Prognostic Value of Pulmonary Hypertension as an Incidental Finding Detected by Echocardiography in Patients Without Known Cardiovascular or Pulmonary Diseases
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Echocardiography Study
2.3. Data Collection
2.4. Statistical Analysis
3. Results
3.1. Study Population
3.2. Echocardiograms
3.3. Survival Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- D’ALto, M.; Bossone, E.; Opotowsky, A.R.; Ghio, S.; Rudski, L.G.; Naeije, R. Strengths and weaknesses of echocardiography for the diagnosis of pulmonary hypertension. Int. J. Cardiol. 2018, 263, 177–183. [Google Scholar] [CrossRef] [PubMed]
- Hoeper, M.M.; Ghofrani, H.-A.; Grünig, E.; Klose, H.; Olschewski, H.; Rosenkranz, S. Pulmonary Hypertension. Dtsch. Ärzteblatt. Int. 2017, 114, 73–84. [Google Scholar] [CrossRef]
- Galiè, N.; Humbert, M.; Vachiery, J.L.; Gibbs, S.; Lang, I.; Torbicki, A.; Hoeper, M. 2015 ESC/ERS guidelines for the diagnosis and treatment of pulmonary hypertension: The Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS). Eur. Heart J. 2016, 37, 67–119. [Google Scholar] [CrossRef] [PubMed]
- Gaggin, H.K.; Januzzi, J.L., Jr. MGH Cardiology Board Review, 2nd ed.; Springer: Berlin/Heidelberg, Germany, 2011. [Google Scholar]
- Harris, H.; Bockhold, C. Improving outcomes for pulmonary hypertension. Nurs Crit Care. 2014, 9, 32–40. [Google Scholar] [CrossRef]
- Augustine, D.X.; Coates-Bradshaw, L.D.; Willis, J.; Harkness, A.; Ring, L.; Grapsa, J.; Coghlan, G.; Kaye, N.; Oxborough, D.; Robinson, S.; et al. Echocardiographic assessment of pulmonary hypertension: A guideline protocol from the British Society of Echocardiography. Echo Res. Pract. 2018, 5, G11–G24. [Google Scholar] [CrossRef] [PubMed]
- McKenna, S.P.; Doughty, N.; Meads, D.M.; Doward, L.C.; Pepke-Zaba, J. The Cambridge Pulmonary Hypertension Outcome Review (CAMPHOR): A Measure of Health-Related Quality of Life and Quality of Life for Patients with Pulmonary Hypertension. Qual. Life Res. 2006, 15, 103–115. [Google Scholar] [CrossRef] [PubMed]
- Hoeper, M.M.; Kramer, T.; Pan, Z.; Eichstaedt, C.A.; Spiesshoefer, J.; Benjamin, N.; Olsson, K.M.; Meyer, K.; Vizza, C.D.; Vonk-Noordegraaf, A.; et al. Mortality in pulmonary arterial hypertension: Prediction by the 2015 European pulmonary hypertension guidelines risk stratification model. Eur. Respir. J. 2017, 50, 1700740. [Google Scholar] [CrossRef] [PubMed]
- Moreira, E.M.; Gall, H.; Leening, M.J.G.; Lahousse, L.; Loth, D.W.; Krijthe, B.P.; Jong, J.C.K.-D.; Brusselle, G.G.; Hofman, A.; Stricker, B.H.; et al. Prevalence of Pulmonary Hypertension in the General Population: The Rotterdam Study. PLoS ONE 2015, 10, e0130072. [Google Scholar] [CrossRef] [PubMed]
- Corris, P.A.; Seeger, W. Call it by the correct name—Pulmonary hypertension not pulmonary arterial hypertension: Growing recognition of the global health impact for a well-recognized condition and the role of the Pulmonary Vascular Research Institute. Am. J. Physiol. Cell. Mol. Physiol. 2020, 318, L992–L994. [Google Scholar] [CrossRef] [PubMed]
- Hoeper, M.M.; Humbert, M.; Souza, R.; Idrees, M.; Kawut, S.M.; Sliwa-Hahnle, K.; Jing, Z.-C.; Gibbs, J.S.R. A global view of pulmonary hypertension. Lancet Respir. Med. 2016, 4, 306–322. [Google Scholar] [CrossRef] [PubMed]
- Frost, A.; Badesch, D.; Gibbs, J.S.R.; Gopalan, D.; Khanna, D.; Manes, A.; Oudiz, R.; Satoh, T.; Torres, F.; Torbicki, A. Diagnosis of pulmonary hypertension. Eur. Respir. J. 2018, 53, 1801904. [Google Scholar] [CrossRef] [PubMed]
- Langridge, J. The ECHO suggests pulmonary hypertension: What next? In Proceedings of the Cardiovascular Respiratory Conference, 2016; Conference paper.
- D’alto, M.; Di Marco, G.M.; D’andrea, A.; Argiento, P.; Romeo, E.; Ferrara, F.; Lamia, B.; Ghio, S.; Rudski, L.G. Invasive and Noninvasive Evaluation for the Diagnosis of Pulmonary Hypertension: How to Use and How to Combine Them Pulmonary hypertension Heart catheterization Echocardiography Invasive Noninvasive. Heart Fail. Clin. 2018, 14, 353–360. [Google Scholar] [CrossRef] [PubMed]
- Anonymous. CardioPulse Articles. Eur. Hear. J. 2016, 37, 4–11. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Opotowsky, A.R.; Ojeda, J.; Rogers, F.; Prasanna, V.; Clair, M.; Moko, L.; Vaidya, A.; Afilalo, J.; Forfia, P.R. A Simple Echocardiographic Prediction Rule for Hemodynamics in Pulmonary Hypertension. Circ. Cardiovasc. Imaging 2012, 5, 765–775. [Google Scholar] [CrossRef] [PubMed]
- Huston, J.H.; Maron, B.A.; French, J.; Huang, S.; Thayer, T.; Farber-Eger, E.H.; Wells, Q.S.; Choudhary, G.; Hemnes, A.R.; Brittain, E.L. Association of Mild Echocardiographic Pulmonary Hypertension With Mortality and Right Ventricular Function. JAMA Cardiol. 2019, 4, 1112–1121. [Google Scholar] [CrossRef] [PubMed]
- Stewart, S.; Chan, Y.-K.; Playford, D.; Harris, S.; Strange, G.A. Incident pulmonary hypertension in 13 488 cases investigated with repeat echocardiography: A clinical cohort study. ERJ Open Res. 2023, 9, 00082-2023. [Google Scholar] [CrossRef] [PubMed]
- Strange, G.; Playford, D.; Stewart, S.; Deague, J.A.; Nelson, H.; Kent, A.; Gabbay, E. Pulmonary hypertension: Prevalence and mortality in the Armadale echocardiography cohort. Heart 2012, 98, 1805–1811. [Google Scholar] [CrossRef] [PubMed]
- Trammell, A.W.; Shah, A.J.; Phillips, L.S.; Hart, C.M. Mortality in US veterans with pulmonary hypertension: A retrospective analysis of survival by subtype and baseline factors. Pulm. Circ. 2019, 9, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Lang, I.M.; Palazzini, M. The burden of comorbidities in pulmonary arterial hypertension. Eur. Hear. J. Suppl. 2019, 21, K21–K28. [Google Scholar] [CrossRef] [PubMed]
- Butrous, G. Pulmonary hypertension: From an orphan disease to a global epidemic. Glob. Cardiol. Sci. Pract. 2020, 2020, e202005. [Google Scholar] [CrossRef] [PubMed]
- Kolte, D.; Lakshmanan, S.; Jankowich, M.D.; Brittain, E.L.; Maron, B.A.; Choudhary, G. Mild pulmonary hypertension is associated with increased mortality: A systematic review and meta-analysis. J. Am. Heart Assoc. 2018, 7, e009729. [Google Scholar] [CrossRef] [PubMed]
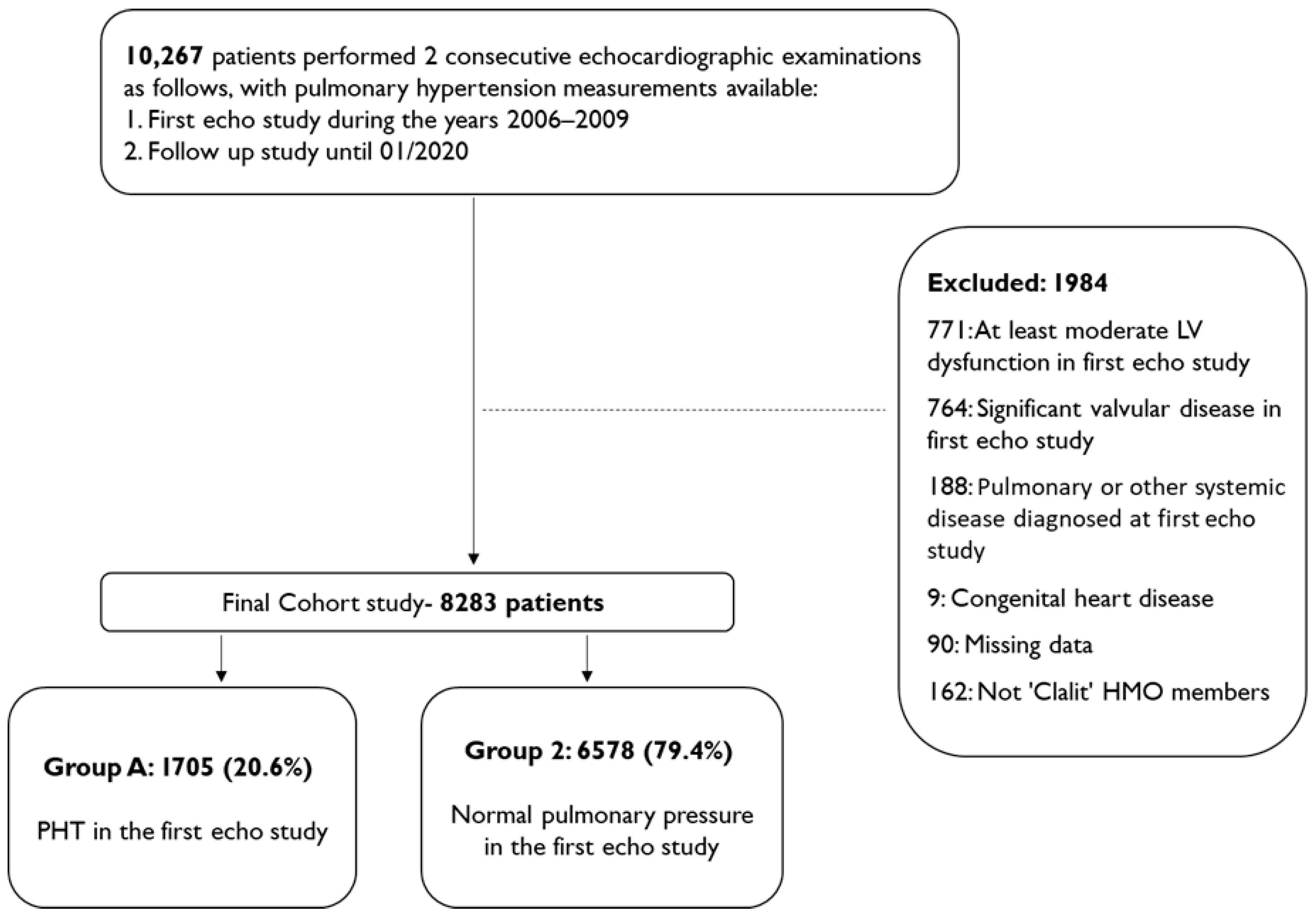
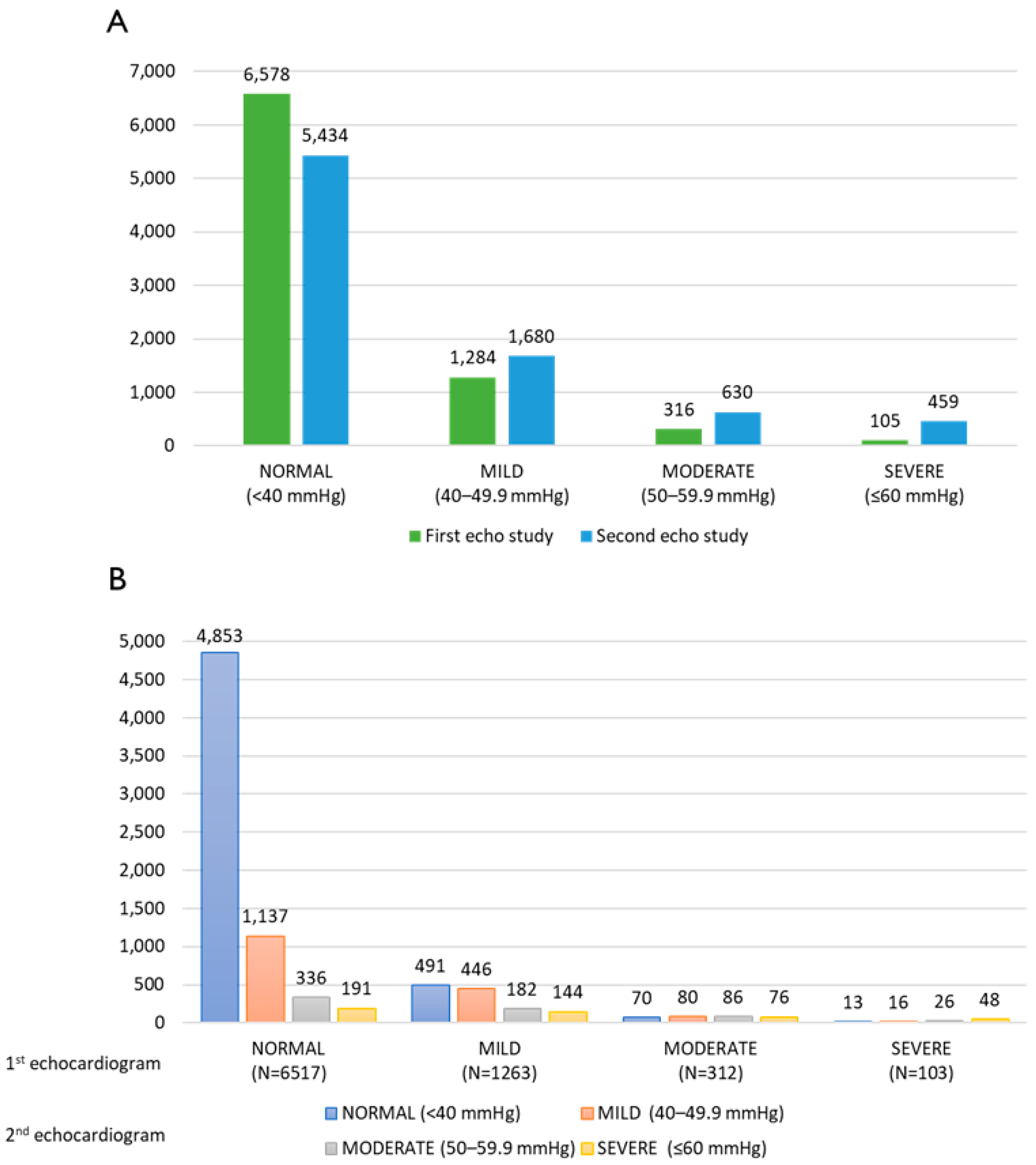
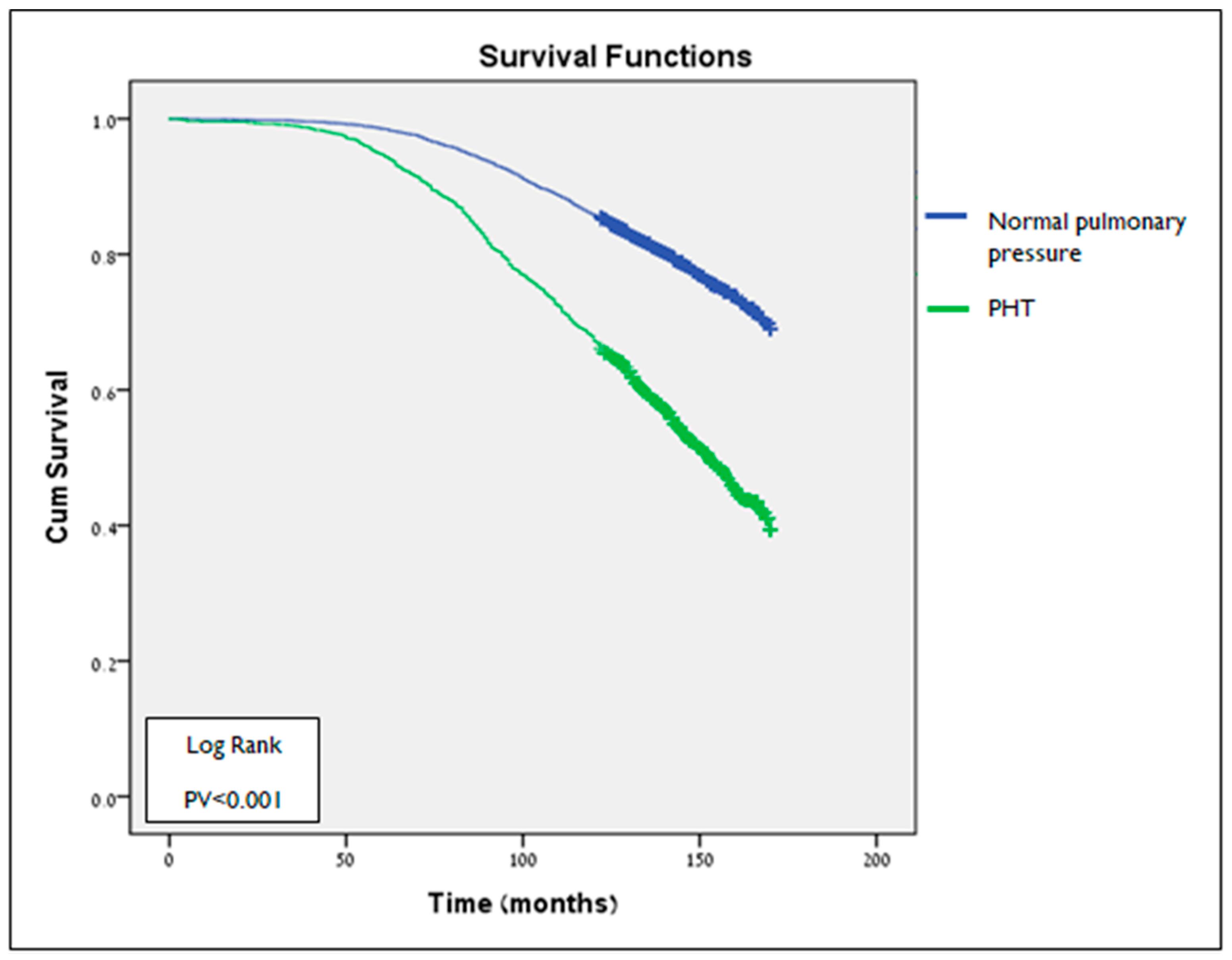
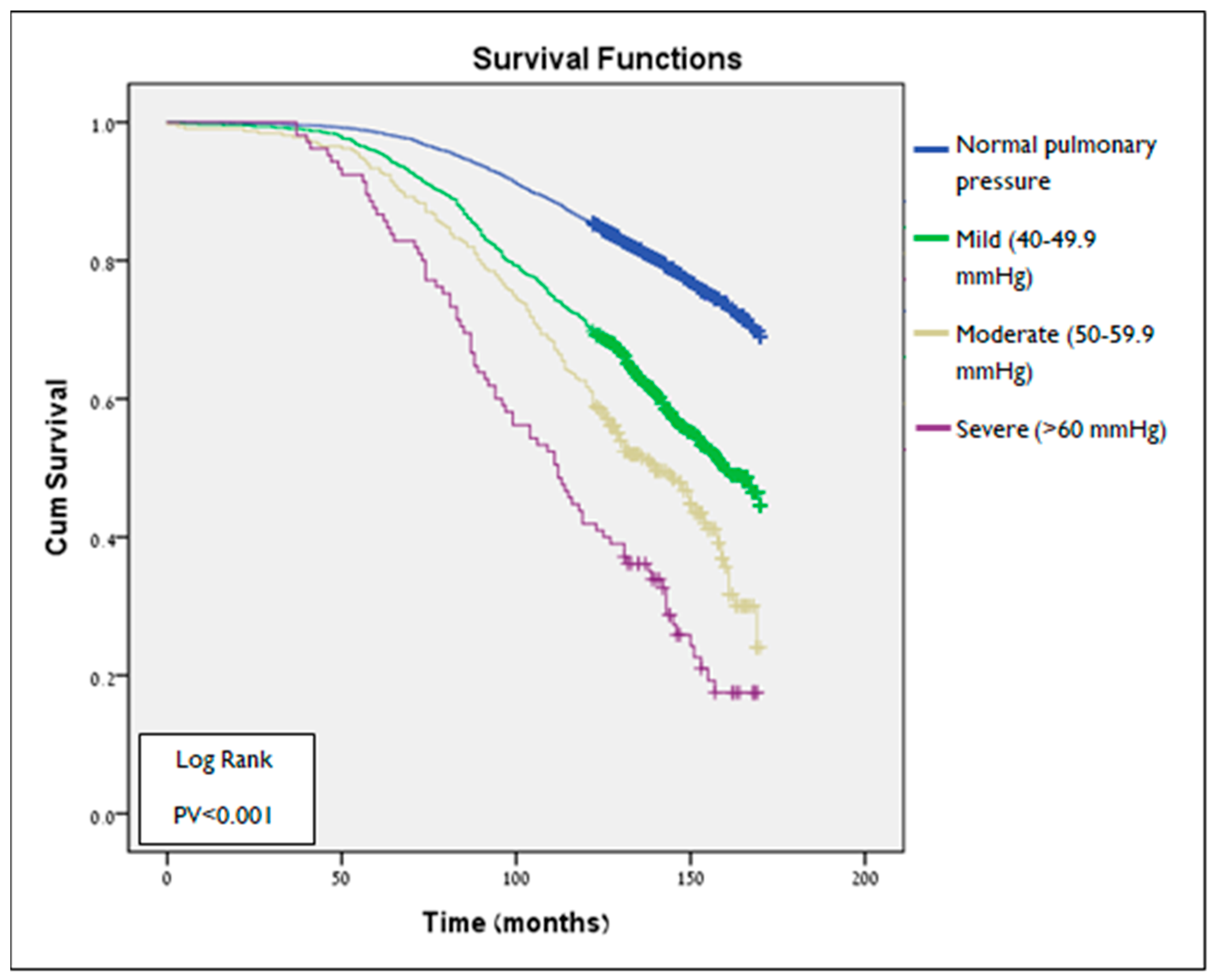
| Characteristics | All Cohort N = 8283 N (%) | PHT Status N (%) | ||
|---|---|---|---|---|
| PHT 1705 (20.6) | Normal Pulmonary Pressure 6578 (79.4) | p-Value | ||
| Age, years at first echocardiogram mean ± SD | 63.41 ± 14.21 | 69.54 ± 11.42 | 61.85 ± 14.4 | <0.001 |
| Female gender | 4778 (57.7) | 1106 (64.9) | 3672 (55.8) | <0.001 |
| Smoking | 626 (7.5) | 104 (6.1) | 522 (7.9) | 0.01 |
| Hypertension | 5945 (71.7) | 1500 (88) | 4445 (67.6) | <0.001 |
| Diabetes mellitus | 2802 (33.8) | 776 (45.5) | 2026 (30.8) | <0.001 |
| Dyslipidemia | 4893 (59) | 1177 (69) | 3716 (56.5) | <0.001 |
| Ischemic heart disease | 3254 (39.3) | 763 (44.8) | 2491 (37.9) | <0.001 |
| Adj. HR | 95% CI | p-Value | |
|---|---|---|---|
| PHT diagnosis in the first echocardiogram | 1.34 | 1.21–1.47 | <0.001 |
| Gender | 0.89 * | 0.82–0.98 | 0.02 |
| Age at first echocardiographic study | 1.07 ** | 1.06–1.08 | <0.001 |
| Socioeconomic index | 1.22 *** | 1.11–1.33 | <0.001 |
| Smoking | 1.14 | 1.01–1.28 | 0.03 |
| Hypertension | 1.20 | 1.03–1.39 | 0.02 |
| Diabetes mellitus | 1.43 | 1.30–1.56 | <0.001 |
| Dyslipidemia | 1.03 | 0.92–1.15 | 0.57 |
| Ischemic heart disease | 1.12 | 1.01–1.23 | 0.03 |
| Event of PE\DVT in the past | 1.49 | 1.25–1.77 | <0.001 |
| Asthma # | 1.06 | 0.91–1.24 | 0.48 |
| COPD # | 0.93 | 0.8–1.08 | 0.36 |
| OSA # | 0.89 | 0.75–1.06 | 0.19 |
| Other chronic disease ****,# | 1.77 | 1.60–1.95 | <0.001 |
| Clinical heart failure # | 1.40 | 1.26–1.55 | <0.001 |
| Adj. HR | 95% CI | p-Value | |
|---|---|---|---|
| Pulmonary pressure in the first echocardiogram | |||
| Normal | Refence Group | ||
| Mild PHT | 1.27 | 1.14–1.41 | <0.001 |
| Moderate PHT | 1.40 | 1.18–1.66 | <0.001 |
| Severe PHT | 2.00 | 1.58–2.54 | <0.001 |
| Gender | 0.89 * | 0.81–0.97 | 0.12 |
| Age at first echocardiographic study | 1.07 ** | 1.06–1.08 | <0.001 |
| Socioeconomic index | 1.21 *** | 1.11–1.33 | <0.001 |
| Smoking | 1.17 | 0.99–1.38 | 0.05 |
| Hypertension | 1.19 | 1.03–1.39 | 0.02 |
| Diabetes mellitus | 1.42 | 1.29–1.55 | <0.001 |
| Dyslipidemia | 1.04 | 0.93–1.16 | 0.47 |
| Ischemic heart disease | 1.11 | 1.01–1.23 | 0.04 |
| Event of PE\DVT in the past | 1.49 | 1.25–1.77 | <0.001 |
| Asthma # | 1.07 | 0.92–1.26 | 0.36 |
| COPD # | 0.94 | 0.81–1.11 | 0.46 |
| OSA # | 0.88 | 0.74–1.04 | 0.12 |
| Other chronic disease ****,# | 1.73 | 1.57–1.92 | <0.001 |
| Clinical heart failure # | 1.38 | 1.24–1.53 | <0.001 |
| LV systolic dysfunction in second echocardiogram | |||
| Normal LV function | Refence Group | ||
| Mild | 1.23 | 1.08–1.41 | 0.003 |
| Mild–moderate to moderate | 1.24 | 1.08–1.42 | 0.002 |
| Moderate–severe to severe | 1.59 | 1.38–1.83 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ashur, A.; Levy, A.; Liel-Cohen, N.; Sergienko, R.; Kobal, S.L. Prognostic Value of Pulmonary Hypertension as an Incidental Finding Detected by Echocardiography in Patients Without Known Cardiovascular or Pulmonary Diseases. J. Clin. Med. 2025, 14, 5044. https://doi.org/10.3390/jcm14145044
Ashur A, Levy A, Liel-Cohen N, Sergienko R, Kobal SL. Prognostic Value of Pulmonary Hypertension as an Incidental Finding Detected by Echocardiography in Patients Without Known Cardiovascular or Pulmonary Diseases. Journal of Clinical Medicine. 2025; 14(14):5044. https://doi.org/10.3390/jcm14145044
Chicago/Turabian StyleAshur, Avia, Amalia Levy, Noah Liel-Cohen, Ruslan Sergienko, and Sergio L. Kobal. 2025. "Prognostic Value of Pulmonary Hypertension as an Incidental Finding Detected by Echocardiography in Patients Without Known Cardiovascular or Pulmonary Diseases" Journal of Clinical Medicine 14, no. 14: 5044. https://doi.org/10.3390/jcm14145044
APA StyleAshur, A., Levy, A., Liel-Cohen, N., Sergienko, R., & Kobal, S. L. (2025). Prognostic Value of Pulmonary Hypertension as an Incidental Finding Detected by Echocardiography in Patients Without Known Cardiovascular or Pulmonary Diseases. Journal of Clinical Medicine, 14(14), 5044. https://doi.org/10.3390/jcm14145044







