Modern Perspectives on Inguinal Hernia Repair: A Narrative Review on Surgical Techniques, Mesh Selection and Fixation Strategies
Abstract
1. Introduction
2. Methodology
3. History
4. Laparoscopic Techniques
5. TAPP Surgical Technique
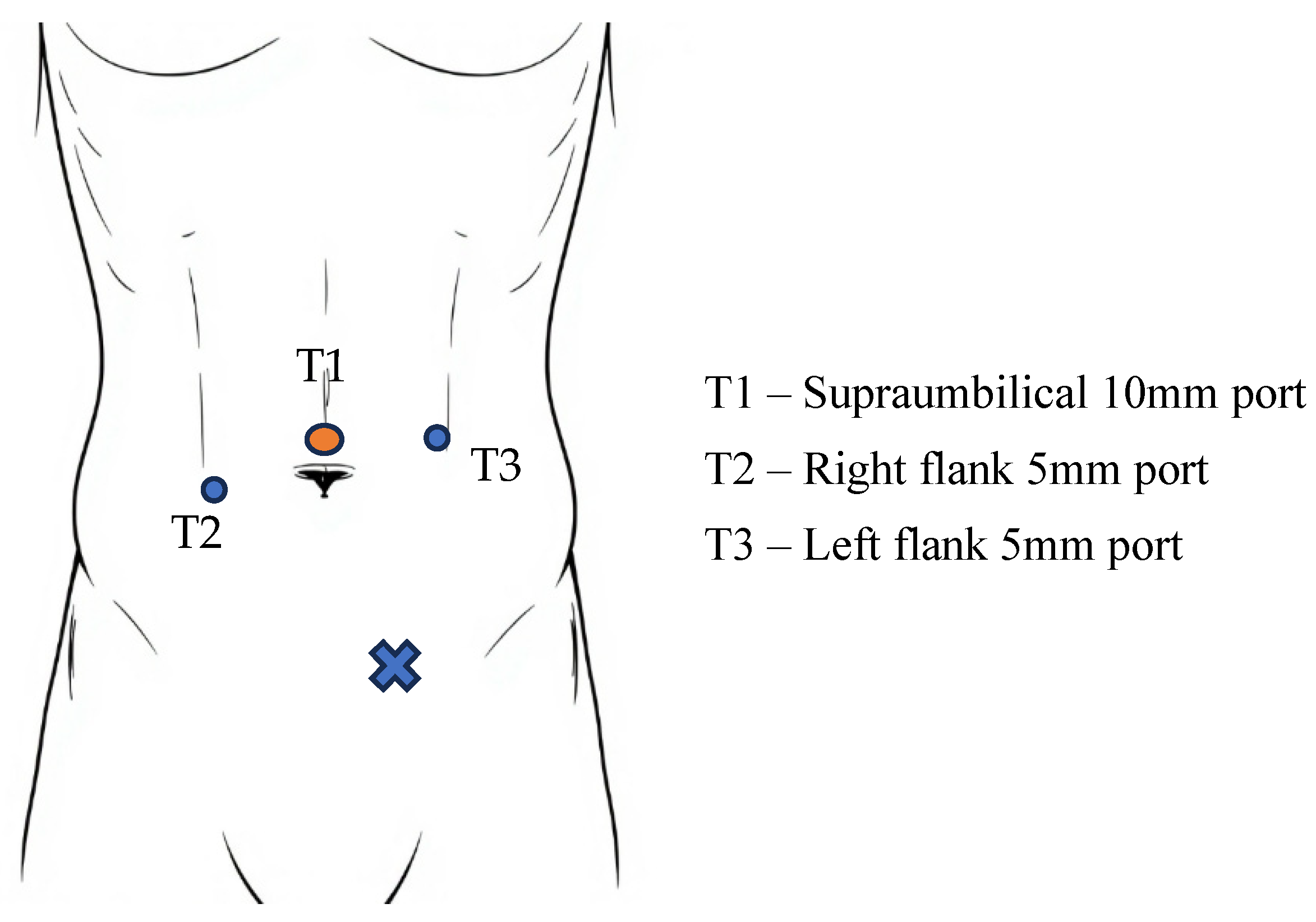
6. TEP Surgical Technique
- Identify the pubic symphysisThe pubic symphysis serves as a central anatomical landmark and reference point for subsequent dissection. Correct identification ensures a midline orientation and facilitates symmetrical mesh positioning.
- Blunt dissection of Cooper’s ligament bilaterallyThis exposes the retropubic (Retzius) space and defines the medial limit of dissection. Careful dissection here helps avoid vascular injury and ensures that the mesh has firm anchorage on both sides, minimizing medial recurrence.
- Identify Hesselbach’s triangle and the three hernia orifices (direct, femoral, obturator)This critical step ensures that all potential sites of herniation are visualized and adequately covered. It promotes a comprehensive repair and prevents future defects by enabling a thorough anatomical assessment.
- Identify and elevate the inferior epigastric vesselsElevation of these vessels exposes the underlying space of Bogros laterally and prevents inadvertent vascular injury. Awareness of this anatomy is key to preventing bleeding and facilitates proper lateral dissection.
- Develop the space of Bogros laterally up to the anterior superior iliac spine (ASIS)This step allows full lateral exposure for appropriate mesh coverage, especially critical in indirect hernias. It helps prevent folding or underlap of the mesh, which could lead to recurrence.
- Dissect the spermatic cord (or round ligament in females)Proper identification and gentle dissection of these structures help avoid trauma to the vas deferens, testicular vessels, or surrounding nerves, reducing the risk of infertility, hematoma, or chronic pain.
- Place a large mesh with adequate overlapThe mesh should cover all anatomical defects with sufficient overlap to prevent recurrence. It should extend medially over the pubic symphysis, laterally to the ASIS, and inferiorly over the femoral canal, with minimal fixation to reduce nerve irritation and postoperative pain.
7. Complications
8. Prosthetic Meshes
9. Fixation Methods
10. Discussion
11. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Aydin, M.; Fikatas, P.; Denecke, C.; Pratschke, J.; Raakow, J. Cost analysis of inguinal hernia repair: The influence of clinical and hernia-specific factors. Hernia 2021, 25, 1129–1135. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wang, J.; Han, X.; Fan, J.; Huang, C.; Dong, Y. Global burden and future trends of inguinal, femoral, and abdominal hernia in older adults: A systematic analysis from the Global Burden of Disease Study 2021. PLoS ONE 2025, 20, e0323790. [Google Scholar]
- Maresova, P.; Peteja, M.; Lerch, M.; Zonca, P.; Kuca, K. Costs of inguinal hernia repair associated with using different medical devices in the Czech Republic. Ther. Clin. Risk Manag. 2016, 12, 1593–1597. [Google Scholar] [CrossRef]
- Garofil, N.D.; Bratucu, M.N.; Zurzu, M.; Paic, V.; Tigora, A.; Prunoiu, V.; Rogobete, A.; Balan, A.; Vladescu, C.; Strambu, V.D.E.; et al. Groin Hernia Repair during the COVID-19 Pandemic-A Romanian Nationwide Analysis. Medicina 2023, 59, 970. [Google Scholar] [CrossRef]
- HerniaSurge Group. International guidelines for groin hernia management. Hernia 2018, 22, 1–165. [Google Scholar] [CrossRef]
- Lockhart, K.; Dunn, D.; Teo, S.; Ng, J.Y.; Dhillon, M.; Teo, E.; van Driel, M.L. Mesh versus non-mesh for inguinal and femoral hernia repair. Cochrane Database Syst. Rev. 2018, 9, CD011517. [Google Scholar] [CrossRef] [PubMed]
- Fischer, J.P.; Basta, M.N.; Mirzabeigi, M.N.; Bauder, A.R.; Fox, J.P.; Drebin, J.A.; Serletti, J.M.; Kovach, S.J. A Risk Model and Cost Analysis of Incisional Hernia After Elective, Abdominal Surgery Based Upon 12,373 Cases: The Case for Targeted Prophylactic Intervention. Ann. Surg. 2016, 263, 1010–1017. [Google Scholar] [CrossRef]
- Flum, D.R.; Horvath, K.; Koepsell, T. Have outcomes of incisional hernia repair improved with time?: A population-based analysis. Ann. Surg. 2003, 237, 129–135. [Google Scholar]
- Bassini, E. Nuovo metodo per la cura radicale dell’ernia inguinale. Atti. Congr. Assoc. Med. Ital. 1887, 2, 380. [Google Scholar]
- Dulucq, J.L.; Wintringer, P.; Mahajna, A. Laparoscopic totally extraperitoneal inguinal hernia repair: Lessons learned from 3100 hernia repairs over 15 years. Surg. Endosc. 2009, 23, 482–486. [Google Scholar] [CrossRef]
- Huerta, S.; Timmerman, C.; Argo, M.; Favela, J.; Pham, T.; Kukreja, S.; Yan, J.; Zhu, H. Open, Laparoscopic, and Robotic Inguinal Hernia Repair: Outcomes and Predictors of Complications. J. Surg. Res. 2019, 241, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Prabhu, A.S.; Carbonell, A.; Hope, W.; Warren, J.; Higgins, R.; Jacob, B.; Blatnik, J.; Haskins, I.; Alkhatib, H.; Tastaldi, L.; et al. Robotic Inguinal vs Transabdominal Laparoscopic Inguinal Hernia Repair: The RIVAL Randomized Clinical Trial. JAMA Surg. 2020, 155, 380–387. [Google Scholar] [CrossRef]
- de’Angelis, N.; Schena, C.A.; Moszkowicz, D.; Kuperas, C.; Fara, R.; Gaujoux, S.; Gillion, J.F.; Gronnier, C.; Loriau, J.; Mathonnet, M.; et al. Robotic surgery for inguinal and ventral hernia repair: A systematic review and meta-analysis. Surg. Endosc. 2024, 38, 24–46. [Google Scholar] [CrossRef]
- Anoldo, P.; Manigrasso, M.; D’Amore, A.; Musella, M.; De Palma, G.D.; Milone, M. Abdominal Wall Hernias-State of the Art of Laparoscopic versus Robotic Surgery. J. Pers. Med. 2024, 14, 100. [Google Scholar] [CrossRef]
- Kockerling, F. Data and outcome of inguinal hernia repair in hernia registers—A review of the literature. Innov. Surg. Sci. 2017, 2, 69–79. [Google Scholar] [CrossRef] [PubMed]
- Deveci, C.D.; Oberg, S.; Rosenberg, J. Definition of Mesh Weight and Pore Size in Groin Hernia Repair: A Systematic Scoping Review of Randomised Controlled Trials. J. Abdom. Wall Surg. JAWS 2023, 2, 11179. [Google Scholar] [CrossRef] [PubMed]
- Calomino, N.; Poto, G.E.; Carbone, L.; Micheletti, G.; Gjoka, M.; Giovine, G.; Sepe, B.; Bagnacci, G.; Piccioni, S.A.; Cuomo, R.; et al. Weighing the benefits: Exploring the differential effects of light-weight and heavy-weight polypropylene meshes in inguinal hernia repair in a retrospective cohort study. Am. J. Surg. 2024, 238, 115950. [Google Scholar] [CrossRef]
- Najm, A.; Niculescu, A.G.; Gaspar, B.S.; Grumezescu, A.M.; Beuran, M. A Review of Abdominal Meshes for Hernia Repair-Current Status and Emerging Solutions. Materials 2023, 16, 7124. [Google Scholar] [CrossRef]
- Cobb, W.S.; Carbonell, A.M.; Kalbaugh, C.L.; Jones, Y.; Lokey, J.S. Infection risk of open placement of intraperitoneal composite mesh. Am. Surg. 2009, 75, 762–767. [Google Scholar]
- Jeroukhimov, I.; Dykman, D.; Hershkovitz, Y.; Poluksht, N.; Nesterenko, V.; Yehuda, A.B.; Stephansky, A.; Zmora, O. Chronic pain following totally extra-peritoneal inguinal hernia repair: A randomized clinical trial comparing glue and absorbable tackers. Langenbeck’s Arch. Surg. 2023, 408, 190. [Google Scholar] [CrossRef]
- Trisca, R.; Oprea, V.; Toma, M.; Bucuri, C.E.; Stancu, B.; Grad, O.; Gherman, C. The Effectiveness of Cyanoacrylates versus Sutures for Mesh Fixation after Lichtenstein Repair (SCyMeLi STUDY) A Systematic Review and Meta-Analyze of Randomized Controlled Trials. Chirurgia 2024, 119, 87–101. [Google Scholar] [CrossRef] [PubMed]
- Dams, A.; Vankeirsbilck, J.; Poelmans, S.; Kerschaever, I.; Borreman, P.; Berwouts, L.; De Mulder, W.; Colle, J.; Beunis, A.; Dhooghe, V.; et al. Cyanoacrylate mesh fixation for laparoscopic inguinal hernia repair: A prospective, multicenter, single-arm study. Surg. Endosc. 2023, 37, 9105–9115. [Google Scholar] [CrossRef]
- Vierstraete, M.; Chastan, P.; Meneghin, A.; Muysoms, F. History of the Creation of Self-Gripping Mesh. J. Abdom. Wall Surg. JAWS 2023, 2, 11330. [Google Scholar] [CrossRef] [PubMed]
- Lv, Y.; Yang, B.; Hao, G.; Wang, Y. Mesh Fixation Versus Nonfixation in Laparoscopic Inguinal Hernia Repair: A Systematic Review and Meta-Analysis. Am. Surg. 2024, 90, 111–121. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Li, J.; Suo, H.; Bai, J. Non-fixation versus fixation of mesh in laparoscopic transabdominal preperitoneal repair of inguinal hernia: A systematic review and meta-analysis of randomized controlled trials. PLoS ONE 2024, 19, e0314334. [Google Scholar] [CrossRef]
- Stabilini, C.; van Veenendaal, N.; Aasvang, E.; Agresta, F.; Aufenacker, T.; Berrevoet, F.; Burgmans, I.; Chen, D.; de Beaux, A.; East, B.; et al. Update of the international HerniaSurge guidelines for groin hernia management. BJS Open 2023, 7. [Google Scholar] [CrossRef]
- Dönmez, A.E.; Goswami, A.G.; Raheja, A.; Bhadani, A.; El Kady, A.E.S.; Alniemi, A.; Awad, A.; Aladl, A.; Younis, A.; Alwali, A. Access to and quality of elective care: A prospective cohort study using hernia surgery as a tracer condition in 83 countries. Lancet Glob. Health 2024, 12, e1094–e1103. [Google Scholar]
- Wang, H.; Wang, X. Laparoscopic Versus Open Hernia Repair for Indirect Inguinal Hernia in Adolescents: A Retrospective Cohort Study. J. Investig. Surg. 2024, 37, 2427382. [Google Scholar]
- Picciochi, M.; Alexander, P.V.; Anyomih, T.; Boumas, N.; Crawford, R.; Enoch Gyamfi, F.; Hopane, N.; Isiagi, M.; Kamarajah, S.; Ledda, V. Provision of inguinal hernia surgery in first-referral hospitals across low-and middle-income countries: Secondary analysis of an international cohort study. World J. Surg. 2025, 49, 374–384. [Google Scholar]
- Abedizadeh, R.; Majidi, F.; Khorasani, H.R.; Abedi, H.; Sabour, D. Colorectal cancer: A comprehensive review of carcinogenesis, diagnosis, and novel strategies for classified treatments. Cancer Metastasis Rev. 2024, 43, 729–753. [Google Scholar] [CrossRef]
- Lau, W.Y. History of treatment of groin hernia. World J. Surg. 2002, 26, 748–759. [Google Scholar] [CrossRef]
- Kingsorth, A.; Sanders, D.L. General Introduction and History of Hernia Surgery. In Management of Abdominal Hernias; LeBlanc, K.A., Kingsnorth, A., Sanders, D.L., Eds.; Springer International Publishing: Cham, Switzerland, 2018; pp. 3–30. [Google Scholar] [CrossRef]
- Patino, J.F. A history of the treatment of hernia. In Hernia, 4th ed.; Nyhus, L.M., Condon, R.E., Eds.; JB Lippincott Company: Philadelphia, PA, USA, 1995; pp. 3–15. [Google Scholar]
- Furtado, M.; Claus, C.M.P.; Cavazzola, L.T.; Malcher, F.; Bakonyi-Neto, A.; Saad-Hossne, R. Systemization of Laparoscopic Inguinal Hernia Repair (Tapp) Based on a New Anatomical Concept: Inverted Y and Five Triangles. Arq. Bras. Cir. Dig. 2019, 32, e1426. [Google Scholar] [CrossRef]
- Haeger, K. The Illustrated History of Surgery; Harold Starke: London, UK, 1989. [Google Scholar]
- Paré, A. The Apologie and Treatise of Ambroise Paré Containing the Voyages Made into Divers Places, with Many of His Writings upon Surgery. Yale J. Biol. Med. 1953, 25, 545. [Google Scholar]
- Nover, A.; Kümmel, W.F. Surgeon and eye doctor Caspar Stromayr and the ophthalmological illustrations in his “Practica copiosa” (1559). Klin. Monatsblatter Augenheilkd. 1984, 185, 321–323. [Google Scholar] [CrossRef]
- Cooper, A.P. Lectures on the Principles and Practice of Surgery; Printed for W. Simpkin and R. Marshall; Blanchard and Lea: Philadelphia, PA, USA, 1827. [Google Scholar]
- Colles, A. Treatise on Surgical Anatomy; Gilbert Hodges: Dublin, Ireland, 1811. [Google Scholar]
- Cloquet, J.; Wantz, G.E. Recherches Anatomiques sur les Hernies de l’abdomen; Hernes de l’abdomen; Chez Méquignon-Marvis, libraire pour la partie de médecine, rue de l’imprimerie de Didot jeune, imprimeur de la faculté de médecine: Paris, France, 1817. [Google Scholar]
- Margotta, R. An Illustrated History of Medicine; Hamlyn: Middlesex, UK, 1968. [Google Scholar]
- Ramjist, J.K.; Dossa, F.; Stukel, T.A.; Urbach, D.R.; Fu, L.; Baxter, N.N. Reoperation for inguinal hernia recurrence in Ontario: A population-based study. Hernia 2019, 23, 647–654. [Google Scholar] [CrossRef] [PubMed]
- McArthur, L.L. Autoplastic sutures in hernia and other diastases. JAMA 1901, 37, 1162–1165. [Google Scholar]
- Devlin, H.B. Management of Abdominal Hernias; Butterworth & Co: London, UK, 1988. [Google Scholar]
- Mair, G.B. Preliminary report on the use of whole skin-grafts as a substitute for fascial sutures in the treatment of herniae. J. Br. Surg. 1945, 32, 381–385. [Google Scholar]
- Baylón, K.; Rodríguez-Camarillo, P.; Elías-Zúñiga, A.; Díaz-Elizondo, J.A.; Gilkerson, R.; Lozano, K. Past, present and future of surgical meshes: A review. Membranes 2017, 7, 47. [Google Scholar] [CrossRef]
- Cumberland, V. A preliminary report on the use of prefabricated nylon weave in the repair of ventral hernia. Med. J. Aust. 1952, 1, 143–144. [Google Scholar]
- Scales, J. Tissue reactions to synthetic materials. Proc. R Soc. Med. 1953, 46, 647–652. [Google Scholar]
- Lichtenstein, I.L.; Shulman, A.G.; Amid, P.K.; Montllor, M.M. The tension-free hernioplasty. Am. J. Surg. 1989, 157, 188–193. [Google Scholar] [PubMed]
- Ger, R.; Monroe, K.; Duvivier, R.; Mishrick, A. Management of indirect inguinal hernias by laparoscopic closure of the neck of the sac. Am. J. Surg. 1990, 159, 370–373. [Google Scholar]
- Iossa, A.; Traumueller Tamagnini, G.; De Angelis, F.; Micalizzi, A.; Lelli, G.; Cavallaro, G. TEP or TAPP: Who, when, and how? Front. Surg. 2024, 11, 1352196. [Google Scholar] [CrossRef]
- Srivastava, N.K.; Yadav, A.S.; Sinha, R. A Comparative Evaluation of Extended Total Extraperitoneal Repair Versus Standard Total Extraperitoneal Repair and Transabdominal Preperitoneal Repair of Inguinal Hernias. J. Soc. Laparoendosc. Surg. 2023, 27, e2023. [Google Scholar] [CrossRef]
- Xie, J.; Koo, D.C.; Lee, M.J.; Sugiyama, G. The evolution of minimally invasive inguinal hernia repairs. Ann. Laparosc. Endosc. Surg. 2024, 9, 10. [Google Scholar] [CrossRef]
- Andresen, K.; Rosenberg, J. Transabdominal pre-peritoneal (TAPP) versus totally extraperitoneal (TEP) laparoscopic techniques for inguinal hernia repair. Cochrane Database Syst. Rev. 2024, 7, CD004703. [Google Scholar] [CrossRef]
- Mita, K.; Kobayashi, N.; Takahashi, K.; Sakai, T.; Shimaguchi, M.; Kouno, M.; Toyota, N.; Hatano, M.; Toyota, T.; Sasaki, J. Anatomical recognition of dissection layers, nerves, vas deferens, and microvessels using artificial intelligence during transabdominal preperitoneal inguinal hernia repair. Hernia 2024, 29, 52. [Google Scholar] [CrossRef]
- Correia de Sa, T.; Jacome, F.; Basto, T.; Costa, M.; Goncalves, A.; Teixeira, N.; Castro Neves, L.; Barros da Silva, J. Transabdominal preperitoneal (TAPP) repair for emergency groin hernia: A systematic review. Hernia 2024, 28, 1005–1015. [Google Scholar] [CrossRef]
- Sivakumar, J.; Chen, Q.; Hii, M.W.; Cullinan, M.; Choi, J.; Steven, M.; Crosthwaite, G. Learning curve of laparoscopic inguinal hernia repair: Systematic review, meta-analysis, and meta-regression. Surg. Endosc. 2023, 37, 2453–2475. [Google Scholar] [CrossRef]
- Peethambaran, M.S.; Rajendran, R.R.; Murthy, N.G. Totally Extraperitoneal Repair of Grynfeltt’s Hernia: Easy Solution for a Rare Problem. Cureus 2024, 16, e74743. [Google Scholar] [CrossRef]
- Baginski, B.; Tran, D.; Ogola, G.; Arnold, D. A single-center retrospective review of laparoscopic totally extraperitoneal versus robotic transabdominal preperitoneal inguinal hernia repair. Proceedings 2024, 37, 897–902. [Google Scholar] [CrossRef] [PubMed]
- Basukala, S.; Shrestha, O.; Chhetri, S.T.; Thapa, N.; Oli, S.; Mehta, B.K.; Pokhrel, N.; Tiwari, B. Transabdominal Preperitoneal (TAPP) Compared to Totally Extraperitoneal (TEP) for Primary Inguinal Hernia Repair in a Military Hospital: A Retrospective Cohort Study. Health Sci. Rep. 2024, 7, e70270. [Google Scholar] [CrossRef] [PubMed]
- Ozel, Y.; Kara, Y.B. Comparison of Clinical Outcomes of Laparoscopic Totally Extraperitoneal (TEP) and Transabdominal Preperitoneal (TAPP) Techniques in Bilateral Inguinal Hernia Repair: A Retrospective Study. Cureus 2024, 16, e69134. [Google Scholar] [CrossRef]
- Dokania, M.K.; Ankur, A.; Agarwal, N.; Jain, A.; Anshu, A.; Singh, R.A.K. Comparison of Perioperative Complication Rates of Total Extraperitoneal and Transabdominal Preperitoneal Repairs in Primary Inguinal Hernia. J. West Afr. Coll. Surg. 2024, 14, 69–75. [Google Scholar] [CrossRef]
- Ielpo, B.; Nunez-Alfonsel, J.; Duran, H.; Diaz, E.; Fabra, I.; Caruso, R.; Malave, L.; Ferri, V.; Barzola, E.; Quijano, Y.; et al. Cost-effectiveness of Randomized Study of Laparoscopic Versus Open Bilateral Inguinal Hernia Repair. Ann. Surg. 2018, 268, 725–730. [Google Scholar] [CrossRef] [PubMed]
- Senol, Z. Evaluation of Surgical Results and Effectiveness of Laparoscopic Transabdominal Preperitoneal and Laparoscopic Totally Extraperitoneal Approaches in Bilateral Inguinal Hernia Repair: A Randomized Analysis. J. Laparoendosc. Adv. Surg. Tech. Part A 2025, 35, 152–155. [Google Scholar] [CrossRef]
- Almutairi, H.; Alshammari, R.S.; Alharbi, M.J.; Althobaiti, D.M.; Alghamdi, R.S.; Alsamiri, S.; Mawash, S.W.; Ahmed, D.A.; Alamoudi, A.A.; Arif, F.Y.; et al. Laparoscopic Management of Inguinal Hernia: A Systematic Review and Updated Network Meta-Analysis of Randomized Controlled Trials. Cureus 2024, 16, e54192. [Google Scholar] [CrossRef]
- Rivas, J.F.; Molina, A.P.R.F.; Carmona, J.M. Transabdominal preperitoneal (TAPP) inguinal hernia repair: How we do it. Ann. Laparosc. Endosc. Surg. 2021, 6, 6. [Google Scholar] [CrossRef]
- Carter, J.; Duh, Q.Y. Laparoscopic repair of inguinal hernias. World J. Surg. 2011, 35, 1519–1525. [Google Scholar] [CrossRef]
- Mubark, M.; Mohammed, H.A.; Mohamed, M.A. Transabdominal pre-peritoneal (TAPP) versus totally extraperitoneal (TEP) laparoscopic techniques for inguinal hernia repair. SVU-Int. J. Med. Sci. 2023, 6, 676–683. [Google Scholar] [CrossRef]
- Daes, J.; Felix, E. Critical View of the Myopectineal Orifice. Ann. Surg. 2017, 266, e1–e2. [Google Scholar] [CrossRef] [PubMed]
- Sözen, S.; Erdem, H. Hernia Surgery; BoD: Norderstedt, Germany, 2022. [Google Scholar]
- Delibegović, S. TEP, STEP BY STEP. South-East Eur. Endo-Surg. J. 2023, 2, 111–122. [Google Scholar] [CrossRef]
- Ferzli, G.; Iskandar, M. Laparoscopic totally extra-peritoneal (TEP) inguinal hernia repair. Ann. Laparosc. Endosc. Surg. 2019, 4, 35. [Google Scholar] [CrossRef]
- Lomanto, D.; Goel, R. Intraoperative Complications During Laparoscopic Hernia Repair. In The SAGES Manual of Hernia Repai; Springer: New York, NY, USA, 2012; pp. 143–156. [Google Scholar]
- Davis, C.J.; Arregui, M.E. Laparoscopic repair for groin hernias. Surg. Clin. 2003, 83, 1141–1161. [Google Scholar]
- Felix, E.L.; Harbertson, N.; Vartanian, S. Laparoscopic hernioplasty: Significant complications. Surg. Endosc. 1999, 13, 328–331. [Google Scholar] [PubMed]
- Sartori, A.; De Luca, M.; Noaro, G.; Piatto, G.; Pignata, G.; Di Leo, A.; Lauro, E.; Andreuccetti, J. Rare intraoperative and postoperative complications after transabdominal laparoscopic hernia repair: Results from the multicenter wall hernia group registry. J. Laparoendosc. Adv. Surg. Tech. 2021, 31, 290–295. [Google Scholar]
- Frydenlund, P.; Ramaswamy, A. Intraoperative and postoperative complications of MIS inguinal hernia repair. In The SAGES Manual of Hernia Surgery; Springer: New York, NY, USA, 2018; pp. 549–558. [Google Scholar]
- Poffenberger, R.J. Laparoscopic repair of intraperitoneal bladder injury: A simple new technique. Urology 1996, 47, 248–249. [Google Scholar]
- Tamme, C.; Scheidbach, H.; Hampe, C.; Schneider, C.; Köckerling, F. Totally extraperitoneal endoscopic inguinal hernia repair (TEP). Surg. Endosc. Other Interv. Tech. 2003, 17, 190–195. [Google Scholar]
- Moreno-Egea, A.; Paredes, P.G.; Perello, J.M.; Campillo-Soto, A.; Baena, E.G.; Muñoz, J.R.O.; Aguayo-Albasini, J.L. Vascular injury by tacks during totally extraperitoneal endoscopic inguinal hernioplasty. Surg. Laparosc. Endosc. Percutaneous Tech. 2010, 20, e129–e131. [Google Scholar]
- Fitzgibbons, R.J., Jr.; Camps, J.; Cornet, D.A.; Nguyen, N.X.; Litke, B.S.; Annibali, R.; Salerno, G.M. Laparoscopic inguinal herniorrhaphy results of a multicenter trial. Ann. Surg. 1995, 221, 3–13. [Google Scholar]
- Lipskar, A.M.; Reiner, M.A. Cord Structure Complications in Inguinal Hernia Surgery; Springer: New York, NY, USA, 2013. [Google Scholar]
- Ghariani, W.; Dougaz, M.W.; Jerraya, H.; Khalfallah, M.; Bouasker, I.; Dziri, C. Recurrence Factors of Groin Hernia: A systematic Review. La Tunis. Medicale 2019, 97, 619–625. [Google Scholar]
- Stan, A.-M.; Preda, S.-D.; Râmboiu, S.; Câråu, D.; Pãtraæcu, Æ.; Cazacu, S.; Biciuæcã, V.; Ætiolicã, A.T.; Andronic, O.; Pãduraru, D.N. Ventral Hernia Repair and Drainage–A Prospective, Observational, and Comparative Study of Outcomes. Chirurgia 2023, 118, 426–434. [Google Scholar]
- Van Veen, R.; Wijsmuller, A.; Vrijland, W.; Hop, W.; Lange, J.; Jeekel, J. Long-term follow-up of a randomized clinical trial of non-mesh versus mesh repair of primary inguinal hernia. J. Br. Surg. 2007, 94, 506–510. [Google Scholar]
- Bittner, R.; Leibl, B.; Kraft, B.; Schwarz, J. One-year results of a prospective, randomised clinical trial comparing four meshes in laparoscopic inguinal hernia repair (TAPP). Hernia 2011, 15, 503–510. [Google Scholar] [PubMed]
- See, C.W.; Kim, T.; Zhu, D. Hernia mesh and hernia repair: A review. Eng. Regen. 2020, 1, 19–33. [Google Scholar]
- Hollinsky, C.; Sandberg, S.; Koch, T.; Seidler, S. Biomechanical properties of lightweight versus heavyweight meshes for laparoscopic inguinal hernia repair and their impact on recurrence rates. Surg. Endosc. 2008, 22, 2679–2685. [Google Scholar] [PubMed]
- Idrees, S.; Jindal, S.; Gupta, M.; Sarangi, R. Surgical meshes–The search continues. Curr. Med. Res. Pract. 2018, 8, 177–182. [Google Scholar]
- Cobb, W.S.; Burns, J.M.; Kercher, K.W.; Matthews, B.D.; Norton, H.J.; Heniford, B.T. Normal intraabdominal pressure in healthy adults. J. Surg. Res. 2005, 129, 231–235. [Google Scholar]
- Kalaba, S.; Gerhard, E.; Winder, J.S.; Pauli, E.M.; Haluck, R.S.; Yang, J. Design strategies and applications of biomaterials and devices for hernia repair. Bioact. Mater. 2016, 1, 2–17. [Google Scholar]
- Brown, C.; Finch, J. Which mesh for hernia repair? Ann. R. Coll. Surg. Engl. 2010, 92, 272–278. [Google Scholar]
- Earle, D.B.; Mark, L.A. Prosthetic material in inguinal hernia repair: How do I choose? Surg. Clin. N. Am. 2008, 88, 179–201. [Google Scholar]
- Li, J.; Ji, Z.; Cheng, T. Lightweight versus heavyweight in inguinal hernia repair: A meta-analysis. Hernia 2012, 16, 529–539. [Google Scholar] [PubMed]
- O’dwyer, P.; Kingsnorth, A.; Molloy, R.; Small, P.; Lammers, B.; Horeyseck, G. Randomized clinical trial assessing impact of a lightweight or heavyweight mesh on chronic pain after inguinal hernia repair. J. Br. Surg. 2005, 92, 166–170. [Google Scholar]
- Melkemichel, M.; Bringman, S.; Nilsson, H.; Widhe, B. Patient-reported chronic pain after open inguinal hernia repair with lightweight or heavyweight mesh: A prospective, patient-reported outcomes study. J. Br. Surg. 2020, 107, 1659–1666. [Google Scholar]
- Bona, S.; Rosati, R.; Opocher, E.; Fiore, B.; Montorsi, M. Pain and quality of life after inguinal hernia surgery: A multicenter randomized controlled trial comparing lightweight vs heavyweight mesh (Supermesh Study). Updates Surg. 2018, 70, 77–83. [Google Scholar] [PubMed]
- Bakker, W.J.; Aufenacker, T.J.; Boschman, J.S.; Burgmans, J.P. Heavyweight mesh is superior to lightweight mesh in laparo-endoscopic inguinal hernia repair: A meta-analysis and trial sequential analysis of randomized controlled trials. Ann. Surg. 2021, 273, 890–899. [Google Scholar] [PubMed]
- DeBord, J.R. The historical development of prosthetics in hernia surgery. Surg. Clin. N. Am. 1998, 78, 973–1006. [Google Scholar]
- Amid, P. Classification of biomaterials and their related complications in abdominal wall hernia surgery. Hernia 1997, 1, 15–21. [Google Scholar]
- Trandafir, A.F.; Popa, D.E.; Vasile, D. Prostheses Used in Laparoscopic Inguinal Hernia Repair: Biocompatibility, Postoperative Complications and Quality of Life–Review of the Literature. Maedica 2017, 12, 202. [Google Scholar]
- Gavlin, A.; Kierans, A.S.; Chen, J.; Song, C.; Guniganti, P.; Mazzariol, F.S. Imaging and treatment of complications of abdominal and pelvic mesh repair. Radiographics 2020, 40, 432–453. [Google Scholar] [CrossRef]
- Spotnitz, W.D.; Falstrom, J.K.; Rodeheaver, G.T. The role of sutures and fibrin sealant in wound healing. Surg. Clin. N. Am. 1997, 77, 651–669. [Google Scholar] [CrossRef]
- Matthew, T.L.; Spotnitz, W.D.; Kron, I.L.; Daniel, T.M.; Tribble, C.G.; Nolan, S.P. Four years’ experience with fibrin sealant in thoracic and cardiovascular surgery. Ann. Thorac. Surg. 1990, 50, 40–43; discussion 43–44. [Google Scholar] [CrossRef] [PubMed]
- Byrne, D.J.; Hardy, J.; Wood, R.A.; McIntosh, R.; Cuschieri, A. Effect of fibrin glues on the mechanical properties of healing wounds. Br. J. Surg. 1991, 78, 841–843. [Google Scholar] [CrossRef]
- Katkhouda, N.; Mavor, E.; Friedlander, M.H.; Mason, R.J.; Kiyabu, M.; Grant, S.W.; Achanta, K.; Kirkman, E.L.; Narayanan, K.; Essani, R. Use of fibrin sealant for prosthetic mesh fixation in laparoscopic extraperitoneal inguinal hernia repair. Ann. Surg. 2001, 233, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi Tofigh, A.; Karimian Ghadim, M.; Bohlooli, M. Comparing suture with N-Hexyl Cyanoacrylate glue for mesh fixation in inguinal hernia repair, a randomised clinical trial. Am. J. Surg. 2021, 222, 203–207. [Google Scholar] [CrossRef]
- Mahardawi, B.; Jiaranuchart, S.; Rochanavibhata, S.; Siriwat, K.; Mattheos, N.; Pimkhaokham, A. Cyanoacrylate tissue adhesive versus silk sutures for mandibular third molar surgery: A systematic review and meta-analysis. Clin. Oral Investig. 2024, 28, 180. [Google Scholar] [CrossRef] [PubMed]
- Giray, C.B.; Atasever, A.; Durgun, B.; Araz, K. Clinical and electron microscope comparison of silk sutures and n-butyl-2-cyanoacrylate in human musosa. Aust. Dent. J. 1997, 42, 255–258. [Google Scholar] [CrossRef]
- Ayyıldız, S.N.; Ayyıldız, A. Cyanoacrylic tissue glues: Biochemical properties and their usage in urology. Urkish J. Urol. 2017, 43, 14. [Google Scholar] [CrossRef]
- Burati, M.; Scaini, A.; Fumagalli, L.A.; Gabrielli, F.; Chiarelli, M. Mesh fixation methods in groin hernia surgery. In Techniques and Innovation in Hernia Surgery; IntechOpen: Rijeka, Croatia, 2019; p. 8. [Google Scholar] [CrossRef]
- Rancke-Madsen, P.; Oberg, S.; Rosenberg, J. Mesh fixation in laparoscopic groin hernia repair: A comprehensive review of techniques and devices. Hernia 2025, 29, 105. [Google Scholar] [CrossRef]
- Wirth, U.; Saller, M.L.; von Ahnen, T.; Kockerling, F.; Schardey, H.M.; Schopf, S. Long-term outcome and chronic pain in atraumatic fibrin glue versus staple fixation of extra light titanized meshes in laparoscopic inguinal hernia repair (TAPP): A single-center experience. Surg. Endosc. 2020, 34, 1929–1938. [Google Scholar] [CrossRef]
- Smith, A.M.; Faulkner, J.D.; Chase, N.; Eckhauser, F.E.; Hope, W.W. The Effect of Tack Fixation Methods on Outcomes in Laparoscopic Ventral Hernia Repair. J. Laparoendosc. Adv. Surg. Tech. Part A 2021, 31, 779–782. [Google Scholar] [CrossRef]
- Reynvoet, E.; Berrevoet, F. Pros and cons of tacking in laparoscopic hernia repair. Surg. Technol. Int. 2014, 25, 136–140. [Google Scholar]
- LeBlanc, K.A. Tack hernia: A new entity. J. Soc. Laparoendosc. Surg. 2003, 7, 383–387. [Google Scholar]
- Firestone, D.E.; Lauder, A.J. Chemistry and mechanics of commonly used sutures and needles. J. Hand Surg. 2010, 35, 486–488, quiz 488. [Google Scholar] [CrossRef]
- Yag-Howard, C. Sutures, needles, and tissue adhesives: A review for dermatologic surgery. Dermatol. Surg. 2014, 40 (Suppl. 9), S3–S15. [Google Scholar] [CrossRef] [PubMed]
- Moy, R.L.; Waldman, B.; Hein, D.W. A review of sutures and suturing techniques. J. Dermatol. Surg. Oncol. 1992, 18, 785–795. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.J.; Kim, J.H.; Kim, C.H.; Lee, G.R.; Lee, Y.S.; Kim, H.J. Single incision laparoscopic totally extraperitoneal hernioplasty: Lessons learned from 1,231 procedures. Ann. Surg. Treat. Res. 2021, 100, 47–53. [Google Scholar] [CrossRef]
- Cugura, J.F.; Kirac, I.; Kulis, T.; Jankovic, J.; Beslin, M.B. First case of single incision laparoscopic surgery for totally extraperitoneal inguinal hernia repair. Acta Clin. Croat. 2008, 47, 249–252. [Google Scholar]
- Kim, J.H.; An, C.H.; Lee, Y.S.; Kim, H.Y.; Lee, J.I. Single incision laparoscopic totally extraperitoneal hernioplasty (SIL-TEP): Experience of 512 procedures. Hernia 2015, 19, 417–422. [Google Scholar] [CrossRef]
- Escobar Dominguez, J.E.; Gonzalez, A.; Donkor, C. Robotic inguinal hernia repair. J. Surg. Oncol. 2015, 112, 310–314. [Google Scholar] [CrossRef]
- Chen, Y.J.; Huynh, D.; Nguyen, S.; Chin, E.; Divino, C.; Zhang, L. Outcomes of robot-assisted versus laparoscopic repair of small-sized ventral hernias. Surg. Endosc. 2017, 31, 1275–1279. [Google Scholar] [CrossRef]
- Waite, K.E.; Herman, M.A.; Doyle, P.J. Comparison of robotic versus laparoscopic transabdominal preperitoneal (TAPP) inguinal hernia repair. J. Robot. Surg. 2016, 10, 239–244. [Google Scholar] [CrossRef] [PubMed]
- Kolachalam, R.; Dickens, E.; D’Amico, L.; Richardson, C.; Rabaza, J.; Gamagami, R.; Gonzalez, A. Early outcomes of robotic-assisted inguinal hernia repair in obese patients: A multi-institutional, retrospective study. Surg. Endosc. 2018, 32, 229–235. [Google Scholar] [CrossRef]
- Charles, E.J.; Mehaffey, J.H.; Tache-Leon, C.A.; Hallowell, P.T.; Sawyer, R.G.; Yang, Z. Inguinal hernia repair: Is there a benefit to using the robot? Surg. Endosc. 2018, 32, 2131–2136. [Google Scholar] [CrossRef] [PubMed]
- Xie, H.; Jia, Y.; Liu, S. Integration of artificial intelligence in clinical laboratory medicine: Advancements and challenges. Interdiscip. Med. 2024, 2, e20230056. [Google Scholar] [CrossRef]
- Abdelmoaty, W.F.; Dunst, C.M.; Neighorn, C.; Swanstrom, L.L.; Hammill, C.W. Robotic-assisted versus laparoscopic unilateral inguinal hernia repair: A comprehensive cost analysis. Surg. Endosc. 2019, 33, 3436–3443. [Google Scholar] [CrossRef]
- Huerta, S.; Garza, A.M. A Systematic Review of Open, Laparoscopic, and Robotic Inguinal Hernia Repair: Management of Inguinal Hernias in the 21st Century. J. Clin. Med. 2025, 14, 990. [Google Scholar]
- Sabbatini, F.; La Regina, D.; Murgante Testa, N.; Senatore, A.M.; Saporito, A.; Pini, R.; Mongelli, F. Hospital costs of robotic-assisted and open treatment of large ventral hernias. Sci. Rep. 2024, 14, 11523. [Google Scholar]
- Hung, T.Y.; Wu, C.C.; Chen, L.S.; Kang, Y.N. Safety of two common laparoscopic inguinal herniorrhaphy approaches: An updated systematic review with meta-analysis of randomized clinical trials. Transl. Androl. Urol. 2020, 9, 2007–2021. [Google Scholar] [CrossRef]
- Aiolfi, A.; Cavalli, M.; Del Ferraro, S.; Manfredini, L.; Lombardo, F.; Bonitta, G.; Bruni, P.G.; Panizzo, V.; Campanelli, G.; Bona, D. Total extraperitoneal (TEP) versus laparoscopic transabdominal preperitoneal (TAPP) hernioplasty: Systematic review and trial sequential analysis of randomized controlled trials. Hernia 2021, 25, 1147–1157. [Google Scholar] [CrossRef]
- Bracale, U.; Melillo, P.; Pignata, G.; Di Salvo, E.; Rovani, M.; Merola, G.; Pecchia, L. Which is the best laparoscopic approach for inguinal hernia repair: TEP or TAPP? A systematic review of the literature with a network meta-analysis. Surg. Endosc. 2012, 26, 3355–3366. [Google Scholar] [CrossRef]
- Löfgren, J.; Nordin, P.; Ibingira, C.; Matovu, A.; Galiwango, E.; Wladis, A. A Randomized Trial of Low-Cost Mesh in Groin Hernia Repair. N. Engl. J. Med. 2016, 374, 146–153. [Google Scholar] [CrossRef] [PubMed]
- Popa, F.; Rosca, O.; Georgescu, A.; Cannistra, C. Reconstruction of the Abdominal Wall in Anatomical Plans. Pre- and Postoperative Keys in Repairing “Cold” Incisional Hernias. Clujul Med. 2016, 89, 117–121. [Google Scholar] [CrossRef] [PubMed]
- Ünek, T.; Sökmen, S.; Egeli, T.; Avkan Oğuz, V.; Ellidokuz, H.; Obuz, F. The results of expanded-polytetrafluoroethylene mesh repair in difficult abdominal wall defects. Asian J. Surg. 2019, 42, 131–143. [Google Scholar] [CrossRef] [PubMed]
- Koehler, R.H.; Begos, D.; Berger, D.; Carey, S.; LeBlanc, K.; Park, A.; Ramshaw, B.; Smoot, R.; Voeller, G. Minimal adhesions to ePTFE mesh after laparoscopic ventral incisional hernia repair: Reoperative findings in 65 cases. J. Soc. Laparoendosc. Surg. 2003, 7, 335–340. [Google Scholar]
- Aydemir Sezer, U.; Sanko, V.; Gulmez, M.; Aru, B.; Sayman, E.; Aktekin, A.; Vardar Aker, F.; Yanıkkaya Demirel, G.; Sezer, S. Polypropylene composite hernia mesh with anti-adhesion layer composed of polycaprolactone and oxidized regenerated cellulose. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 99, 1141–1152. [Google Scholar] [CrossRef]
- Sanbhal, N.; Saitaer, X.; Li, Y.; Mao, Y.; Zou, T.; Sun, G.; Wang, L. Controlled Levofloxacin Release and Antibacterial Properties of β-Cyclodextrins-Grafted Polypropylene Mesh Devices for Hernia Repair. Polymers 2018, 10, 493. [Google Scholar] [CrossRef]
- Pérez-Köhler, B.; Benito-Martínez, S.; Rodríguez, M.; García-Moreno, F.; Pascual, G.; Bellón, J.M. Experimental study on the use of a chlorhexidine-loaded carboxymethylcellulose gel as antibacterial coating for hernia repair meshes. Hernia 2019, 23, 789–800. [Google Scholar] [CrossRef]
- Wang, Y.; Cao, Z.; Cheng, R.; Qin, M.; Zhang, D.; Deng, L.; Chen, X.; Cui, W. Immunomodulated electrospun fibrous scaffolds via bFGF camouflage for pelvic regeneration. Appl. Mater. Today 2019, 15, 570–581. [Google Scholar] [CrossRef]
- Miao, Y.-H.; Wang, X.; Zhao, X.-M.; Hu, Y.-W.; Liu, X.; Deng, D.-W. Co-assembly strategies of natural plant compounds for improving their bioavailability. Food Med. Homol. 2025, 2, 9420022. [Google Scholar] [CrossRef]
- Zhu, Y.X.; Jia, H.R.; Jiang, Y.W.; Guo, Y.; Duan, Q.Y.; Xu, K.F.; Shan, B.H.; Liu, X.; Chen, X.; Wu, F.G. A red blood cell-derived bionic microrobot capable of hierarchically adapting to five critical stages in systemic drug delivery. Exploration 2024, 4, 20230105. [Google Scholar] [CrossRef] [PubMed]
- Sanders, D.L.; Waydia, S. A systematic review of randomised control trials assessing mesh fixation in open inguinal hernia repair. Hernia 2014, 18, 165–176. [Google Scholar] [CrossRef]
- Sajid, M.S.; Ladwa, N.; Kalra, L.; McFall, M.; Baig, M.K.; Sains, P. A meta-analysis examining the use of tacker mesh fixation versus glue mesh fixation in laparoscopic inguinal hernia repair. Am. J. Surg. 2013, 206, 103–111. [Google Scholar] [CrossRef] [PubMed]
- Fenger, A.Q.; Helvind, N.M.; Pommergaard, H.C.; Burcharth, J.; Rosenberg, J. Fibrin sealant for mesh fixation in laparoscopic groin hernia repair does not increase long-term recurrence. Surg. Endosc. 2016, 30, 986–992. [Google Scholar] [CrossRef]
- Helvind, N.M.; Andresen, K.; Rosenberg, J. Lower reoperation rates with the use of fibrin sealant versus tacks for mesh fixation. Surg. Endosc. 2013, 27, 4184–4191. [Google Scholar] [CrossRef] [PubMed]
- Pascual, G.; Mesa-Ciller, C.; Rodríguez, M.; Pérez-Köhler, B.; Gómez-Gil, V. Pre-clinical assay of the tissue integration and mechanical adhesion of several types of cyanoacrylate adhesives in the fixation of lightweight polypropylene meshes for abdominal hernia repair. PLoS ONE 2018, 13, e0206515. [Google Scholar] [CrossRef]
- Agarwal, P.K.; Sutrave, T.; Kaushal, D.; Vidua, R.; Malik, R.; Maurya, A.P. Comparison of Postoperative Chronic Groin Pain After Repair of Inguinal Hernia Using Nonabsorbable Versus Absorbable Sutures for Mesh Fixation. Cureus 2023, 15, e35562. [Google Scholar] [CrossRef]
- Bilaloglu, M.H. The effect of absorbable vs. non-absorbable fixation on early complications in laparoscopic transabdominal preperitoneal (TAPP) inguinal herniorrhaphy. Ann. Med. Res. 2024, 31, 627–632. [Google Scholar]
- Khan, R.M.A.; Bughio, M.; Ali, B.; Hajibandeh, S.; Hajibandeh, S. Absorbable versus non-absorbable tacks for mesh fixation in laparoscopic ventral hernia repair: A systematic review and meta-analysis. Int. J. Surg. 2018, 53, 184–192. [Google Scholar] [CrossRef]
- Sajid, M.S.; McFall, M.R.; Whitehouse, P.A.; Sains, P.S. Systematic review of absorbable vs non-absorbable sutures used for the closure of surgical incisions. World J. Gastrointest. Surg. 2014, 6, 241–247. [Google Scholar] [CrossRef]
- Radu, P.; Brătucu, M.; Garofil, D.; Pasnicu, C.; Iorga, C.; Popa, F.; Strâmbu, V. Molecular factors of failure in incisional hernia surgery. Chirurgia 2013, 108, 193–198. [Google Scholar] [PubMed]
- Klosterhalfen, B.; Junge, K.; Klinge, U. The lightweight and large porous mesh concept for hernia repair. Expert Rev. Med. Devices 2005, 2, 103–117. [Google Scholar] [CrossRef] [PubMed]
- Radu, P.; Brătucu, M.; Garofil, D.; Goleanu, V.; Popa, F.; Strâmbu, V. The Role of Collagen Metabolism in the Formation and Relapse of Incisional Hernia. Chirurgia 2015, 110, 224–230. [Google Scholar]
- Parker, S.G.; Mallett, S.; Quinn, L.; Wood, C.P.J.; Boulton, R.W.; Jamshaid, S.; Erotocritou, M.; Gowda, S.; Collier, W.; Plumb, A.A.O.; et al. Identifying predictors of ventral hernia recurrence: Systematic review and meta-analysis. BJS Open 2021, 5, zraa071. [Google Scholar] [CrossRef]
- Arrey, E.; Young, T.; Alford, A. A Comprehensive Review of the Evolution of Minimally Invasive Hernia Repair: Historical Milestones to Modern Clinical Practice. Curr. Surg. Rep. 2024, 13, 2. [Google Scholar]
- McCormack, K.; Wake, B.; Perez, J.; Fraser, C.; Cook, J.; McIntosh, E.; Vale, L.; Grant, A. Laparoscopic surgery for inguinal hernia repair: Systematic review of effectiveness and economic evaluation. Health Technol. Assess. 2005, 9, 1–203, iii–iv. [Google Scholar] [PubMed]
- Li, X.; Li, Y.-J.; Dong, H.; Wang, D.-C.; Wei, J. Meta-analysis of the effectiveness and safety of robotic-assisted versus laparoscopic transabdominal preperitoneal repair for inguinal hernia. PLoS ONE 2024, 19, e0298989. [Google Scholar]
- Simons, M.P.; Aufenacker, T.; Bay-Nielsen, M.; Bouillot, J.L.; Campanelli, G.; Conze, J.; de Lange, D.; Fortelny, R.; Heikkinen, T.; Kingsnorth, A.; et al. European Hernia Society guidelines on the treatment of inguinal hernia in adult patients. Hernia 2009, 13, 343–403. [Google Scholar] [CrossRef]
- Lund, H.; Spanager, L.; Winther, A.C.R.; Gierløff, M.; Sunekær, K.; Kleif, J.; Bertelsen, C.A. Recurrence and complications after laparoscopic inguinal hernia repair using a self-adherent mesh: A patient-reported follow-up study. Surg. Endosc. 2025, 39, 2464–2470. [Google Scholar]
- Nikkolo, C.; Lillsaar, T.; Vaasna, T.; Kirsimägi, Ü.; Lepner, U. Ten-Year Results of Inguinal Hernia Open Mesh Repair. J. Abdom. Wall Surg. 2025, 4, 14384. [Google Scholar]
- Kabaoglu, B.; Sobutay, E.; Bilgic, C. Postoperative Outcomes and Recurrence Rate in Laparoscopic Tep Inguinal Hernia Repairs Using Partially Absorbable Meshes: A Retrospective Single-Surgeon Study Over a 5-Year Period. Med. Bull. Sisli Etfal Hosp. 2024, 58, 276–283. [Google Scholar]
- Viera, O.J.; Florin, J.L.; Morales, K.E. Long-term outcomes of robotic inguinal hernia repair (r-TAPP): A retrospective review of 434 consecutive cases by a single surgeon with 3–8 years of follow-up. J. Robot. Surg. 2025, 19, 1–5. [Google Scholar]
- Mulita, F.; Parchas, N.; Solou, K.; Tchabashvili, L.; Gatomati, F.; Iliopoulos, F.; Maroulis, I. Postoperative pain scores after open inguinal hernia repair: Comparison of three postoperative analgesic regimens. Med. Arch. 2020, 74, 355. [Google Scholar] [PubMed]
- Coppens, S.; Gidts, J.; Huynen, P.; Van De Velde, M.; Joshi, G. Pain management after open inguinal hernia repair: An updated systematic review and procedure-specific postoperative pain management (PROSPECT/ESRA) recommendations. Acta Anaesthesiol. Belg. 2020, 71 (Suppl. 1), 45–56. [Google Scholar]
- Sahoo, R.K.; Pradhan, A.; Samanta, P.; Senapati, L.K.; Satapathy, G.C. Effect of ultrasound-guided ilioinguinal-iliohypogastric nerve block on chronic pain in patients undergoing open inguinal hernia surgery under spinal anesthesia: A randomized double-blind study. Korean J. Pain 2024, 37, 332–342. [Google Scholar]



| Region | Most Common Technique | Laparoscopic Access | Robotic Access | Mesh Availability |
|---|---|---|---|---|
| North America | TAPP, TEP, OMR | Widespread | Moderate–High | Lightweight, composite, 3D meshes |
| Western Europe | TAPP, TEP, SIL-TEP, OMR | Widespread | Moderate | Broad access to all mesh types |
| Eastern Europe | TAPP, TEP, OMR | Moderate | Low | Mainly polypropylene |
| Latin America | OMR, some TEP | Limited–Moderate | Rare | Limited lightweight mesh options |
| Sub-Saharan Africa | OMR | Rare | Very Rare | Often lacks mesh or uses reused polypropylene |
| South and Southeast Asia | OMR, some TAPP/TEP | Limited | Rare | Highly variable by country and institution |
|
|
|
|
|
|
|
|
| Non-mesh techniques |
|
| Open mesh techniques |
|
| Laparo-endoscopic mesh techniques |
|
| Indications | Contraindications |
|---|---|
|
|
| Intraoperative | Postoperative |
|---|---|
|
|
| Pore Type | Pore Size (mm) |
|---|---|
| Microporous | <0.10 |
| Small pore | 0.10–0.60 |
| Medium pore | 0.60–1 |
| Large pore | 1–2 |
| Very large pore | >2 |
| Mesh Type | Density (g/m2) | Advantages | Disadvantages | Ref. |
|---|---|---|---|---|
| Ultra-lightweight | <35 |
|
| [87] |
| Lightweight | 35–50 |
|
| [94] |
| Medium weight | 50–90 |
|
| [92] |
| Heavyweight | >90 |
|
| [92] |
| Type of Mesh | Material | Constitution | Pore Size (mm) | Properties |
|---|---|---|---|---|
| Totally macroporous | Polypropylene | Monofilament | >3 mm |
|
| Totally microporous | Polytetrafluoroethylene (CTPTFE) | Patches | <0.06 |
|
| Macroporous with microfilament or microporous components | Polyester | Plated multifilament threads | >0.075 |
|
| Mixed | Polypropylene + polyglecaprone-25 (Ultrapro) | Monofilament | >3 mm |
|
| Polypropylene + titanium (Ti-Mesh) | Monofilament | >1 mm |
| |
| Polyester + collagen layer (composite) | Multifilament | >3 mm |
| |
| Polyester + polylactic acid micro-grips (Progrip) | Monofilament | >1 mm |
|
| Fixation Type | Manufacturer | Material |
|---|---|---|
| Fibrin Glue | Tissel (Baxter, Bucharest, Romania) | Human fibrinogen, synthetic aprotinin, human albumin, L-histidine, niacinamide, polysorbate 80, sodium citrate dihydrate, human thrombin, calcium chloride dihydrate, sodium chloride, water |
| Evicel (RxList, Bucharest, Romania) | Human fibrinogen, arginine hydrochloride, glycine, sodium chloride, sodium citrate, calcium chloride, human thrombin, human albumin, mannitol, sodium acetate, water | |
| Cyanoacrylic Glue | Histoacryl (B. Braun, Bucharest, Romania) | Monomeric N-butyl-2-cyanoacrylate |
| Glubran-2 (GEM Srl, Bucharest, Romania) | N-butyl-2-cyanoacrylate, Monomeric N-butyl-2-cyanoacrylate |
| Product (Manufacturer) | Type of Tack | Material | Penetration Length | Resorption |
|---|---|---|---|---|
| ProTack (MedTronic, Bucharest, Romania) | Helical titanium tack | Titanium | 3–4 mm | Non-absorbable |
| PermaFix (Bard, Bucharest, Romania) | Helical nontitanium | Polyacetal | 6.7 mm | Non-absorbable |
| AbsorbaTack (Medtronic, Bucharest, Romania) | Absorbable | Poly(glycolide-co-L-lactide) | 5.1 mm | 3–5 months (complete 12 months) |
| PermaSorb (Bard, Bucharest, Romania) | Absorbable | Poly(D,L)-lactide | 6.4 mm | 16 months |
| SorbaFix (Bard, Bucharest, Romania) | Absorbable | Poly(D,L)-lactide | 6.7 mm | 12 months |
| Product | Manufacturer | Type of Suture | Material |
|---|---|---|---|
| Nylon Ethilon | Dolphin Sutures Ethicon, Bucharest, Romania | Non-absorbable | Polyamide |
| Prolene Surgipro | J&J, Ethicon Covidien, Bucharest, Romania | Non-absorbable | Polypropylene |
| Ti-Cron | Covidien, Bucharest, Romania | Non-absorbable | Polyester |
| Monocryl | J&J Ethicon, Bucharest, Romania | Absorbable | Poliglecaprone |
| Polysorb | Covidien, Bucharest, Romania | Absorbable | LactomerTM |
| Vicryl | J&J, Orion Sutures, Bucharest, Romania | Absorbable | Polyglactin |
| Dexon | N.A, Medtronic, Bucharest, Romania | Absorbable | Polyglycolic acid |
| Maxon | Medtronic, Bucharest, Romania | Absorbable | Polyglyconate |
| PDS | J&J, Ethicon, Bucharest, Romania | Absorbable | Polydioxanone |
| Technique | Advantages | Disadvantages |
|---|---|---|
| Open (Lichtenstein) |
|
|
| TAPP |
|
|
| TEP |
|
|
| SIL-TEP |
|
|
| Robotic |
|
|
| Technique/Mesh Type | Chronic Pain | Recurrence Rate | Foreign-Body Sensation | References |
|---|---|---|---|---|
| Open mesh (Lichtenstein) | Up to ~15% | ~1.8–2.1% (5–10 y) | Moderate–high | [159] |
| Lightweight mesh (open/laparoscopic) | Reduced vs. HW mesh | Similar to HW | Low | [160] |
| TEP + partially absorbable mesh | 0% | 0.67% at 5 y | None reported | [161] |
| r-TAPP + mesh | Low | ~0.5% | Low | [162] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tigora, A.; Radu, P.A.; Garofil, D.N.; Bratucu, M.N.; Zurzu, M.; Paic, V.; Ioan, R.G.; Surlin, V.; Margaritescu, D.; Badoiu, S.C.; et al. Modern Perspectives on Inguinal Hernia Repair: A Narrative Review on Surgical Techniques, Mesh Selection and Fixation Strategies. J. Clin. Med. 2025, 14, 4875. https://doi.org/10.3390/jcm14144875
Tigora A, Radu PA, Garofil DN, Bratucu MN, Zurzu M, Paic V, Ioan RG, Surlin V, Margaritescu D, Badoiu SC, et al. Modern Perspectives on Inguinal Hernia Repair: A Narrative Review on Surgical Techniques, Mesh Selection and Fixation Strategies. Journal of Clinical Medicine. 2025; 14(14):4875. https://doi.org/10.3390/jcm14144875
Chicago/Turabian StyleTigora, Anca, Petru Adrian Radu, Dragos Nicolae Garofil, Mircea Nicolae Bratucu, Mihai Zurzu, Vlad Paic, Raluca Gabriela Ioan, Valeriu Surlin, Dragos Margaritescu, Silviu Constantin Badoiu, and et al. 2025. "Modern Perspectives on Inguinal Hernia Repair: A Narrative Review on Surgical Techniques, Mesh Selection and Fixation Strategies" Journal of Clinical Medicine 14, no. 14: 4875. https://doi.org/10.3390/jcm14144875
APA StyleTigora, A., Radu, P. A., Garofil, D. N., Bratucu, M. N., Zurzu, M., Paic, V., Ioan, R. G., Surlin, V., Margaritescu, D., Badoiu, S. C., Popa, F., Strambu, V., & Ramboiu, S. (2025). Modern Perspectives on Inguinal Hernia Repair: A Narrative Review on Surgical Techniques, Mesh Selection and Fixation Strategies. Journal of Clinical Medicine, 14(14), 4875. https://doi.org/10.3390/jcm14144875