The Influence of Diabetes on Orthodontic Treatment: A Systematic Review of the Clinical Considerations and Challenges in Response
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Study Selection
2.3. Data Extraction
2.4. Quality Analysis
3. Results
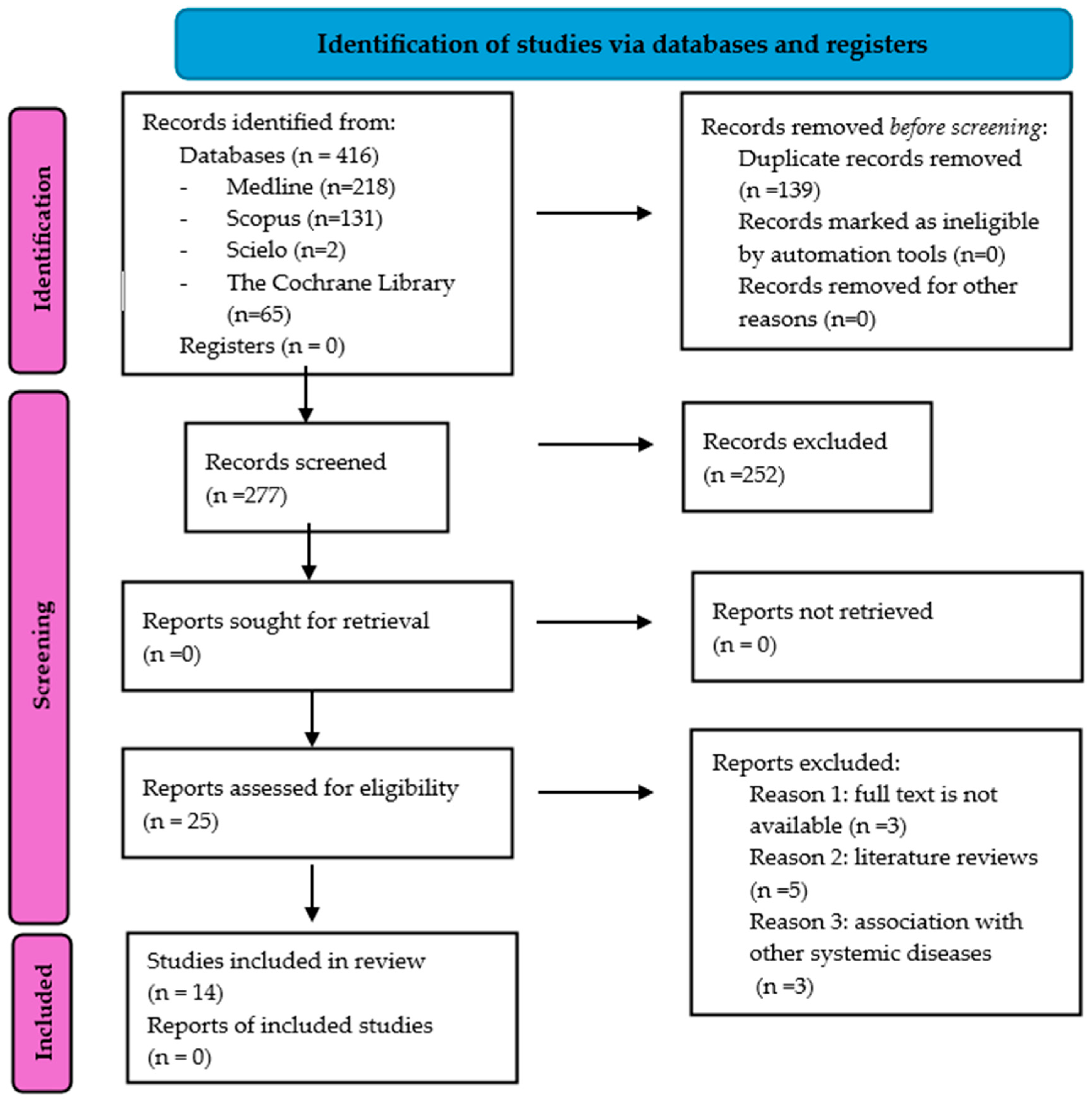
3.1. Study Selection and Flow Diagram
3.2. Data Extraction
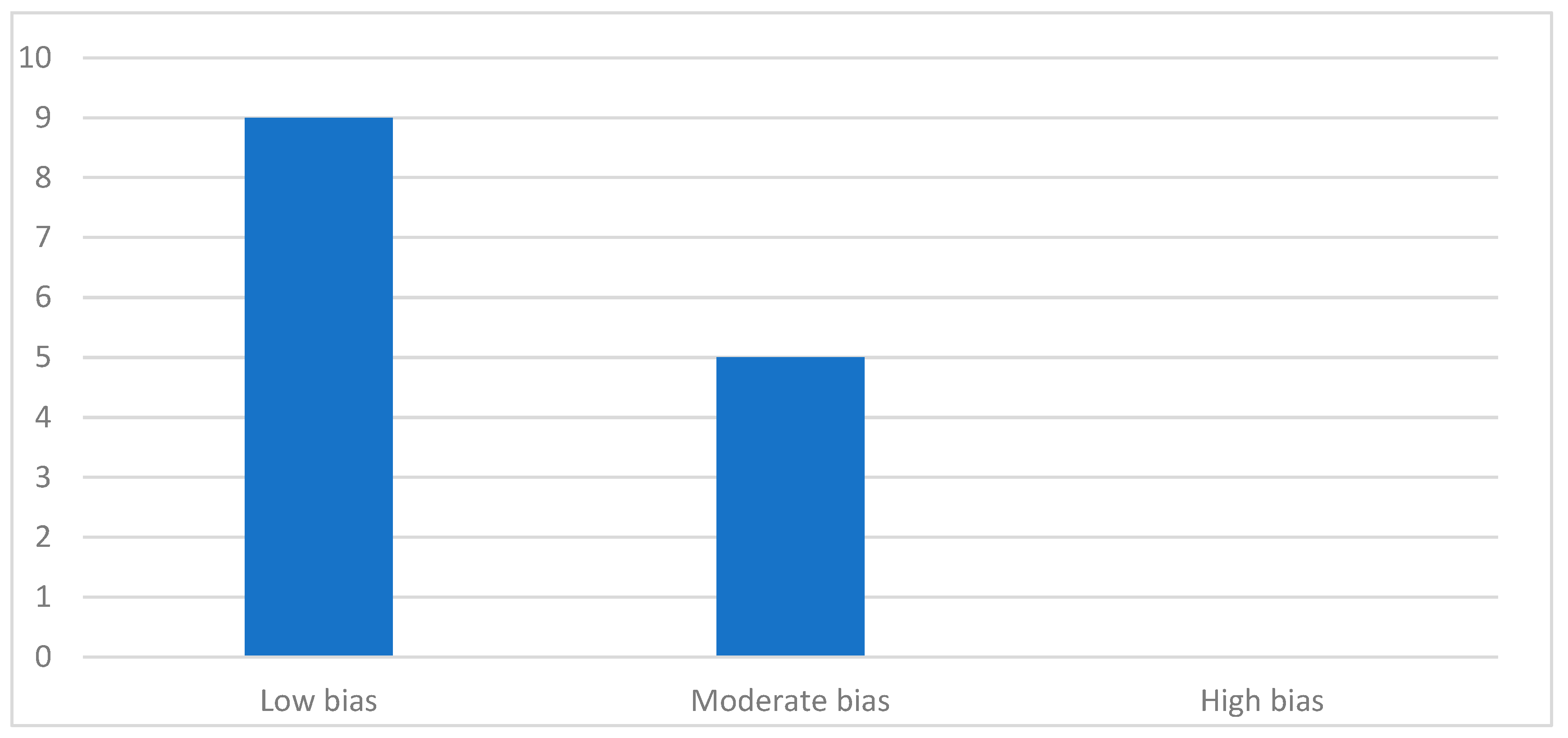
3.3. Quality Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Harreiter, J.; Roden, M. Diabetes mellitus: Definition, classification, diagnosis, screening and prevention (Update 2023). Wien Klin. Wochenschr 2023, 135 (Suppl. S1), 7–17. [Google Scholar] [PubMed]
- Ikegami, H.; Hiromine, Y.; Noso, S. Insulin-dependent diabetes mellitus in older adults: Current status and future prospects. Geriatr. Gerontol. Int. 2022, 22, 549–553. [Google Scholar]
- Sun, H.; Saeedi, P.; Karuranga, S.; Pinkepank, M.; Ogurtsova, K.; Duncan, B.B.; Stein, C.; Basit, A.; Chan, J.C.; Mbanya, J.C.; et al. IDF Diabetes Atlas: Global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res. Clin. Pract. 2022, 183, 109119. [Google Scholar]
- Schleicher, E.; Gerdes, C.; Petersmann, A.; Müller-Wieland, D.; Müller, U.A.; Freckmann, G.; Heinemann, L.; Nauck, M.; Landgraf, R. Definition, Classification and Diagnosis of Diabetes Mellitus. Exp. Clin. Endocrinol. Diabetes 2022, 130, S1–S8. [Google Scholar] [PubMed]
- American Diabetes Association Professional Practice Committee. 2. Diagnosis and Classification of Diabetes: Standards of Care in Diabetes-2025. Diabetes Care 2025, 48 (Suppl. S1), S27–S49. [Google Scholar]
- Ceriello, A.; Prattichizzo, F. Variability of risk factors and diabetes complications. Cardiovasc. Diabetol. 2021, 20, 101. [Google Scholar]
- Rumie Carmi, H.K.; Dominguez-Menendez, G.; Araya, M.; Martinez-Aguayo, A. [New Insulins for Type 1 Diabetes treatment]. Andes Pediatr. 2023, 94, 278–285. [Google Scholar]
- Carrasco-Sanchez, F.J.; Fernandez-Rodriguez, J.M.; Ena, J.; Gomez-Huelgas, R.; Carretero-Gomez, J.; Diabetes, Obesity and Nutrition Group of the Spanish Society of Internal Medicine. Medical treatment of type 2 diabetes mellitus: Recommendations of the Diabetes, Obesity and Nutrition Group of the Spanish Society of Internal Medicine. Rev. Clin. Esp. 2021, 221, 101–108. [Google Scholar] [PubMed]
- Scribante, A.; Butera, A.; Alovisi, M. Customized Minimally Invasive Protocols for the Clinical and Microbiological Management of the Oral Microbiota. Microorganisms 2022, 10, 675. [Google Scholar] [CrossRef]
- Ayesha, I.E.; Monson, N.R.; Klair, N.; Patel, U.; Saxena, A.; Patel, D.; Venugopal, S.; Ayesha, I.E. Probiotics and Their Role in the Management of Type 2 Diabetes Mellitus (Short-Term Versus Long-Term Effect): A Systematic Review and Meta-Analysis. Cureus 2023, 15, e46741. [Google Scholar]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [PubMed]
- Stang, A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur. J. Epidemiol. 2010, 25, 603–605. [Google Scholar]
- Munn, Z.; Barker, T.H.; Moola, S.; Tufanaru, C.; Stern, C.; McArthur, A.; Stephenson, M.; Aromataris, E. Methodological quality of case series studies: An introduction to the JBI critical appraisal tool. JBI Evid. Synth. 2020, 18, 2127–2133. [Google Scholar] [PubMed]
- Hooijmans, C.R.; Rovers, M.M.; de Vries, R.B.; Leenaars, M.; Ritskes-Hoitinga, M.; Langendam, M.W. SYRCLE’s risk of bias tool for animal studies. BMC Med. Res. Methodol 2014, 14, 43. [Google Scholar]
- Ying, Q.; Jiang, Y.; Sun, C.; Zhang, Y.; Gao, R.; Liu, H.; Liu, H.; Guo, J.; Li, M. AGEs impair osteogenesis in orthodontic force-induced periodontal ligament stem cells through the KDM6B/Wnt self-reinforcing loop. Stem Cell Res. Ther. 2024, 15, 431. [Google Scholar] [PubMed]
- Alshahrani, A.A. Effect of type 2 diabetes mellitus in adults undergoing fixed orthodontic treatment on proinflammatory chemokine profile and levels of advanced glycation in gingival crevicular fluid. Eur. Rev. Med. Pharmacol. Sci. 2022, 26, 8351–8357. [Google Scholar] [CrossRef] [PubMed]
- Alqerban, A. Levels of proinflammatory chemokines and advanced glycation end products in patients with type-2 diabetes mellitus undergoing fixed orthodontic treatment. Angle Orthod. 2021, 91, 105–110. [Google Scholar]
- Kamran, M.A. Salivary and crevicular fluid proinflammatory cytokines and advanced glycation end products in patients with different glycemic levels undergoing fixed orthodontic treatment. Angle Orthod. 2024, 94, 233–239. [Google Scholar]
- Plut, A.; Sprogar, Š.; Drevenšek, G.; Hudoklin, S.; Zupan, J.; Marc, J.; Drevenšek, M. Bone remodeling during orthodontic tooth movement in rats with type 2 diabetes. Am. J. Orthod. Dentofac. Orthop. 2015, 148, 1017–1025. [Google Scholar]
- Wang, M.; Qiu, Y.; Gao, L.; Qi, F.; Bi, L. The impact of IGF-1 on alveolar bone remodeling and BMP-2 expression in orthodontic tooth movement in diabetic rats. Adv. Clin. Exp. Med. 2023, 32, 349–356. [Google Scholar]
- Arita, K.; Hotokezaka, H.; Hashimoto, M.; Nakano-Tajima, T.; Kurohama, T.; Kondo, T.; Darendeliler, M.A.; Yoshida, N. Effects of diabetes on tooth movement and root resorption after orthodontic force application in rats. Orthod. Craniofacial Res. 2016, 19, 83–92. [Google Scholar]
- Santamaria, M., Jr.; Bagne, L.; Zaniboni, E.; Santamaria, M.P.; Jardini, M.A.N.; Felonato, M.; Dos Santos, G.M.T.; Mendonça, F.A.S.; Esquisatto, M.A.M. Diabetes mellitus and periodontitis: Inflammatory response in orthodontic tooth movement. Orthod. Craniofacial Res. 2020, 23, 27–34. [Google Scholar]
- Ferreira, C.L.; da Rocha, V.C.; da Silva Ursi, W.J.; De Marco, A.C.; Santamaria Jr, M.; Santamaria, M.P.; Jardini, M.A.N. Periodontal response to orthodontic tooth movement in diabetes-induced rats with or without periodontal disease. J. Periodontol. 2018, 89, 341–350. [Google Scholar]
- Vicente, A.; Bravo-Gonzalez, L.A.; Navarro, J.A.; Buendia, A.J.; Camacho-Alonso, F. Effects of diabetes on oxidative stress, periodontal ligament fiber orientation, and matrix metalloproteinase 8 and 9 expressions during orthodontic tooth movement. Clin. Oral Investig. 2021, 25, 1383–1394. [Google Scholar]
- Santamaria-Jr, M.; do Nascimento, E.R.A.; Bagne, L.; Calsa, B.; Esquisatto, M.A.M. Pulpal outcomes in orthodontic tooth movement in diabetes mellitus. Odontology 2021, 109, 921–929. [Google Scholar] [PubMed]
- Sun, J.; Du, J.; Feng, W.; Lu, B.; Liu, H.; Guo, J.; Amizuka, N.; Li, M. Histological evidence that metformin reverses the adverse effects of diabetes on orthodontic tooth movement in rats. J. Mol. Histol. 2017, 48, 73–81. [Google Scholar] [PubMed]
- Mena Laura, E.E.; Cestari, T.M.; Almeida, R.; Pereira, D.S.; Taga, R.; Garlet, G.P.; Assis, G.F. Metformin as an add-on to insulin improves periodontal response during orthodontic tooth movement in type 1 diabetic rats. J. Periodontol. 2019, 90, 920–931. [Google Scholar]
- Qi, J.; Kitaura, H.; Shen, W.R.; Ogawa, S.; Ohori, F.; Noguchi, T.; Marahleh, A.; Nara, Y.; Adya, P.; Mizoguchi, I. Effect of a DPP-4 Inhibitor on Orthodontic Tooth Movement and Associated Root Resorption. BioMed Res. Int. 2020, 2020, 7189084. [Google Scholar]
- Curto, A.; Gomez-Polo, C.; Curto, D.; Munoz-Bruguier, M.; Lorenzo-Luengo, M.C.; Montero, J. Influence of the metabolic control in patients with type 1 diabetes on their oral health status and the need for orthodontic treatment in a group of Spanish children (aged 6–12 years): A cross-sectional study. BMC Oral Health 2025, 25, 155. [Google Scholar]
- Mhaske, A.R.; Parhad, S.M.; Sattar, S.A.; Pandey, A.; Fafat, K.K.; Tekale, P.D. Awareness of Orthodontists Toward Management of Orthodontic Patients Suffering with Diabetes Mellitus in Central India Population: A Cross-Sectional Survey. J. Pharm Bioallied Sci. 2024, 16 (Suppl. S3), S2285–S2287. [Google Scholar]
- Huang, D.; Li, Y.; Chen, S.; Wang, H.; Jiang, Y.; Wei, Y.; Lin, H.; Zou, S. The onset of adenosine monophosphate-activated protein kinase activity on orthodontic tooth movement in rats with type 2 diabetes. Eur. J. Oral Sci. 2023, 131, e12955. [Google Scholar] [PubMed]
- Chen, S.; Huang, D.; Zhu, L.; Jiang, Y.; Guan, Y.; Zou, S.; Li, Y. Contribution of diabetes mellitus to periodontal inflammation during orthodontic tooth movement. Oral Dis. 2024, 30, 650–659. [Google Scholar] [PubMed]
- Mariș, M.; Bucur, S.M.; Mariș, M.; Păcurar, M.; Chibelean, M.; Nenovici, D.; Earar, K. Correlation between HbA1c Levels and Periodontal Bacterial Load in Diabetic Patients with Fixed Retainers. Curr. Health Sci. J. 2024, 50, 570–576. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bensch, L.; Braem, M.; Van Acker, K.; Willems, G. Orthodontic treatment considerations in patients with diabetes mellitus. Am. J. Orthod. Dentofac. Orthop. 2003, 123, 74–78. [Google Scholar]
- AlAlmadih, A.; Al-Zayer, M.; Dabel, S.; Alkhalaf, A.; Al Mayyad, A.; Bardisi, W.; Alshammari, S.; Alsihati, Z. Orthodontic Treatment Consideration in Diabetic Patients. J. Clin. Med. Res. 2018, 10, 77–81. [Google Scholar][Green Version]
- Koletsi, D.; Iliadi, A.; Papageorgiou, S.N.; Konrad, D.; Eliades, T. Evidence on the effect of uncontrolled diabetes mellitus on orthodontic tooth movement. A systematic review with meta-analyses in pre-clinical in- vivo research. Arch Oral Biol. 2020, 115, 104739. [Google Scholar]
- Robin, V.; Wim, T.; Maria, C.L.; Isabelle, L. Probiotics for maintaining oral health during fixed orthodontic treatment: A systematic review and meta-analysis. Int. J. Dent. Hyg. 2025, 23, 100–113. [Google Scholar][Green Version]
| Criterion | Type 1 Diabetes | Type 2 Diabetes |
|---|---|---|
| Etiology | Autoimmune genetic predisposition. | Genetic predisposition, multifactorial. |
| Prevalence | <10% of cases. | Common, >90% of cases. |
| Age of Onset | Mainly during childhood or adolescence, except for LADA. | Mainly at older age, although increasingly earlier onset. |
| Body Weight | Mostly normal. | Mostly overweight. |
| Symptoms | Common. | Less common. Slow onset and often accompanied by secondary conditions. |
| Tendency for Diabetic Ketoacidosis | Yes. | None or mild. |
| Databases | Search Field | Results |
|---|---|---|
| Medline (PubMed) | 1# “diabetes”, “diabetes mellitus”, “diabetic”, “hyperglycemia” | 106,373 |
| 2# “orthodontic”, “orthodontics”, “orthodontic treatment”, “braces”, “orthodontic therapy” | 10,584 | |
| 3# “tooth movement”, “orthodontic force”, “bone remodeling”, “periodontal response”, “treatment response”, “outcome”, “clinical considerations”, “inflammation” | 338,644 | |
| 1# AND 2# AND 3# | 218 | |
| SCOPUS | 1# “diabetes”, “diabetes mellitus”, “diabetic”, “hyperglycemia” | 1,055,158 |
| 2# “orthodontic”, “orthodontics”, “orthodontic treatment”, “braces”, “orthodontic therapy” | 68,498 | |
| 3# “tooth movement”, “orthodontic force”, “bone remodeling”, “periodontal response”, “treatment response”, “outcome”, “clinical considerations”, “inflammation” | 326 | |
| 1# AND 2# AND 3# | 131 | |
| Scielo | 1# “diabetes” fueron: “diabetes”, “diabetes mellitus”, “diabetic”, “hyperglycemia” | 15,684 |
| 2# “orthodontic”, “orthodontics”, “orthodontic treatment”, “braces”, “orthodontic therapy” | 613 | |
| 3# “tooth movement”, “orthodontic force”, “bone remodeling”, “periodontal response”, “treatment response”, “outcome”, “clinical considerations”, “inflammation” | 70 | |
| 1# AND 2# AND 3# | 2 | |
| The Cochrane Library | 1# “diabetes”, “diabetes mellitus”, “diabetic”, “hyperglycemia” | 130,694 |
| 2# “orthodontic”, “orthodontics”, “orthodontic treatment”, “braces”, “orthodontic therapy” | 9619 | |
| 3# “tooth movement”, “orthodontic force”, “bone remodeling”, “periodontal response”, “treatment response”, “outcome”, “clinical considerations”, “inflammation” | 933,269 | |
| 1# AND 2# AND 3# | 65 |
| Author and Year | Type of Study | Number of Participants or Number of Rats and Comparison | Type of Diabetes | Age | Induction Model | Aspects Analyzed | Conclusions |
|---|---|---|---|---|---|---|---|
| Ying et al. (2024) [15] | Experimental study using animal models | Animal model: Rats. Five groups of male Wistar rats, each consisting of five samples. Comparison included. | Type 2 | 7-week-old rats. | Chemical induction | Influence of diabetes on the cellular and molecular mechanisms through which advanced glycation end-products are synthesized, affecting the osteogenic differentiation of periodontal ligament stem cells under orthodontic force. | Diabetes affects orthodontic treatment at the cellular and molecular level through the accumulation of advanced glycation end-products, impairing the ability of periodontal ligament stem cells to form bones. |
| Alshahrani et al. (2022) [16] | Cross-sectional study | 50 individuals. 25 with type 2 diabetes and 25 non-diabetics. Comparison included. | Type 2 | 25–55 years old. | N/A | Levels of advanced glycation end-products and pro-inflammatory chemokines accumulated in the gingival crevicular fluid of type 2 diabetic patients undergoing orthodontic treatment were analyzed. | Patients with type 2 diabetes mellitus under fixed orthodontic treatment exhibit a biochemical profile in gingival crevicular fluid associated with a heightened pro-inflammatory response and increased gingival bleeding. |
| Alqerban et al. (2021) [17] | Case–control study | 40 participants. 20 with type 2 diabetes and 20 non-diabetics. Comparison included. | Type 2 | Mean age between 26 and 27 years. | N/A | Effect of type 2 diabetes on pro-inflammatory chemokine profiles and advanced glycation end-product levels in gingival crevicular fluid during orthodontic treatment. | Type 2 diabetic patients under fixed orthodontic treatment present elevated levels of pro-inflammatory chemokines and advanced glycation end-products in gingival crevicular fluid, resulting in increased bleeding and an intensified inflammatory response. |
| Kamran et al. (2024) [18] | Case–control study | 75 subjects: 25 non-diabetic, 25 prediabetic, and 25 with type 2 diabetes. Comparison included. | Type 2 | Mean age between 25 and 32 years. | N/A | Influence of diabetes on the deposition of advanced glycation end-products and pro-inflammatory cytokines in gingival crevicular fluid and saliva. | Patients with type 2 diabetes under fixed orthodontic treatment show elevated levels of pro-inflammatory biomarkers in gingival crevicular fluid, associated with greater bleeding and an exacerbated inflammatory response. |
| Alja Plut et al. (2015) [19] | Experimental study using animal models | Animal model: Rats. 48 rats. 24 male Wistar rats (healthy controls) and 24 male GK rats (type 2 diabetic). Comparison included. | Type 2 | Age of rats not specified. | GK rat | Effects of type 2 diabetes on bone remodeling during orthodontic tooth movement. Dental movement in diabetic rats was also measured. | Diabetes influences alveolar bone remodeling, causing a reduction in bone formation and increased resorption in response to orthodontic force. |
| Wang et al. (2023) [20] | Experimental study using animal models | Animal model: Rats. 60 Sprague Dawley rats are divided into 3 groups (20 per group): control, diabetic, and IGF-1 group. Comparison included. | Type 1 | 3-month-old rats. | Chemical induction | Influence of insulin-like growth factor 1 (IGF-1) on alveolar bone remodeling during orthodontic tooth movement in diabetic rats. | Diabetes reduces tooth movement and negatively affects bone remodeling during orthodontic treatment. It also increases inflammation and osteoclastic activity. IGF-1 treatment mitigates these adverse effects by improving bone remodeling. |
| Arita et al. (2016) [21] | Experimental study using animal models | Animal model: Rats. 23 male Sprague-Dawley rats: 7 control, 9 diabetic, 7 diabetic + insulin. Comparison included. | Type 1 | 10-week-old rats. | Chemical induction | Influence of diabetes and insulin administration on tooth movement and root resorption. | Diabetes reduces the rate of tooth movement and the extent of root resorption during orthodontic treatment. Glycemic control with insulin can largely reverse these effects. |
| Milton Santamaria et al. (2019) [22] | Experimental study using animal models | Animal model: Rats. 40 male Wistar rats divided into 4 groups (10 per group): healthy rats with OTM; rats with periodontitis and OTM; diabetic rats with OTM; and rats with both periodontitis, diabetes, and OTM. Comparison included. | Type 1 and 2 | 90-day-old rats. | Chemical induction | How diabetes alters the inflammatory response of gingival tissue and alveolar bone during orthodontic movement. | Diabetes increases local inflammatory response during orthodontic movement, resulting in less efficient tooth movement compared to non-diabetic conditions. |
| Lopes Ferreira et al. (2018) [23] | Experimental study using animal models | Animal model: Rats. 40 male Wistar rats divided into diabetic and non-diabetic groups. Comparison included. | Type 1 | 90-day-old rats. | Chemical induction | Histological periodontal responses to orthodontic tooth movement in rats with induced diabetes. Also analyzed were bone loss, bone density, amount of tooth movement, bone resorption, and periodontal ligament destruction. | Diabetes has a negative effect on the supporting dental bone, leading to greater bone loss during orthodontic treatment. |
| Ascensión Vicente et al. (2020) [24] | Experimental study using animal models | Animal model: Rats. 60 male Sprague-Dawley rats divided into 3 groups: 20 normoglycemic, 20 untreated diabetic, and 20 insulin-treated diabetics. Comparison included. | Type 1 | Adult rats (exact age not specified). | Chemical induction | Influence of diabetes, with or without treatment (simulating type 1 diabetes), on various aspects of orthodontic tooth movement: oxidative stress levels, orientation of periodontal ligament fibers, matrix metalloproteinase expression, and amount of tooth movement. | In untreated diabetic rats, orthodontic force causes increased inflammation, oxidative stress, disorganization of the periodontal ligament, and greater expression of matrix-degrading enzymes. Insulin largely reverses these effects. |
| Alves do Nascimento et al. (2021) [25] | Experimental study using animal models | Animal model: Rats. 40 male Wistar rats divided into 4 groups: 10 healthy controls without OTM; 10 diabetics without OTM; 10 non-diabetics with OTM; and 10 diabetics with OTM. Comparison included. | Type 1 | 90-day-old rats. | Chemical induction | Pulpal responses to orthodontic tooth movement in rats with type 1 diabetes mellitus. | Pulp tissue of teeth subjected to orthodontic movement in the presence of diabetes mellitus shows reduced adaptive and reparative capacity. |
| Sun et al. (2017) [26] | Experimental study using animal models | Animal model: Rats. 30 Wistar rats divided into 3 groups: 10 normoglycemic controls, 10 with type 2 diabetes, and 10 with type 2 diabetes treated with metformin. Comparison included. | Type 2 | Age not specified. | Chemical induction | Effects of metformin, a drug used to treat type 2 diabetes, orthodontic tooth movement, alveolar bone remodeling, and root resorption. | Type 2 diabetes in rats increases osteoclast number and activity, leading to greater tooth movement under orthodontic treatment. Metformin reduces osteoclast activity, enhances osteoblast function, and restores osteocyte function, thus normalizing tooth movement. |
| Ever Elias Mena et al. (2019) [27] | Experimental study using animal models | Animal model: Rats. 80 male Albinus Wistar rats divided into 4 groups: normoglycemic controls, untreated type 1 diabetics, type 1 diabetics treated with insulin, and type 1 diabetics treated with insulin and metformin. Comparison included. | Type 1 | 8-week-old rats. | Chemical induction | Effect of metformin (used in addition to insulin) on periodontal response during orthodontic tooth movement in type 1 diabetic rats. Also evaluated movement pattern, amount of tooth movement, and alveolar bone changes. | Untreated type 1 diabetes causes severe periodontal damage and an altered pattern of tooth movement under orthodontic force. Antidiabetic treatment (insulin or insulin plus metformin) reduces this damage, producing a response like that of non-diabetic rats. |
| Qi et al. (2020) [28] | Experimental study using animal models | Animal model: Mice. 24 male C57BL6/J mice divided into 3 groups: OTM + DPP-4 inhibitor; OTM + phosphate-buffered saline; control. Comparison included. | Type 2 | 8–10 weeks old. | Chemical induction | Effects of DPP-4 inhibitors (used to treat type 2 diabetes) on tooth movement distance and root resorption. | DPP-4 inhibitors, drugs used to treat type 2 diabetes, may negatively affect orthodontic treatment by reducing tooth movement and root resorption. |
| Case–Control Studies (NOS) | Selection | Comparability | Exposure | Total Score |
|---|---|---|---|---|
| Alqerban et al. [17] |    |   |   | 7  |
| Kamran et al. [18] |    |   |   | 7  |
| Article Title | Clear Inclusion Criteria | Subjects and Setting Described | Exposure Measured Validly | Standard Criteria for Condition | Confounding Factors Identified | Strategies to Deal with Confounding | Outcomes Measured Validly | Appropriate Statistical Analysis | Overall Appraisal | % |
|---|---|---|---|---|---|---|---|---|---|---|
| Alshahrani et al. (2022) [16] | Yes  | Yes  | Yes  | Yes  | Yes  | No  | Yes  | Yes  | Include  | 87.5 |
| Study | 1. Appropriate Random Allocation | 2. Similar Baseline Characteristics | 3. Allocation Concealment | 4. Blinding of Personal/Care Givers | 5. Blinding of Outcome Assessors | 6. Incomplete Data Adequately Handled | 7. Selective Reporting Avoided | 8. Free from Other Biases | 9. Funding Without Conflict of Interest | 10. Appropriate Experimental Design | Overall Risk |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Ying et al. [15] | No  | Yes  | Unclear  | No  | No  | Yes  | Yes  | Yes  | Yes  | Yes  | Moderate  |
| Alja et al. [19] | Yes  | Unclear  | Yes  | No  | Unclear  | Yes  | Yes  | Yes  | Yes  | Yes  | Low  |
| Wang et al. [20] | Yes  | Yes  | Unclear  | No  | Unclear  | Yes  | Yes  | Yes  | Yes  | Yes  | Low  |
| Arita et al. [21] | Yes  | Yes  | Unclear  | No  | No  | Yes  | Yes  | No  | Yes  | Yes  | Moderate  |
| Milton Santamaría et al. [22] | Yes  | No  | Unclear  | Unclear  | Yes  | Yes  | No  | No  | Yes  | Yes  | Moderate  |
| Lopes Ferreira et al. [23] | Yes  | Yes  | Unclear  | Yes  | Unclear  | No  | Yes  | Yes  | Yes  | Yes  | Low  |
| Ascensión Vicente et al. [24] | Unclear  | Unclear  | Yes  | Yes  | Yes  | No  | Yes  | Yes  | Yes  | Yes  | Low  |
| Alves do Nascimento et al. [25] | Yes  | Yes  | Unclear  | Unclear  | Yes  | No  | No  | Yes  | Yes  | Yes  | Moderate  |
| Sun et al. [26] | Unclear  | Unclear  | Yes  | Yes  | Yes  | No  | Yes  | Yes  | Yes  | Yes  | Low  |
| Ever Elías Mena et al. [27] | Yes  | Yes  | Unclear  | No  | Unclear  | No  | Yes  | Yes  | Yes  | Yes  | Moderate  |
| Qi et al. [28] | Unclear  | Yes  | Unclear  | Unclear  | Yes  | Yes  | Yes  | Yes  | Yes  | Yes  | Low  |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
García-Rios, P.; Rodríguez-Lozano, F.J.; Guerrero-Gironés, J.; Pecci-Lloret, M.R.; Oñate-Sánchez, R.E.; Pérez-Guzmán, N. The Influence of Diabetes on Orthodontic Treatment: A Systematic Review of the Clinical Considerations and Challenges in Response. J. Clin. Med. 2025, 14, 4879. https://doi.org/10.3390/jcm14144879
García-Rios P, Rodríguez-Lozano FJ, Guerrero-Gironés J, Pecci-Lloret MR, Oñate-Sánchez RE, Pérez-Guzmán N. The Influence of Diabetes on Orthodontic Treatment: A Systematic Review of the Clinical Considerations and Challenges in Response. Journal of Clinical Medicine. 2025; 14(14):4879. https://doi.org/10.3390/jcm14144879
Chicago/Turabian StyleGarcía-Rios, Paula, Francisco Javier Rodríguez-Lozano, Julia Guerrero-Gironés, Miguel R. Pecci-Lloret, Ricardo E. Oñate-Sánchez, and Nuria Pérez-Guzmán. 2025. "The Influence of Diabetes on Orthodontic Treatment: A Systematic Review of the Clinical Considerations and Challenges in Response" Journal of Clinical Medicine 14, no. 14: 4879. https://doi.org/10.3390/jcm14144879
APA StyleGarcía-Rios, P., Rodríguez-Lozano, F. J., Guerrero-Gironés, J., Pecci-Lloret, M. R., Oñate-Sánchez, R. E., & Pérez-Guzmán, N. (2025). The Influence of Diabetes on Orthodontic Treatment: A Systematic Review of the Clinical Considerations and Challenges in Response. Journal of Clinical Medicine, 14(14), 4879. https://doi.org/10.3390/jcm14144879