often extends beyond the macroscopically visible tumors [
11]. This infiltrative growth pattern is frequently associated with localized immune responses at the invasive front, including the accumulation of tumor-infiltrating lymphocytes. These local immune interactions may, in turn, contribute to systemic inflammation, as indicated by elevated serum CRP levels [
12]. Serum albumin level is also recognized as a marker reflecting malnutrition, impaired immune function, and chronic inflammation. It has been identified as an independent predictor of cancer-related mortality and treatment outcomes [
13,
14].
Based on these observations, the Glasgow Prognostic Score (GPS) was developed as an inflammation- and nutrition-based composite index, calculated using serum CRP and albumin levels [
15]. GPS is simple, noninvasive, and easily applicable in routine clinical settings. It has been widely validated as an independent prognostic factor in various malignancies, including colorectal and esophageal cancers [
16,
17,
18]. GPS has also been evaluated in cancers treated with definitive radiotherapy or chemoradiotherapy, showing prognostic value in esophageal squamous cell carcinoma and inoperable non-small cell lung cancer [
19,
20]. However, its prognostic role in HPSCC remains poorly defined, particularly in patients treated with curative radiotherapy. Given the high toxicity burden and treatment complexity associated with definitive radiotherapy, there is a clear need for simple and reliable tools to stratify patients by prognosis before treatment.
This retrospective study aimed to evaluate the association between pretreatment GPS and survival outcomes in patients with HPSCC treated with curative radiotherapy at a single institution. By validating the clinical utility of GPS in this setting, we seek to provide evidence to support its use as a practical prognostic marker that may help optimize treatment strategies and facilitate personalized care in HPSCC management.
2. Materials and Methods
This single-institution retrospective study was designed to evaluate the prognostic value of the GPS in patients diagnosed with HPSCC treated with curative radiotherapy. The study was approved by the Research Ethics Committee of the Faculty of Medicine of the University of Tokyo (No. 3372-7), and all patient data were anonymized to ensure confidentiality.
Patients included in this study had a pathologically confirmed diagnosis of HPSCC, were treated with curative-intent radiotherapy between June 2015 and February 2024, and had complete medical records, including pretreatment blood tests. Patients were excluded if they had previously undergone treatment for HNC, showed evidence of distant metastasis at the time of diagnosis, or had incomplete clinical or laboratory data. Data were collected from electronic medical records and included demographic information such as age and sex; clinical characteristics such as tumor stage and nodal status; treatment details such as the use of concurrent chemotherapy and upfront neck dissection; and follow-up information, including recurrence and survival status.
After completion of treatment, all patients were followed closely through a collaborative effort between our department and board-certified otolaryngologists. During the first year after treatment, follow-up visits were scheduled approximately every 1–2 months. In the second year, patients were seen every 2–3 months, and thereafter every 3–6 months based on clinical status. Each visit included a physical examination and flexible fiberoptic laryngopharyngoscopy. CT scans of the neck and chest were performed every 3–6 months to monitor for regional or distant metastases.
All patients were treated with intensity-modulated radiotherapy using tomotherapy (Accuray Inc., Sunnyvale, CA, USA), employing a three-level simultaneous integrated boost technique with curative intent. The total dose and fractionation schedule were determined to be 70 Gy in 35 fractions based on institutional protocols. All patients completed radiotherapy as planned, with a total dose of 70 Gy in 35 fractions. Computed tomography (CT) simulation for radiation therapy planning was performed with the patient immobilized using a thermoplastic mask. Gross tumor volume (GTV) included the primary tumor and enlarged lymph nodes. The high-risk clinical target volume (CTV 70 Gy) was defined as the GTV plus a 5–10 mm margin. The intermediate-risk volume (CTV 59.5 Gy) included prophylactic lymph node levels on the ipsilateral side of the involved lymph nodes, and the low-risk volume (CTV 54 Gy) included prophylactic lymph node levels on the contralateral side. These radiotherapy principles were based on international consensus recommendations regarding both primary tumor targeting and prophylactic lymph nodes [
21,
22]. The planning target volume was generated by adding a 3 mm margin to each CTV. The GPS was calculated based on pretreatment blood parameters, specifically CRP and albumin levels. A GPS of 0 was assigned when the CRP was ≤1.0 mg/dL and the albumin was ≥3.5 g/dL. A GPS of 1 was assigned when the CRP level was >1.0 mg/dL or the albumin level was <3.5 g/dL. A GPS of 2 was assigned when the CRP level was >1.0 mg/dL and the albumin level was <3.5 g/dL.
All statistical analyses were performed using R software (version 4.1.2; R Foundation for Statistical Computing, Vienna, Austria). Continuous variables are summarized as median ranges, depending on the data distribution. Categorical variables are presented as frequencies and percentages. OS was defined as the time from the start of radiotherapy for HPSCC to death from any cause or the date of the last follow-up. Progression-free survival (PFS) was defined as the time from the initiation of radiotherapy for HPSCC to the first occurrence of disease progression or death from any cause, whichever occurred first. Survival analyses were conducted using the Kaplan–Meier method, and survival curves were compared using the log-rank test. Multivariate analysis was performed using a Cox proportional hazards model to identify independent prognostic factors. The following clinical variables were included simultaneously in a multivariate model: age, sex, performance status, tumor subsite, clinical stage, chemotherapy, upfront neck dissection, and Glasgow Prognostic Score. Hazard ratios (HRs), 95% confidence intervals (CIs), and p-values were calculated. Variables were selected based on clinical relevance rather than univariate significance, to control for potential confounders. Statistical significance was defined as a p-value < 0.05.
3. Results
This study included 98 patients with histologically verified HPSCC who underwent definitive radiotherapy, retrospectively analyzed (
Table 1).
The median patient age was 68 years (range, 41–89 years). The cohort consisted of 90 men and 8 women. The Eastern Cooperative Oncology Group (ECOG) performance status was 0 in 81 patients and 1 in 17. The primary tumor was located in the pyriform sinus, posterior pharyngeal wall, and post-cricoid region in 78, 10, and 10 patients, respectively. The clinical stage was I in 16 patients, II in 34, III in 14, and IVA/IVB in 34. Fifty-two patients received chemotherapy, and eleven underwent upfront neck dissection prior to radiotherapy. Regarding chemotherapy, the majority of patients (49 out of 52) received concurrent cisplatin (CDDP) during radiotherapy using triweekly regimens. Among these, the most common protocol was a cumulative dose of 240 mg/m
2, typically administered in three cycles on days 1, 22, and 43 of radiotherapy. Three others received alternative systemic therapy: one patient was treated with weekly docetaxel, one with cetuximab, and one received induction chemotherapy with the DCF regimen (docetaxel, cisplatin, and 5-FU) prior to radiotherapy. Upfront neck dissection was performed in 11 patients as part of a planned treatment strategy, based on multidisciplinary tumor board discussions. Indications included radiological suspicion of extranodal extension or bulky nodal disease [
23]. Patients were stratified based on their GPS, with 74 patients categorized as having a GPS of 0, 18 as having a GPS of 1, and 6 as having a GPS of 2. Pretreatment liver function tests were available for 96, 97, and 86 patients for aspartate aminotransferase (AST), alanine aminotransferase (ALT), and total bilirubin (T-Bil), respectively. The median AST level was 23 U/L (range: 11–105 U/L), with 18 patients exceeding the institutional upper normal limit of 30 U/L. The median ALT level was 15 U/L (range: 4–78 U/L), with 7 patients above the normal threshold of 30 U/L. The median T-Bil level was 0.7 mg/dL (range: 0.2–1.8 mg/dL), and three patients had elevated levels above 1.5 mg/dL.
The median follow-up period was 36.2 months (range: 3.5–98.2 months). At 3 years, the OS rate was 78.7% (95% CI: 68.2–86.1) in the entire cohort, and the median OS was not reached (lower bound: 71.4 months). At 3 years, the PFS rate was 51.7% (95% CI: 40.4–61.8) in the entire cohort, with a median PFS of 40.2 months (95% CI: 23.0–NA).
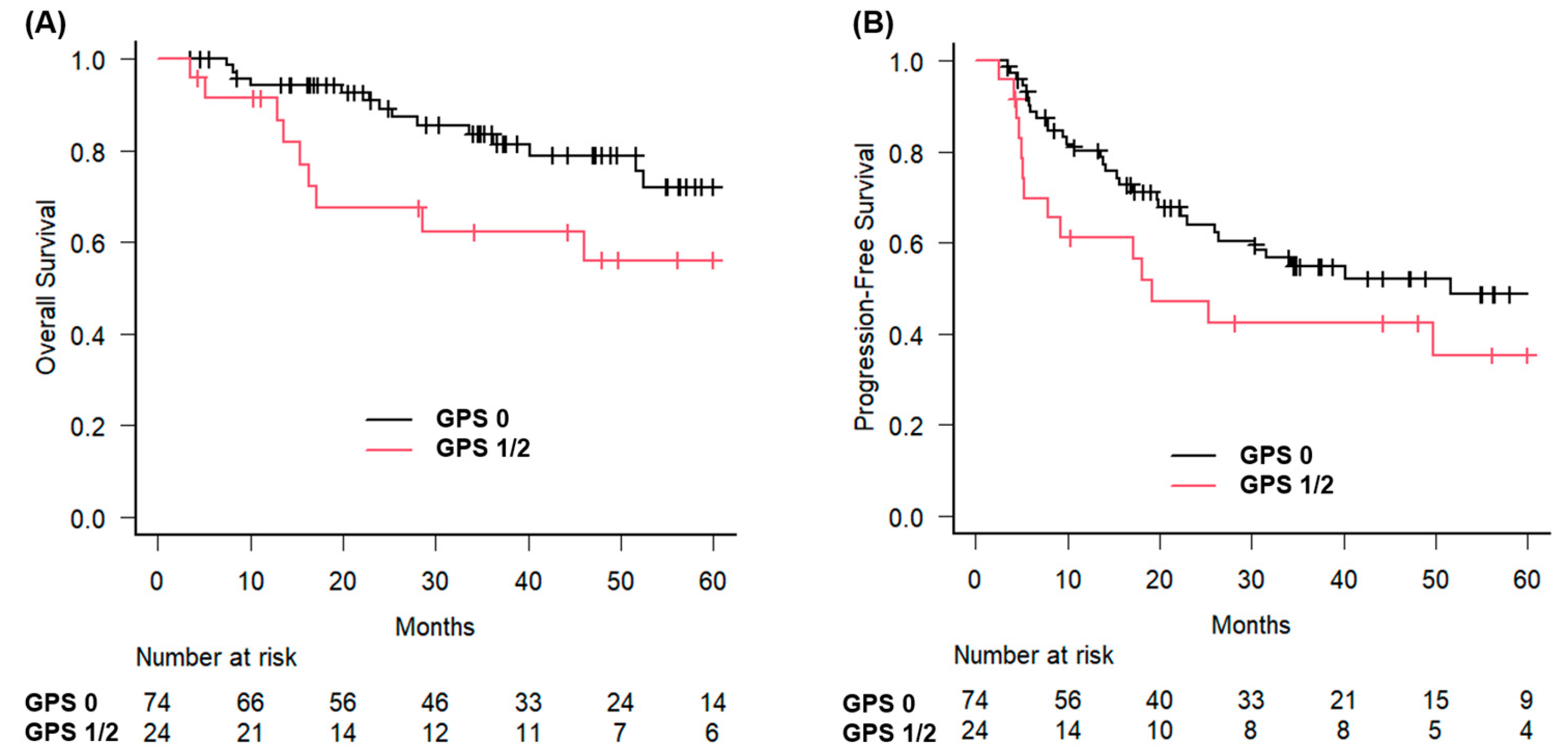
The 3-year OS rates were analyzed using the GPS. For patients with GPS 0, the 3-year OS rate was 83.6% (95% CI: 71.5–90.9%), and the median OS was not reached. In contrast, patients with GPS 1 or 2 had a 3-year OS rate of 62.2% (95% CI: 38.3–79.1%), and the median OS was 71.4 months (95% CI: 16.3 months–not estimable) (
Figure 1).
The difference in OS between the groups was statistically significant (
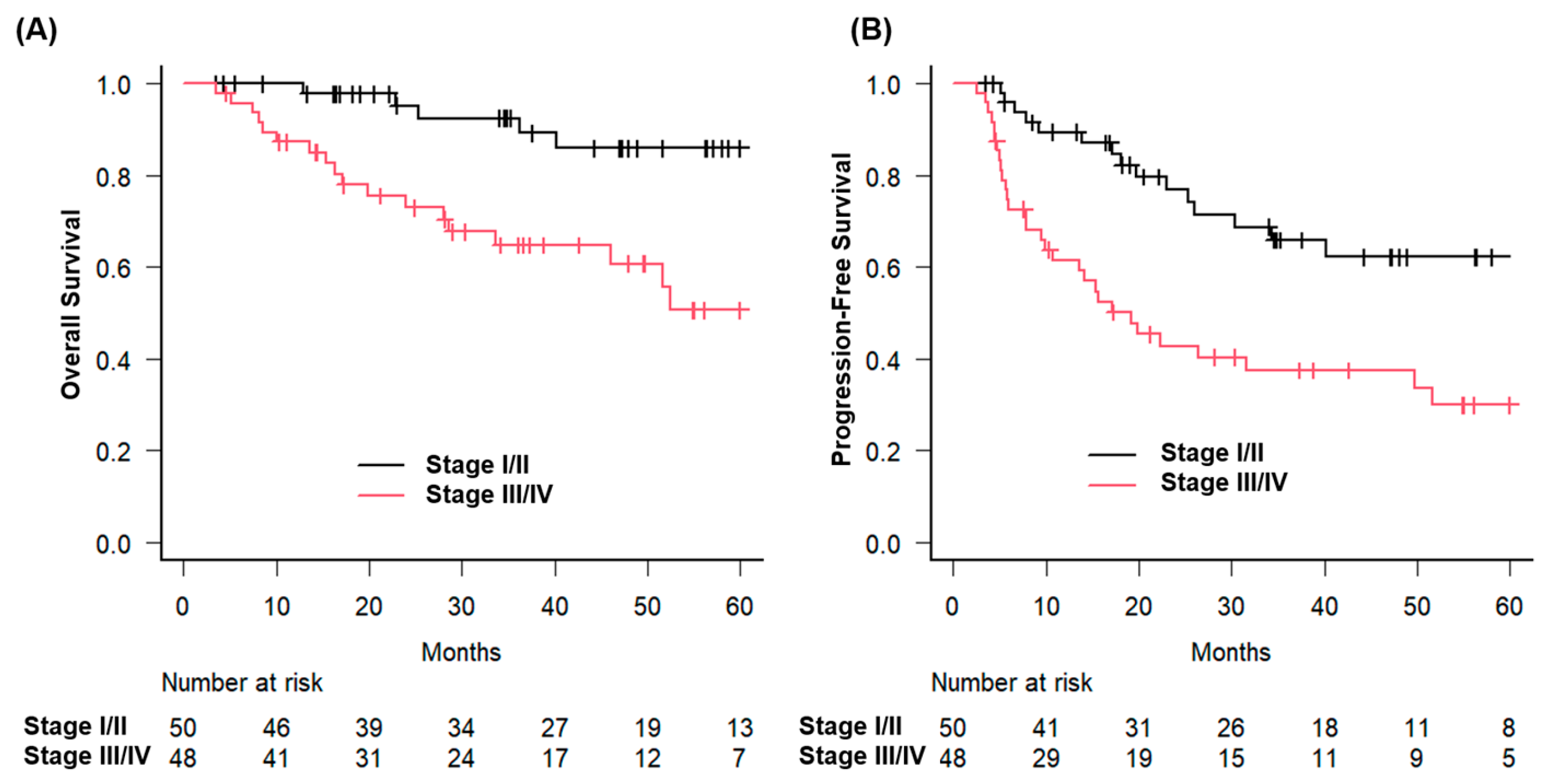
p = 0.023). Similarly, patients with early-stage disease had a 3-year OS of 92.5%, compared with 64.7% for those with advanced-stage disease (
p = 0.0021) (
Figure 2).
In addition, patients with ECOG performance status 0 had a 3-year survival rate of 85.6% compared with 47.1% for those with status 1 (
p < 0.001) (
Figure 3).
In the univariate analysis for OS, an elevated GPS (0 vs. 1 or 2) was significantly associated with worse OS, with a hazard ratio (HR) of 2.485 (95% CI: 1.103–5.600;
p = 0.028), which remained significant in the multivariate analysis (HR: 2.622; 95% CI: 1.027–6.697;
p = 0.044) (
Table 2).
Regarding other factors, univariate analysis showed that performance status (HR: 4.826, 95% CI: 2.105–11.063, p < 0.001) and clinical stage (HR: 3.863, 95% CI: 1.529–9.764, p = 0.004) were significantly associated with poorer prognosis. In multivariate analysis, performance status (HR: 6.034, 95% CI: 2.292–15.890, p < 0.001), clinical stage (HR: 21.840, 95% CI: 4.879–97.810, p < 0.001), and chemotherapy (HR: 0.091, 95% CI: 0.020–0.411, p = 0.002) remained independently associated with prognosis.
In the Cox proportional hazards analysis for PFS, GPS (0 vs. 1 or 2) was not statistically significant in univariate (HR: 1.687; 95% CI: 0.912–3.121;
p = 0.096) or multivariate analysis (HR, 1.415; 95% CI, 0.718–2.787;
p = 0.316), although it showed a trend toward a worse prognosis (
Table 3).
Clinical stage was an independent predictor of PFS in univariate (HR: 2.525, p = 0.002) and multivariate analyses (HR: 4.627, p = 0.001). Chemotherapy demonstrated a significant protective effect in the multivariate analysis (HR: 0.330; 95% CI: 0.126–0.860; p = 0.023) despite being non-significant in univariate analysis.
4. Discussion
This study evaluated the prognostic value of the GPS in patients with HPSCC treated with curative radiotherapy. Our findings suggest that the GPS provides additional prognostic information that complements conventional clinical factors. Patients with a higher GPS (1 or 2) had a significantly worse OS than those with a GPS of 0. These findings are consistent with those of previous studies across various cancers, where an elevated GPS was associated with poor prognosis, reflecting the influence of systemic inflammation and malnutrition. The GPS is based on a simple combination of CRP and albumin levels, serving as a marker of systemic inflammation and nutritional status, respectively. In recent years, revised versions of the GPS have been proposed. McMillan reported that the modified Glasgow Prognostic Score (mGPS) has been validated as an independent prognostic indicator across various cancers in over 60 studies [
15]. Moreover, the high-sensitivity modified Glasgow Prognostic Score (HS-mGPS) has been proposed to provide a superior prognostic value compared with the mGPS [
24].
In our cohort, 47% of patients did not receive concurrent chemotherapy. This was largely due to the inclusion of early-stage cases (Stages I–II), for whom radiotherapy alone is often considered appropriate. In addition, chemotherapy was omitted in some patients due to age, comorbidities, or patient preference. Although all patients had good performance status, treatment decisions were made based on a comprehensive clinical assessment, including factors not fully captured by PS alone.
Recent studies have demonstrated that the GPS and its modifications, such as the mGPS and HS-mGPS, can serve as significant prognostic indicators for radiotherapy. In patients with muscle-invasive bladder cancer undergoing radiotherapy, pretreatment mGPS has been shown to predict OS, with 2-year OS rates of 85.1% for mGPS 0, 71.3% for mGPS 1, and 60.9% for mGPS 2 (
p = 0.003) [
25]. In a retrospective study involving 207 patients with non-small cell lung cancer treated with stereotactic body radiation therapy, the mGPS was found to be an independent predictor of prognosis [
26]. Patients with a high mGPS (1–2) exhibited significantly worse outcomes, including lower OS (median OS: 33.3 vs. 64.5 months;
p = 0.003) and PFS (median PFS: 23.8 vs. 39 months;
p = 0.008). Ishikawa et al. reported that in a cohort of 180 patients undergoing whole-brain radiotherapy, a pretreatment GPS ≥ 1 was significantly associated with worse survival outcomes [
27] (median survival time: 6.1 months), even after adjusting for established prognostic factors such as Karnofsky performance status and Graded Prognostic Assessment.
HPSCC, along with other head and neck cancers (HNC), is associated with a high risk of malnutrition and systemic inflammation due to tumor location, treatment-related toxicities, and impaired oral intake [
28,
29,
30]. Nutritional status can significantly affect treatment tolerance and clinical outcomes. The United Kingdom National Multidisciplinary Guidelines emphasize the importance of nutritional support in managing HNC, noting that many patients are already malnourished at diagnosis and most require nutritional intervention during treatment [
31]. At our institution, structured nutritional support is routinely considered, particularly for patients receiving concurrent chemoradiotherapy. As a general policy, prophylactic percutaneous endoscopic gastrostomy PEG placement is performed before the initiation of chemoradiotherapy. However, due to the retrospective design of our study, detailed data on the proportion of patients who received PEG or other forms of enteral nutrition were not consistently available. In this context, the pretreatment GPS may serve as a practical surrogate indicator reflecting the underlying nutritional and inflammatory status in patients with HPSCC.
Our findings demonstrated that a higher GPS, based on pretreatment CRP and albumin levels, was significantly associated with poorer survival in patients with HPSCC. While GPS is a practical and integrative biomarker reflecting both systemic inflammation and nutritional status, other indices such as the Prognostic Nutritional Index (PNI) and the neutrophil-to-lymphocyte ratio (NLR) have also been shown to predict outcomes in HNC. For example, a meta-analysis by Luan et al. confirmed that a low PNI was significantly associated with worse OS, and PFS, and elevated NLR has similarly been linked to inferior survival in both general HNC populations and hypopharyngeal cancer specifically [
32]. Justesen et al. performed a retrospective cohort study of 1370 patients with oropharyngeal squamous cell carcinoma and found that higher pretreatment NLR was independently associated with worse OS and recurrence-free survival [
33]. In Kuo et al.’s retrospective study of 120 patients with hypopharyngeal cancer treated with definitive chemoradiotherapy, high pretreatment NLR (≥4) was significantly associated with advanced tumor stage, poor treatment response, and worse PFS and OS [
34]. These findings suggest that multiple pretreatment biomarkers may have complementary prognostic value, and further studies are needed to compare their relative and combined utility.
While the present study focused on pretreatment risk stratification, longitudinal nutritional management throughout the course of treatment may also play a critical role in improving patient outcomes. Previous studies have demonstrated that structured nutritional interventions, such as medical nutrition therapy and individualized counseling, can reduce weight loss, preserve body composition, and enhance both treatment tolerance and quality of life [
35,
36]. Notably, Molnár et al. reported that extended durations of nutritional support were associated with significantly improved overall survival [
37]. These findings highlight the importance of not only assessing pretreatment nutritional status but also providing continuous, longitudinal nutritional care as an integral part of comprehensive cancer management.
This study had several limitations. This retrospective design introduced potential biases and restricted our ability to establish causality. Additionally, as this was a single-institution study, the generalizability of our findings may be limited. The sample size, which was sufficient to detect significant associations, may have affected the broader applicability of our results. Furthermore, we were unable to assess patients’ nutritional status or sarcopenia using standardized tools such as NRS-2002 and SARC-F, as these assessments were not routinely performed in our cohort. Future multicenter studies with larger cohorts are necessary to validate our findings, assess the generalizability of the GPS in predicting HPSCC outcomes, and incorporate validated screening tools to enhance the evaluation of patient-related prognostic factors.
5. Conclusions
Our study supports the use of the GPS as a promising prognostic tool for patients with HPSCC who are undergoing curative radiotherapy. Higher GPS values are associated with worse overall outcomes, independent of traditional clinical factors. The simplicity and cost-effectiveness of the GPS make it a practical addition to clinical practice, aiding in risk stratification and treatment planning. Further research is needed to validate these results and explore the potential integration of the GPS into clinical practice guidelines for HPSCC.
Author Contributions
Conceptualization, A.K. and H.Y.; methodology, A.K., M.M. and Y.S.; software, S.S.; validation, A.K., S.S. and K.K.; formal analysis, A.K.; investigation, Y.K., M.M., K.Y. and K.K.; resources, H.Y.; data curation, S.S. and Y.K.; writing—original draft preparation, A.K. and S.S.; writing—review and editing, Y.S., K.Y. and H.Y.; visualization, S.S.; supervision, H.Y. and Y.S.; project administration, A.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Ethical approval was obtained from the Research Ethics Committee of the Graduate School of Medicine and Faculty of Medicine, University of Tokyo (approval number: 3372-7 and approval date 14 September 2023), and the study was performed in compliance with the Declaration of Helsinki.
Informed Consent Statement
The requirement for informed consent was waived due to the retrospective nature of the study.
Data Availability Statement
Data supporting the findings of this study are available from the corresponding author on reasonable request.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Owens, D.; Paleri, V.; Jones, A.V. Head and neck cancer explained: An overview of management pathways. Br. Dent. J. 2022, 233, 721–725. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Garneau, J.C.; Bakst, R.L.; Miles, B.A. Hypopharyngeal cancer: A state of the art review. Oral Oncol. 2018, 86, 244–250. [Google Scholar] [CrossRef] [PubMed]
- Mura, F.; Bertino, G.; Occhini, A.; Benazzo, M. Surgical treatment of hypopharyngeal cancer: A review of the literature and proposal for a decisional flow-chart. Acta Otorhinolaryngol. Ital. 2013, 33, 299–306. [Google Scholar] [PubMed] [PubMed Central]
- Katano, A.; Yamashita, H. Early-stage hypopharyngeal squamous cell carcinoma treated with radical radiotherapy at a uniform dose of 70 Gy in 35 fractions: A single-center study. Eur. Arch. Otorhinolaryngol. 2024, 281, 4401–4407. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Fang, S.; Ye, L.; Cai, W.; Xiang, W.; Qi, Y.; Wu, H.; Yang, C.; Zhang, R.; Liu, Y.; et al. Optimal treatment strategy and prognostic analysis for hypopharyngeal squamous-cell carcinoma patients with T3-T4 or node-positive: A population-based study. Eur. J. Surg. Oncol. 2023, 49, 1162–1170. [Google Scholar] [CrossRef] [PubMed]
- Prabhash, K.; Babu, G.; Chaturvedi, P.; Kuriakose, M.; Birur, P.; Anand, A.K.; Kaushal, A.; Mahajan, A.; Syiemlieh, J.; Singhal, M.; et al. Indian clinical practice consensus guidelines for the management of very advanced disease of squamous cell carcinoma of head and neck. Indian J. Cancer 2020, 57, S22–S25. [Google Scholar] [CrossRef] [PubMed]
- Zheng, K.; Liu, X.; Ji, W.; Lu, J.; Cui, J.; Li, W. The Efficacy of Different Inflammatory Markers for the Prognosis of Patients with Malignant Tumors. J. Inflamm. Res. 2021, 14, 5769–5785. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lee, H.M.; Lee, H.J.; Chang, J.E. Inflammatory Cytokine: An Attractive Target for Cancer Treatment. Biomedicines 2022, 10, 2116. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chen, Y.; Cong, R.; Ji, C.; Ruan, W. The prognostic role of C-reactive protein in patients with head and neck squamous cell carcinoma: A meta-analysis. Cancer Med. 2020, 9, 9541–9553. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Katano, A.; Takahashi, W.; Yamashita, H.; Yamamoto, K.; Ando, M.; Yoshida, M.; Saito, Y.; Abe, O.; Nakagawa, K. The impact of elevated C-reactive protein level on the prognosis for oro-hypopharynx cancer patients treated with radiotherapy. Sci. Rep. 2017, 7, 17805. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ho, C.M.; Ng, W.F.; Lam, K.H.; Wei, W.J.; Yuen, A.P. Submucosal tumor extension in hypopharyngeal cancer. Arch. Otolaryngol. Head Neck Surg. 1997, 123, 959–965. [Google Scholar] [CrossRef] [PubMed]
- Aguilar-Cazares, D.; Chavez-Dominguez, R.; Marroquin-Muciño, M.; Perez-Medina, M.; Benito-Lopez, J.J.; Camarena, A.; Rumbo-Nava, U.; Lopez-Gonzalez, J.S. The systemic-level repercussions of cancer-associated inflammation mediators produced in the tumor microenvironment. Front. Endocrinol. 2022, 13, 929572. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tang, Q.; Li, X.; Sun, C.R. Predictive value of serum albumin levels on cancer survival: A prospective cohort study. Front. Oncol. 2024, 14, 1323192. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Suzuki, S.; Taguchi, Y.; Kitabayashi, T.; Sato, N.; Kaya, H.; Abe, T.; Endo, T.; Suzuki, H.; Kawasaki, Y.; Yamada, T. Serum Albumin as an Independent Predictor of Long-Term Survival in Patients with Recurrent and Metastatic Head and Neck Squamous Cell Carcinoma Treated with Nivolumab. J. Clin. Med. 2024, 13, 2456. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- McMillan, D.C. The systemic inflammation-based Glasgow Prognostic Score: A decade of experience in patients with cancer. Cancer Treat. Rev. 2013, 39, 534–540. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.F.; Zhao, Q.; Chen, Q.X. Prognostic significance of Glasgow prognostic score in patients undergoing esophagectomy for esophageal squamous cell carcinoma. Saudi J. Gastroenterol. 2014, 20, 48–53. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Nozoe, T.; Matono, R.; Ijichi, H.; Ohga, T.; Ezaki, T. Glasgow Prognostic Score (GPS) can be a useful indicator to determine prognosis of patients with colorectal carcinoma. Int. Surg. 2014, 99, 512–517. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Aoyama, T.; Yukawa, N.; Saito, A. Clinical Impact of Nutrition and Inflammation Assessment Tools in Colorectal Cancer Treatment. Anticancer. Res. 2024, 44, 1335–1351. [Google Scholar] [CrossRef] [PubMed]
- Forrest, L.M.; McMillan, D.C.; McArdle, C.S.; Angerson, W.J.; Dagg, K.; Scott, H.R. A prospective longitudinal study of performance status, an inflammation-based score (GPS) and survival in patients with inoperable non-small-cell lung cancer. Br. J. Cancer 2005, 92, 1834–1836. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kimura, J.; Kunisaki, C.; Makino, H.; Oshima, T.; Ota, M.; Oba, M.; Takagawa, R.; Kosaka, T.; Ono, H.A.; Akiyama, H.; et al. Evaluation of the Glasgow Prognostic Score in patients receiving chemoradiotherapy for stage III and IV esophageal cancer. Dis. Esophagus 2016, 29, 1071–1080. [Google Scholar] [CrossRef] [PubMed]
- Grégoire, V.; Ang, K.; Budach, W.; Grau, C.; Hamoir, M.; Langendijk, J.A.; Lee, A.; Le, Q.T.; Maingon, P.; Nutting, C.; et al. Delineation of the neck node levels for head and neck tumors: A 2013 update. DAHANCA, EORTC, HKNPCSG, NCIC CTG, NCRI, RTOG, TROG consensus guidelines. Radiother. Oncol. 2014, 110, 172–181. [Google Scholar] [CrossRef] [PubMed]
- Grégoire, V.; Evans, M.; Le, Q.T.; Bourhis, J.; Budach, V.; Chen, A.; Eisbruch, A.; Feng, M.; Giralt, J.; Gupta, T.; et al. Delineation of the primary tumour Clinical Target Volumes (CTV-P) in laryngeal, hypopharyngeal, oropharyngeal and oral cavity squamous cell carcinoma: AIRO, CACA, DAHANCA, EORTC, GEORCC, GORTEC, HKNPCSG, HNCIG, IAG-KHT, LPRHHT, NCIC CTG, NCRI, NRG Oncology, PHNS, SBRT, SOMERA, SRO, SSHNO, TROG consensus guidelines. Radiother. Oncol. 2018, 126, 3–24. [Google Scholar] [CrossRef] [PubMed]
- Katano, A.; Yamashita, H.; Saito, Y.; Kobayashi, K. The role of upfront neck dissection in definitive radiotherapy for locally advanced hypopharyngeal squamous cell carcinoma: A single-center retrospective analysis. Head Neck 2024, 46, 2815–2823. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.H.; Tsai, Y.T.; Chen, K.Y.; Yap, W.K.; Luan, C.W. Utility of High-Sensitivity Modified Glasgow Prognostic Score in Cancer Prognosis: A Systemic Review and Meta-Analysis. Int. J. Mol. Sci. 2023, 24, 1318. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kikuchi, K.; Nakamura, R.; Segawa, T.; Oikawa, H.; Ariga, H. Modified Glasgow prognostic score can predict survival of muscle invasive bladder cancer patients after radiotherapy. J. Radiat. Res. 2020, 61, 616–621. [Google Scholar] [CrossRef] [PubMed Central]
- Chen, Z.; Nonaka, H.; Onishi, H.; Nakatani, E.; Sato, Y.; Funayama, S.; Watanabe, H.; Komiyama, T.; Kuriyama, K.; Marino, K.; et al. Modified Glasgow Prognostic Score is predictive of prognosis for non-small cell lung cancer patients treated with stereotactic body radiation therapy: A retrospective study. J. Radiat. Res. 2021, 62, 457–464. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ishikawa, Y.; Umezawa, R.; Yamamoto, T.; Takahashi, N.; Takeda, K.; Suzuki, Y.; Kishida, K.; Omata, S.; Teramura, S.; Ito, K.; et al. Glasgow prognostic score for assessing the efficacy of whole-brain radiation therapy in cases of recursive partitioning analysis class 2 and class 3 multiple brain metastases: A retrospective study. Acta Neurol. Belg. 2024, 124, 231–239. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wallmander, C.; Bosaeus, I.; Silander, E.; Berg, M.; Cange, H.H.; Nyman, J.; Hammerlid, E. Malnutrition in patients with advanced head and neck cancer: Exploring the Global Leadership Initiative on Malnutrition (GLIM) criteria, energy balance and health-related quality of life. Clin. Nutr. ESPEN 2025, 66, 332–342. [Google Scholar] [CrossRef] [PubMed]
- Arihara, Y.; Takada, K.; Murase, K.; Kawamura, K.; Kakiuchi, A.; Kurose, M.; Sasaki, T.; Ogi, K.; Yamazaki, M.; Miyazaki, A.; et al. Inflammation and malnutrition as markers of poor outcomes in head and neck cancer patients treated with nivolumab. Acta Otolaryngol. 2023, 143, 714–720. [Google Scholar] [CrossRef] [PubMed]
- Kapała, A.; Różycka, K.; Grochowska, E.; Gazi, A.; Motacka, E.; Folwarski, M. Cancer, malnutrition and inflammatory biomarkers. Why do some cancer patients lose more weight than others? Contemp. Oncol. 2025, 29, 45–54. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Talwar, B.; Donnelly, R.; Skelly, R.; Donaldson, M. Nutritional management in head and neck cancer: United Kingdom National Multidisciplinary Guidelines. J. Laryngol. Otol. 2016, 130, S32–S40. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Luan, C.W.; Tsai, Y.T.; Yang, H.Y.; Chen, K.Y.; Chen, P.H.; Chou, H.H. Pretreatment prognostic nutritional index as a prognostic marker in head and neck cancer: A systematic review and meta-analysis. Sci. Rep. 2021, 11, 17117. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Justesen, M.M.; Jakobsen, K.K.; Bendtsen, S.K.; Garset-Zamani, M.; Mordhorst, C.; Carlander, A.F.; Gothelf, A.B.; Grønhøj, C.; von Buchwald, C. Pretreatment Neutrophil-to-Lymphocyte Ratio as a Prognostic Marker for the Outcome of HPV-Positive and HPV-Negative Oropharyngeal Squamous Cell Carcinoma. Viruses 2023, 15, 198. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kuo, C.; Hsueh, W.T.; Wu, Y.H.; Yang, M.W.; Cheng, Y.J.; Pao, T.H.; Tsai, M.H. The Role of Pretreatment Serum Neutrophil-to-Lymphocyte Ratio in Hypopharyngeal Cancer Treated with Definitive Chemoradiotherapy: A Pilot Study. Sci. Rep. 2019, 9, 1618. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Leis, C.; Arthur, A.E.; Chen, X.; Greene, M.W.; Frugé, A.D. Systematic Review of Nutrition Interventions to Improve Short Term Outcomes in Head and Neck Cancer Patients. Cancers 2023, 15, 822. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cardellini, S.; Deantoni, C.L.; Paccagnella, M.; Casirati, A.; Pontara, A.; Marinosci, A.; Tresoldi, M.; Giordano, L.; Chiara, A.; Dell’Oca, I.; et al. The impact of nutritional intervention on quality of life and outcomes in patients with head and neck cancers undergoing chemoradiation. Front. Oncol. 2024, 14, 1475930. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Molnár, A.; Pálfi, E.; Belák, B.; Blasszauer, C.; Reibl, D.; Lövey, J. Positive correlation between persistence of medical nutrition therapy and overall survival in patients with head and neck cancer. Pathol. Oncol. Res. 2024, 30, 1611664. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
| Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).