Refining Patient Selection Criteria for LV-Only Fusion Pacing in Cardiac Resynchronization Therapy: A Systematic Review
Abstract
1. Introduction
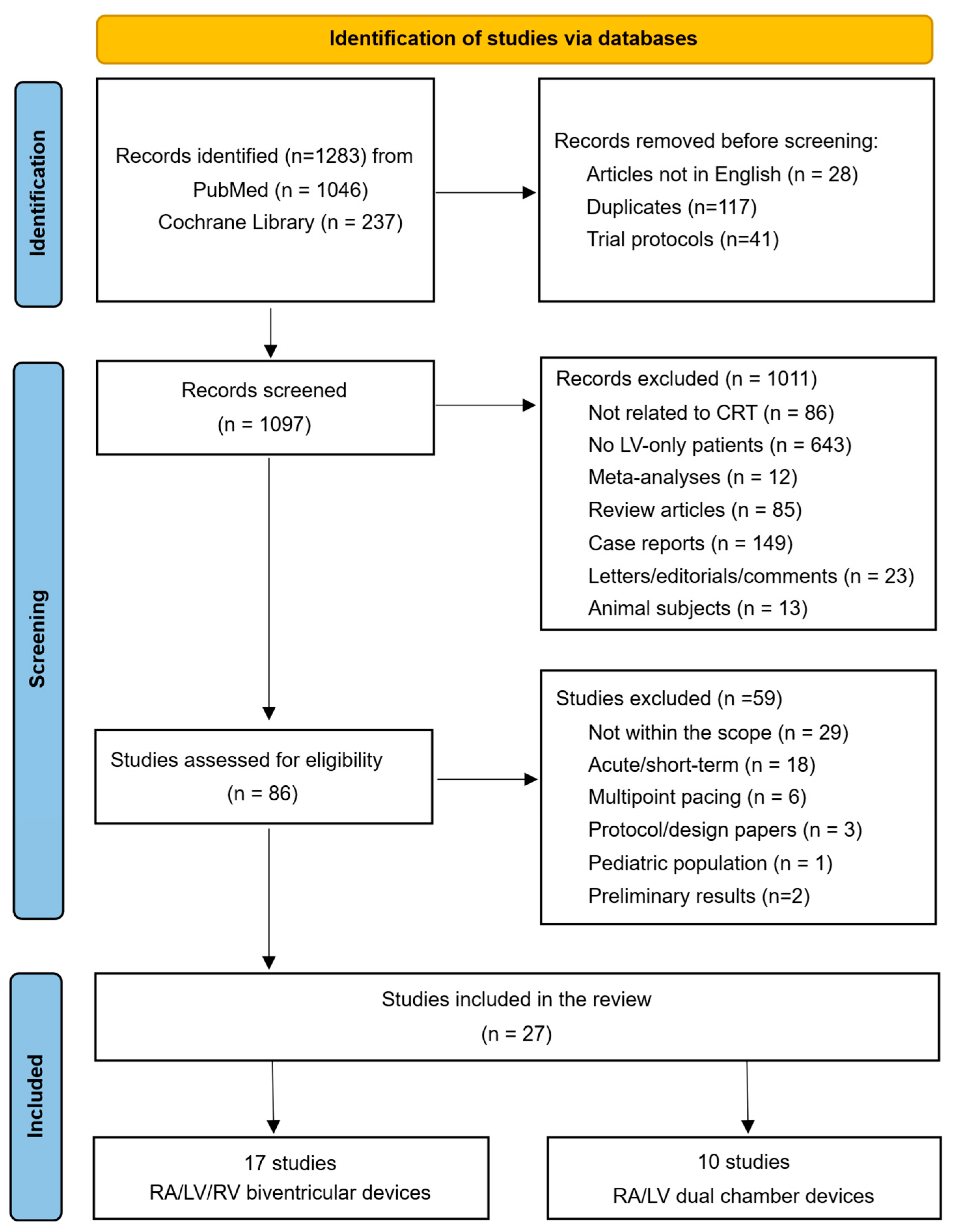
2. Materials and Methods
- –
- P (Population): adult HF patients, LVEF ≤ 35%, symptomatic despite GDMT, and with evidence of electrical dyssynchrony (LBBB with QRS duration ≥ 120 ms);
- –
- I (Intervention): LV-only fCRTp achieved either through RA/LV/RV biventricular devices or RA/LV dual-chamber devices (without a right ventricular lead);
- –
- C (Comparison): conventional BiVp;
- –
- O (Outcomes): all-cause and cardiovascular mortality, functional response to CRT, echocardiographic reverse remodeling, super-responder rates, heart failure-related hospitalizations, incidence of arrhythmic events or need for device upgrade to either CRT-D or BiVp in LV-only fCRTp groups, and device longevity.
3. Results
| Scheme 2001. | Trial Name (if Applicable) | Study Design | Number of Patients | Inclusion Criteria | Exclusion Criteria for LV Only | FOI Method | FU | Results |
|---|---|---|---|---|---|---|---|---|
| Touiza, 2001 [15] | - | prospective, observational | total = 33 LV-only fCRTp = 18 | ischemic and nonischemic etiology, QRS ≥ 140 ms, LBBB, SR | - | Echo | 6 months | similar response; mortality: 7 deaths (3 in BiV, 4 in LV group); only 1 sudden death (BiV group) |
| Etienne, 2001 [16] | - | prospective, observational | total = 23 LV-only fCRTp = 13 | ischemic and nonischemic etiology, NYHA class III–IV LVEF ≤ 40%, LBBB | patients without hemodynamic improvement during acute LV-based pacing (test response) | Echo | 8 ± 3 months | similar response; no difference in response between patients in SR and AF |
| Gasparini, 2006 [17] | BELIEVE | parallel-group randomized controlled trial | total = 69 LV-only fCRTp = 36 | ischemic and nonischemic etiology; NYHA class II-IV, LBBB, ICD indication | AF; pm dependency | Echo | 12 months | similar response; no difference in adverse events; no AV block at FU |
| Rao, 2007 [13] | DECREASE-HF | parallel-group randomized controlled trial | total = 306 BiVp (vv = 0) vs. BiV (VVi optimized) vs. LV-only fCRT 1:1:1 | ischemic and nonischemic etiology; NYHA class III-IV; ICD indication; 2 weeks of VDD mode before randomization | β-blocker therapy for ≤90 days | None | 6 months | similar response; BiV (VV = 0) demonstrated a greater reduction in LVESD; no significant differences in adverse events |
| Sirker, 2007 [18] | LOLA-ROSE | crossover randomized controlled trial | total = 18 | ischemic and nonischemic etiology; NYHA class III-IV; LBBB, LV mechanical dyssynchrony | - | Echo | 2 × 8 weeks | similar response; no difference in adverse events |
| Valzania, 2008 [19] | - | parallel-group randomized controlled trial | total = 22 | ischemic and nonischemic etiology, NYHA class III-IV, LBBB | AF; 2nd- or 3rd-degree AV block, severe renal dysfunction | Echo | 3 months | similar response |
| Boriani, 2010 [20] | B-LEFT HF | parallel-group randomized controlled trial | total = 176 LV-only fCRT = 86 | ischemic and nonischemic etiology; NYHA class III-IV; ICD indication | AF, 2nd- or 3rd-degree AV block, pacing for bradycardia | - | 6 months | similar response; no difference in adverse events |
| Sedlacek, 2010 [21] | - | parallel-group randomized controlled trial | total = 40 | nonischemic etiology; NYHA class III-IV | AF, 2nd- or 3rd-degree AV block, pacing for bradycardia | Echo | 3 years | greater improvement in LVEF, LV remodeling in BiVp; higher CV mortality in LV-only fCRT, higher incidence of arrhythmic events, and a greater necessity for CRT-D upgrade in LV-only fCRT |
| Thibault, 2011 [14] | GREATER-EARTH | crossover randomized controlled trial | total = 121 | ischemic and nonischemic etiology, 6 min walk test distance ≤ 400 m, ICD indication, PRi ≤ 250 ms; Ap/Vp < 5% in “run-in” test; | AF, 2nd- or 3rd-degree AV block, pacing for bradycardia | EKG | 2 × 6 months | similar response; 17.1% of BiV non-responders improved with LV-only fCRTp |
| Martin, 2012 [22] | ADAPTIV-CRT | parallel-group randomized controlled trial | total = 522 aBiV/LV-only fCRTp = 219 | ischemic and nonischemic etiology, NYHA class III-IV, ICD indication | AF, 2nd- and 3rd-degree AV block | aCRT | 9.7 ± 3 months | significant improvement in clinical response in aCRT group; no significant difference in cardiac output, HF hospitalizations, or mortality rates between aCRT and BiV pacing (Echo-optimized) |
| Birnie, 2013 [23] | ADAPTIV-CRT | parallel-group randomized controlled trial | total = 478 aBiV/LV-only fCRTp = 142 | ischemic and nonischemic etiology, ICD indication | AF | aCRT | 1 year | LV-only fCRTp ≥ 50%: a greater improvement in NYHA class, LVEF, and LV remodeling; significant reduction in mortality and HF hospitalizations |
| Burns, 2017 [24] | ADAPTIV-CRT | parallel-group randomized controlled trial | total = 161 LV-only fCRTp = 70 | ischemic and nonischemic etiology, LBBB, ICD indication | AF, AV block | aCRT | 12 months | greater improvement in LVEF, global LV myocardial strain in septal and apical regions with LV-only pacing; 77% responders in the LV-only group; (66% responders in BiV group) |
| Faghfourian, 2017 [25] | - | crossover randomized controlled trial | total = 44 | ischemic and nonischemic etiology, NYHA class II-IV, LBBB: 38 patients Non-LBBB: 7 patients, “preserved AV conduction” | AF, 3rd-degree AV block | - | 2 × 3 months | similar response |
| Gwag, 2019 [26] | - | retrospective, observational | total = 155 convBiV = 129; aBiV = 11; aLV-only fCRT = 15 | ischemic and nonischemic etiology, NYHA class II-IV, LBBB; CRT-D (94.2%) | AF | aCRT/Echo/EKG in convBiV | 27.5 months | LVEF after 6 months: highest in aLV-only fCRT group; super-responders: 58.3% in aLV-only fCRT vs. 36.3% in aCRTBiV vs. convBiV 14.3%; no SCD or ICD upgrades in LV-only fCRTp group |
| Hsu, 2019 [27] | - | retrospective, observational | total = 37.450 aCRT = 11.566 convBiV = 25.884 | ischemic and nonischemic etiology, NYHA class II-IV | AF | aCRT | 15.5± 9.1 months | higher LV-only fCRTp % was associated with lower AF incidence: 1.3% AF (LV-only > 92%) vs. 22.4% AF (0–5% LV-only) |
| Su, 2021 [28] | - | parallel-group randomized controlled trial | total = 73 nLV-only fCRTp = 34 | ischemic and nonischemic etiology, NYHA class II-III; LBBB, PRi ≤ 200 ms | AF, AV block, pacing for bradycardia | aCRT * | 6 months | higher super-response rate in LV-only fCRTp group (68.4%) vs. BiVp group (36.4%); no significant differences in adverse outcomes |
| Vatasescu, 2023 [29] | - | retrospective, observational | total = 622 nLV-only fCRTp = 408 | ischemic and nonischemic etiology; NYHA class II-IV; PRi < 250 ms; Wenckebach point during atrial pacing < 500 ms | AF | EKG | 6 months | the super-responder rate was highest in the LV-only pacing (41.91%) compared with BiVp group (9.81%) |
| Study, Year | Trial Name (if Applicable) | Number of LV-Only Patients | Inclusion Criteria | Exclusion Criteria | Type of Device Used | FOI Method | FU | Outcomes |
|---|---|---|---|---|---|---|---|---|
| Auricchio, 2003 [30] | PATH-CHF II trial | 86 | ischemic and nonischemic etiology; NYHA class III-IV | AF, 2nd- or 3rd-degree AV block, indication for pacing or ICD therapy | RA/LV DDD device | Echo | 1–3 months -pacing ON/1 month-pacing OFF | in patients with QRSd ≥ 150 ms, there is a significant increase in LVEF, improvement in NYHA class and quality of life, no AV block, and no need for upgrade to ICD |
| Blanc, 2004 [31] | PATH-CHF II trial | 22 | ischemic and nonischemic etiology, NYHA class III-IV, LBBB | AF, AV block, indication for pacing | RA/LV DDD device | Echo | 12 months | NYHA class improved by 40%; LVEF improved by 22%; reverse remodeling (LVEDD reduced by 5%, LVESD reduced by 10%, MR area reduced by 40%; mortality: 23% (5 from HF, 2 SCD) |
| Butter, 2006 [32] | - | 29 | ischemic and nonischemic etiology, NYHA class III-IV; LBBB | AF, 2nd- or 3rd-degree AV block, ICD indication | RA/LV VDD device | EKG | 12 months | LVEF increased from 23 ± 7% to 34 ± 9%; QRS duration shortened significantly from 167 ± 21 ms to 140 ± 17 ms; clinical improvement: NYHA class, no AV block, no need for upgrade to ICD |
| Gopi, 2014 [33] | - | 5 | nonischemic DCM, NYHA class III-IV, LBBB | AF, AV block, bradycardia, renal dysfunction (serum creatinine > 1.5 mg/dL) | RA/LV VDD device | EKG | 6 months | QRS d shortened from 174 ± 17 ms to 128 ± 10.9 ms; LVEF improved from 25 ± 6% to 34 ± 6%; reverse remodeling (LVEDD decreased from 73.2 ± 12 mm to 65.8 ± 9.6 mm; LVESD reduced from 65 ± 12 mm to 54 ± 10 mm; no complications |
| Zhao, 2017 [34] | - | 30 | ischemic and nonischemic etiology, NYHA class II-IV, LBBB; “preserved AV conduction” | AV block | RA/RV/LV device with deactivated RV lead; RA/LV DDD device | EKG and Echo/RAAVD | 7.86 ± 3.67 months | clinical improvement: NYHA class, 6MWT, peak VO2; LVEF improved by 9.7%(conv BiVp 6.8%); QRS d shortened from 167.5 ms to 139.2 ms in LV-only fCRTp; no significant difference in mortality |
| Pu, 2017 [35] | - | 36 | ischemic and nonischemic etiology, NYHA class II-IV, LBBB; PQ interval < 220 ms | AF, 2nd- or 3rd-degree AV block | RA/RV/LV device with deactivated RV lead; RA/LV DDD device | EKG and Echo/RAAVD | 13 months | QRS d shortened from 182 ± 20 ms to 132 ± 9.8 ms; LVEF improved from 27% ± 6% to 41% ± 9%; reverse remodeling (MRA reduction from 4.3 ± 1.2 cm2 to 1.9 ± 1.1 cm2); no significant difference in mortality; device longevity comparison between LV-only fusion p and convBiV-CRT: 6.9 ± 0.3 years to 3.7 ± 0.2 years |
| Cozma, 2018 [36] | - | 55 | nonischemic etiology, NYHA class II-III, LBBB; PRi ≤ 250 ms | ischemic etiology, AF, 2nd- or 3rd-degree AV block/ history of syncope/ Wenckebach point < 500 ms; ICD indication | RA/LV DDD device | EKG during rest and during stress test | 35 ± 18 months | significant LV reverse remodeling (LVEDV decreased from 243.2 ± 82 mL to 193.7 ± 81 mL; reduction in mitral regurgitation severity was noted in 38 patients (69%); LVEF improved from 27 ± 5.2% to 38 ± 7.9%; one patient developed 2nd degree AV block and upgrade to a triple-chamber CRT-p needed |
| Goanta, 2022 [37] | - | 83 | nonischemic etiology, NYHA class II-III, LBBB; PRi < 240 ms | ischemic etiology, AF, 2nd- or 3rd-degree AV block/ ICD indication; Wenckebach point < 500 ms; structural cardiomyopathies or channelopathies with a risk of SCD | RA/LV DDD device | EKG during rest and during stress test | 6 months | SRs: 25/83 (31%) with ≥30% LVESV reduction and LVEF ≥ 45%; SR predictors: higher baseline LVEF, lower PASP, less severe MR; no SCD in SR group; no need for ICD upgrade |
| Gurgu, 2024 [38] | - | 62 | nonischemic etiology, NYHA class III-IV, LBBB; preserved AV conduction | ischemic etiology, AF, ICD indication | RA/LV DDD device | Echo | 45 ± 19 months. | LVEF, NYHA class, significantly improved; improvement in diastolic dysfunction (64%); CRT-P upgrade for AV block: 4 pts (5.5%); no SCD, and no ICD upgrades were required |
| Vacarescu, 2024 [39] | - | 73 | nonischemic etiology, NYHA class II-III, LBBB; PRi < 240 ms | ischemic etiology, AF, 2nd- or 3rd-degree AV block/history of syncope; ICD indication; structural cardiomyopathies or channelopathies with a risk of SCD | RA/LV DDD device | EKG during rest and during stress test | 6.4 years ± 27 months | LVEF improved from 27.9 ± 5.1% to 40.4 ± 8.5%; reduction in mitral regurgitation severity in 69%; LA responder = significant LA reverse remodeling; AV block and upgrade to a triple-chamber CRT-p needed in 4%; mortality: 7% (2 from HF/3 extracardiac causes) |
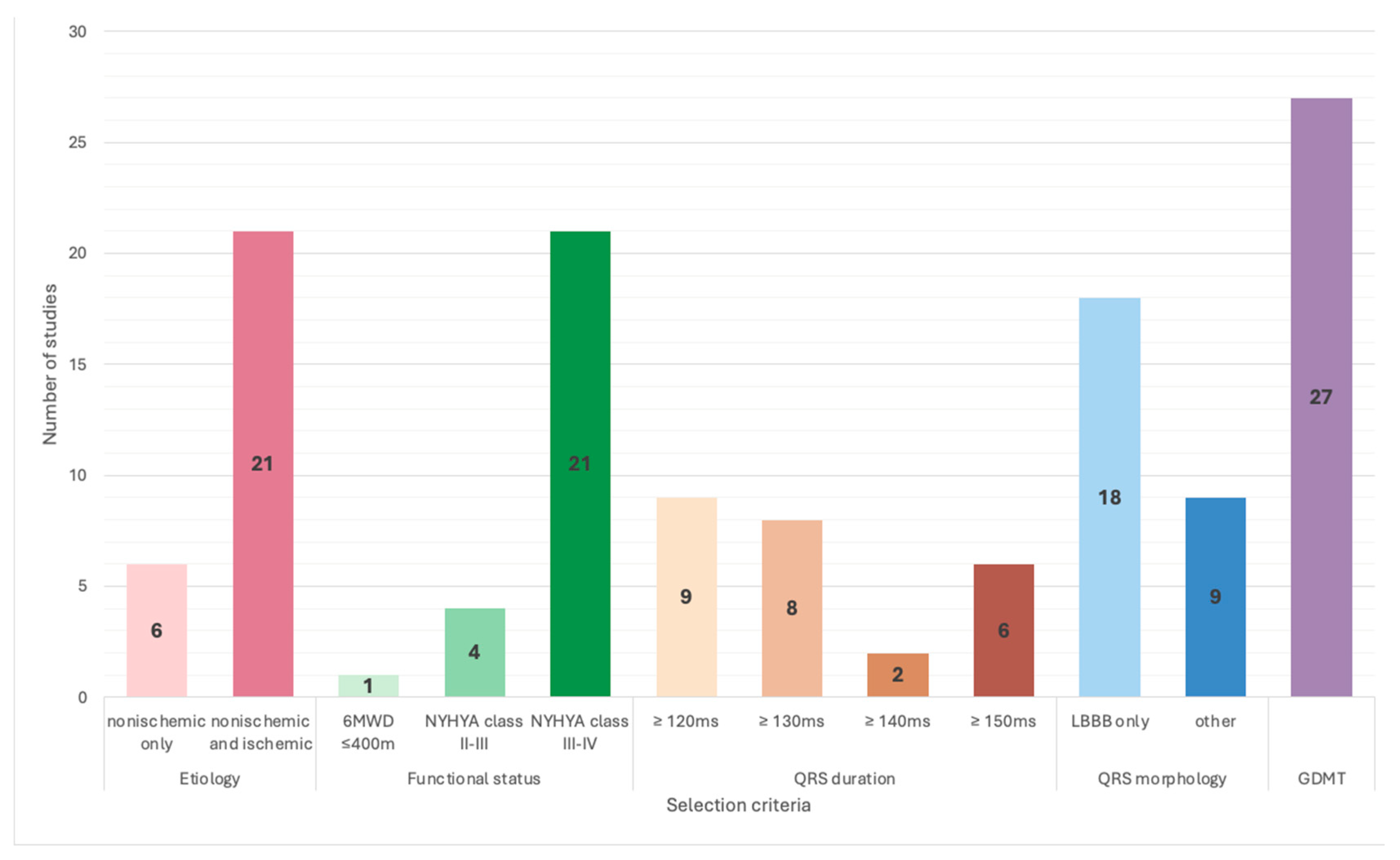
| Specific Selection Criteria for LV Only | ||||||||
|---|---|---|---|---|---|---|---|---|
| Inclusion Criteria | Exclusion Criteria | |||||||
| Criteria | Number of Studies | References | Criteria | Number of Studies | References | |||
| sinus rhythm | 26/27 | [13,14,15,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39]; | atrial fibrillation | paroxysmal | 9/27 | [17,25,26,31,33,35,37,38,39] | ||
| persistent | 9/27 | [14,17,20,21,25,26,28,33,35] | ||||||
| permanent | 24/27 | [14,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,38,39] | ||||||
| Normal AV conduction | PR interval functional tests | PRi ≤ 200 ms | 9/27 | [22,23,24,26,27,28,30,32,34] | chronotropic insufficiency/pacing for bradycardia/pacemaker dependency | 10/27 | [13,14,17,19,20,21,22,28,31,33] | |
| PRi < 240 ms | 2/27 | [37,39] | heart rate at baseline > 100 bpm | 5/27 | [22,23,24,26,27] | |||
| PRi < 250 ms | 3/27 | [14,29,36] | 2nd- or 3rd-degree AV block | 18/27 | [14,19,20,21,22,24,25,28,30,31,32,33,34,35,36,37,38,39] | |||
| PQi < 220 ms | 1/27 | [35] | others | |||||
| Wenckebach point during atrial pacing > 500 ms | 3/27 | [29,36,37] | structural cardiomyopathies or channelopathies with a risk of SCD | 2/27 | [37,39] | |||
| 2–8 weeks of “run-in” test before randomization | 1/27 | [14] | LV dyssynchrony on Echo | 1/27 | [18] | |||
| improvement during acute LV-based pacing (test response) before CRT | 1/27 | [16] | ICD indication in secondary prevention (for RA/LV devices) | 6/10 | [30,32,36,37,38,39] | |||
| 2 weeks of VDD testing mode before randomization | 1/27 | [13] | Severe renal dysfunction | 3/27 | [19,33,34] | |||
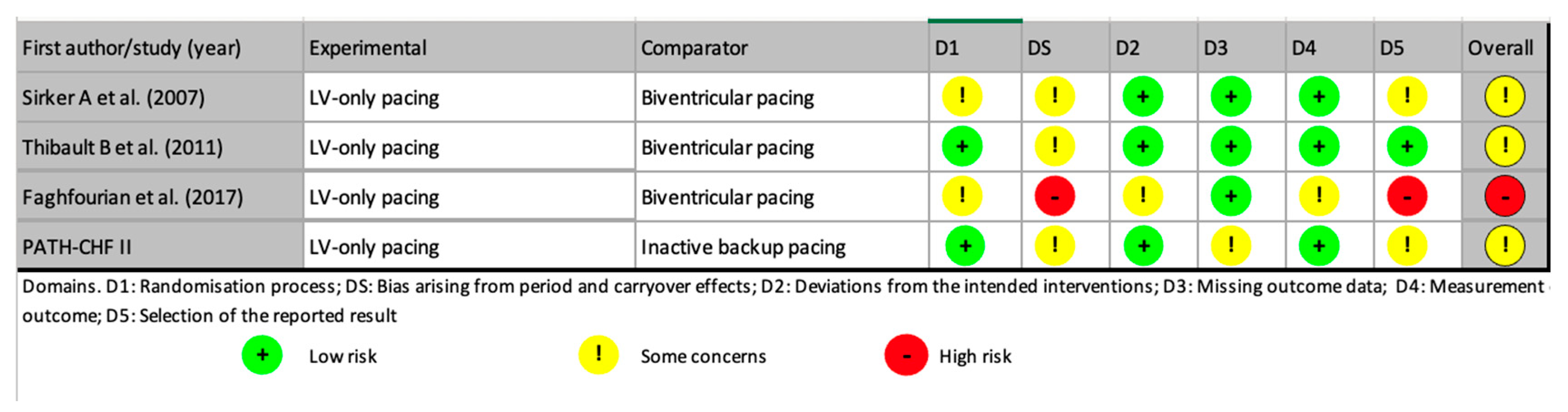
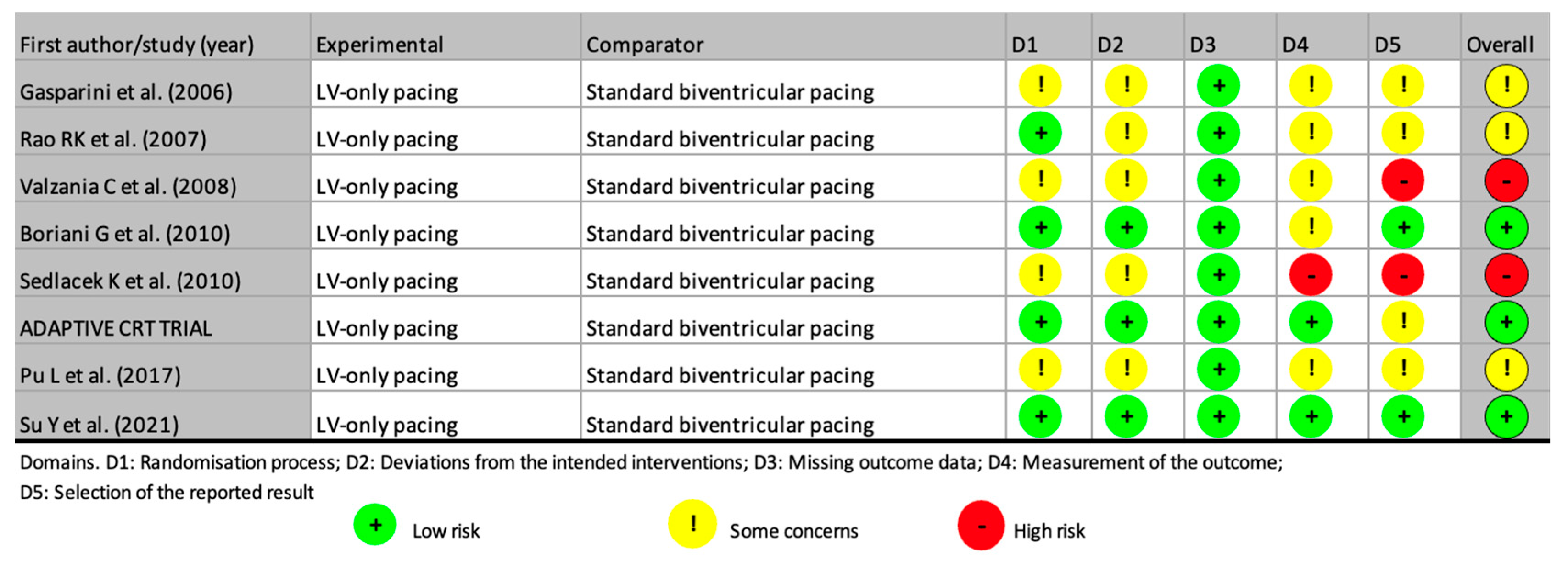
- Risk of bias and quality assessment
3.1. Studies Reporting Similar Outcomes Between LV-Only Fusion Pacing and BiVp
3.2. Studies Reporting Inferior Outcomes with LV-Only Fusion Pacing
3.3. Studies Reporting Superior Outcomes with LV-Only Fusion Pacing
3.4. True LV-Only Fusion CRT Using RA/LV Dual-Chamber Devices
4. Discussion
4.1. Variability in Defining Preserved AV Conduction
4.2. Ischemic vs. Nonischemic Cardiomyopathy: Implications for LV-Only Fusion CRT
4.3. Managing Supraventricular Arrhythmias to Preserve Constant Fusion Pacing
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| aCRT | adaptive cardiac resynchronization therapy |
| AF | atrial fibrillation |
| AV | atrioventricular |
| AV block | atrioventricular block |
| BiVp | biventricular pacing |
| CCS | clinical composite score |
| convBiVp | conventional biventricular pacing |
| CRT-D | cardiac resynchronization therapy with defibrillator |
| CRT-P | cardiac resynchronization therapy with pacemaker |
| DCM | dilated cardiomyopathy |
| DDD device | dual-chamber pacing device |
| DSP | desmoplakin gene |
| Echo | echocardiography |
| EKG | electrocardiogram |
| fCRTp | cardiac resynchronization therapy with fusion pacing |
| FOI | fusion-optimized interval |
| FLNC | filamin C gene |
| FU | follow-up |
| HF | heart failure |
| HR | hazard ratio |
| HFrEF | heart failure with reduced ejection fraction |
| ICD | implantable cardioverter-defibrillator |
| PRi | PR interval |
| LA | left atrium |
| LMNA | laminin A/C gene |
| LV | left ventriclel |
| LVEDD | left ventricular end-diastolic diameter |
| LVEDV | left ventricular end-diastolic volume |
| LGE | late gadolinium enhancement |
| LVEF | left ventricular ejection fraction |
| LVESD | left ventricular end-systolic diameter |
| LVESV | left ventricular end-systolic volume |
| MR | mitral regurgitation |
| MRA | mitral regurgitation area |
| MRI | magnetic resonance imaging |
| NYHA | New York Heart Association |
| PASP | pulmonary artery systolic pressure |
| pm dependency | pacemaker dependency |
| PQ interval/PRi | PR intervalPR interval |
| QRSd | QRS duration |
| RA | right atrium |
| RAAVD | right atrial-based AV delay optimization |
| RV | right ventricle |
| SCD | sudden cardiac death |
| SR | sinus rhythm/super-responder (depending on context) |
| VDD | pacing mode with atrial sensing and ventricular pacing |
| Vp | ventricular pacing |
| VV = 0 | interventricular pacing delay (simultaneous BiV pacing) |
| 6MWT | 6 min walk test |
| VO2 | oxygen consumption |
Appendix A
References
- Dhesi, S.; Lockwood, E.; Sandhu, R.K. Troubleshooting Cardiac Resynchronization Therapy in Nonresponders. Can. J. Cardiol. 2017, 33, 1060–1065. [Google Scholar] [CrossRef] [PubMed]
- Gage, R.M.; Burns, K.V.; Vatterott, D.B.; Kubo, S.H.; Bank, A.J. Pacemaker optimization in nonresponders to cardiac resynchronization therapy: Left ventricular pacing as an available option. PACE—Pacing Clin. Electrophysiol. 2012, 35, 685–694. [Google Scholar] [CrossRef] [PubMed]
- Tops, L.F.; Schalij, M.J.; Bax, J.J. The Effects of Right Ventricular Apical Pacing on Ventricular Function and Dyssynchrony. Implications for Therapy. J. Am. Coll. Cardiol. 2009, 54, 764–776. [Google Scholar] [CrossRef] [PubMed]
- Birnie, D.H.; Tang, A.S.L. If it is not broken, don’t fix it: Avoidance of right ventricular pacing in cardiac resynchronization therapy. J. Cardiovasc. Electrophysiol. 2017, 18, 505–506. [Google Scholar] [CrossRef]
- Lee, K.L.; Burnes, J.E.; Mullen, T.J.; Hettrick, D.A.; Tse, H.F.; Lau, C.P. Avoidance of right ventricular pacing in cardiac resynchronization therapy improves right ventricular hemodynamics in heart failure patients. J. Cardiovasc. Electrophysiol. 2007, 18, 497–504. [Google Scholar] [CrossRef] [PubMed]
- Varma, N.; Jia, P.; Ramanathan, C.; Rudy, Y. RV electrical activation in heart failure during right, left, and biventricular pacing. JACC Cardiovasc. Imaging 2010, 3, 567–575. [Google Scholar] [CrossRef]
- Burri, H.; Prinzen, F.W.; Gasparini, M.; Leclercq, C. Left univentricular pacing for cardiac resynchronization therapy. Europace 2017, 19, 912–919, Erratum in Europace, 2017, 19, 1415. [Google Scholar] [CrossRef]
- Varma, N. Therapy for cardiac resynchronization: When left ventricular-only “fusion” pacing is not enough. HeartRhythm Case Rep. 2020, 6, 963–964. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sapp, J.L.; Sivakumaran, S.; Redpath, C.J.; Khan, H.; Parkash, R.; Exner, D.V.; Healey, J.S.; Thibault, B.; Sterns, L.D.; Lam, N.H.N.; et al. Long-Term Outcomes of Resynchronization-Defibrillation for Heart Failure. N. Engl. J. Med. 2024, 390, 212–220. [Google Scholar] [CrossRef]
- Bordachar, P.; Lafitte, S.; Reuter, S.; Garrigue, S.; Sanders, P.; Roudaut, R.; Jaïs, P.; Haïssaguerre, M.; Clementy, J. Biventricular pacing and left ventricular pacing in heart failure: Similar hemodynamic improvement despite marked electromechanical differences. J. Cardiovasc. Electrophysiol. 2004, 15, 1342–1347. [Google Scholar] [CrossRef]
- Eschalier, R.; Ploux, S.; Lumens, J.; Whinnett, Z.; Varma, N.; Meillet, V.; Ritter, P.; Jaïs, P.; Haïssaguerre, M.; Bordachar, P.; et al. Detailed analysis of ventricular activation sequences during right ventricular apical pacing and left bundle branch block and the potential implications for cardiac resynchronization therapy. Heart Rhythm. 2015, 12, 137–143. [Google Scholar] [CrossRef] [PubMed]
- Waddingham, P.H.; Lambiase, P.; Muthumala, A.; Rowland, E.; Chow, A.W.C. Fusion pacing with biventricular, left ventricular-only and multipoint pacing in cardiac resynchronisation therapy: Latest evidence and strategies for use. Arrhythmia Electrophysiol. Rev. 2021, 10, 91–100. [Google Scholar] [CrossRef]
- Rao, R.K.; Kumar, U.N.; Schafer, J.; Viloria, E.; De Lurgio, D.; Foster, E. Reduced ventricular volumes and improved systolic function with cardiac resynchronization therapy: A randomized trial comparing simultaneous biventricular pacing, sequential biventricular pacing, and left ventricular pacing. Circulation 2007, 115, 2136–2144. [Google Scholar] [CrossRef] [PubMed]
- Thibault, B.; Harel, F.; Ducharme, A.; White, M.; Ellenbogen, K.A.; Frasure-Smith, N.; Roy, D.; Philippon, F.; Dorian, P.; Talajic, M.; et al. Evaluation of resynchronization therapy for heart failure in patients with a QRS duration greater than 120 ms (GREATER-EARTH) trial: Rationale, design, and baseline characteristics. Can. J. Cardiol. 2011, 27, 779–786. [Google Scholar] [CrossRef]
- Touiza, A.; Etienne, Y.; Gilard, M.; Fatemi, M.; Mansourati, J.; Blanc, J.J. Long-term left ventricular pacing: Assessment and comparison with biventricular pacing in patients with severe congestive heart failure. J. Am. Coll. Cardiol. 2007, 38, 1966–1970. [Google Scholar] [CrossRef] [PubMed]
- Etienne, Y.; Mansourati, J.; Touiza, A.; Gilard, M.; Bertault-Valls, V.; Guillo, P.; Boschat, J.; Blanc, J.J.; Fatemi, M.; Nelson, G.S.; et al. Evaluation of left ventricular function and mitral regurgitation during left ventricular-based pacing in patients with heart failure, Eur. J. Heart Fail. 2001, 3, 441–447. [Google Scholar] [CrossRef]
- Gasparini, M.; Bocchiardo, M.; Lunati, M.; Ravazzi, P.A.; Santini, M.; Zardini, M.; Signorelli, S.; Passardi, M.; Klersy, C.; BELIEVE Investigators. Comparison of 1-year effects of left ventricular and biventricular pacing in patients with heart failure who have ventricular arrhythmias and left bundle-branch block: The Bi vs Left Ventricular Pacing: An International Pilot Evaluation on Heart Failure Patients with Ventricular Arrhythmias (BELIEVE) multicenter prospective randomized pilot study. Am. Heart J. 2006, 152, 155.e1–155.e7. [Google Scholar] [CrossRef]
- Sirker, A.; Thomas, M.; Baker, S.; Shrimpton, J.; Jewell, S.; Lee, L.; Rankin, R.; Griffiths, V.; Cooter, N.; James, R.; et al. Cardiac resynchronization therapy: Left or left-and-right for optimal symptomatic effect—The LOLA ROSE study. Europace 2007, 9, 862–868. [Google Scholar] [CrossRef]
- Valzania, C.; Rocchi, G.; Biffi, M.; Martignani, C.; Bertini, M.; Diemberger, I.; Biagini, E.; Ziacchi, M.; Domenichini, G.; Saporito, D.; et al. Left ventricular versus biventricular pacing: A randomized comparative study evaluating mid-term electromechanical and clinical effects. Echocardiography 2008, 25, 141–148. [Google Scholar] [CrossRef]
- Boriani, G.; Kranig, W.; Donal, E.; Calo, L.; Casella, M.; Delarche, N.; Fernandez Lozano, I.; Ansalone, G.; Biffi, M.; Boulogne, E.; et al. A randomized double-blind comparison of biventricular versus left ventricular stimulation for cardiac resynchronization therapy: The Biventricular versus Left Univentricular Pacing with ICD Back-up in Heart Failure Patients (B-LEFT HF) trial. Am. Heart J. 2010, 159, 1052–1058.e1. [Google Scholar] [CrossRef]
- Sedláček, K.; Burianová, L.; Mlčochová, H.; Peichl, P.; Marek, T.; Kautzner, J. Isolated left ventricular pacing results in worse long-term clinical outcome when compared with biventricular pacing: A single-centre randomized study. Europace 2010, 12, 1762–1768. [Google Scholar] [CrossRef]
- Martin, D.O.; Lemke, B.; Birnie, D.; Krum, H.; Lee, K.L.-F.; Aonuma, K.; Gasparini, M.; Starling, R.C.; Milasinovic, G.; Rogers, T.; et al. Investigation of a novel algorithm for synchronized left-ventricular pacing and ambulatory optimization of cardiac resynchronization therapy: Results of the adaptive CRT trial. Heart Rhythm 2012, 9, 11. [Google Scholar] [CrossRef] [PubMed]
- Birnie, D.; Lemke, B.; Aonuma, K.; Krum, H.; Lee, K.L.-F.; Gasparini, M.; Starling, R.C.; Milasinovic, G.; Gorcsan, J.; Houmsse, M.; et al. Clinical outcomes with synchronized left ventricular pacing: Analysis of the adaptive CRT trial. Heart Rhythm 2013, 10, 1368–1374. [Google Scholar] [CrossRef] [PubMed]
- Burns, K.V.; Gage, R.M.; Curtin, A.E.; Gorcsan, J.; Bank, A.J. Left ventricular-only pacing in heart failure patients with normal atrioventricular conduction improves global function and left ventricular regional mechanics compared with biventricular pacing: An adaptive cardiac resynchronization therapy sub-study. Eur. J. Heart Fail. 2017, 19, 1335–1343. [Google Scholar] [CrossRef]
- Faghfourian, B.; Homayoonfar, S.; Rezvanjoo, M.; Poorolajal, J.; Emam, A.H. Comparison of hemodynamic effects of biventricular versus left ventricular only pacing in patients receiving cardiac resynchronization therapy: A before-after clinical trial. J. Arrhythm. 2017, 33, 127–129. [Google Scholar] [CrossRef] [PubMed]
- Gwag, H.B.; Park, Y.; Lee, S.S.; Kim, J.S.; Park, K.M.; On, Y.K.; Park, S.J. Efficacy of Cardiac Resynchronization Therapy Using Automated Dynamic Optimization and Left Ventricular-only Pacing. J. Korean Med. Sci. 2019, 34, 27. [Google Scholar] [CrossRef]
- Hsu, J.C.; Birnie, D.; Stadler, R.W.; Cerkvenik, J.; Feld, G.K.; Birgersdotter-Green, U. Adaptive cardiac resynchronization therapy is associated with decreased risk of incident atrial fibrillation compared to standard biventricular pacing: A real-world analysis of 37,450 patients followed by remote monitoring. Heart Rhythm 2019, 16, 983–989. [Google Scholar] [CrossRef]
- Su, Y.; Hua, W.; Shen, F.; Zou, J.; Tang, B.; Chen, K.; Liang, Y.; He, L.; Zhou, X.; Zhang, X.; et al. Left ventricular-only fusion pacing versus cardiac resynchronization therapy in heart failure patients: A randomized controlled trial. Clin. Cardiol. 2021, 44, 1225. [Google Scholar] [CrossRef]
- Vătăşescu, R.G.; Târtea, G.C.; Iorgulescu, C.; Cojocaru, C.; Deaconu, A.; Badiul, A.; Goanță, E.V.; Bogdan, Ș.; Cozma, D. Predictors for Super-Responders in Cardiac Resynchronization Therapy. Am. J. Ther. 2023, 31, e13. [Google Scholar] [CrossRef]
- Auricchio, A.; Stellbrink, C.; Butter, C.; Sack, S.; Vogt, J.; Misier, A.R.; Böcker, D.; Block, M.; Kirkels, J.H.; Kramer, A.; et al. Clinical Efficacy of Cardiac Resynchronization Therapy Using Left Ventricular Pacing in Heart Failure Patients Stratified by Severity of Ventricular Conduction Delay. J. Am. Coll. Cardiol. 2003, 42, 2109–2116. [Google Scholar] [CrossRef]
- Blanc, J.J.; Bertault-Valls, V.; Fatemi, M.; Gilard, M.; Pennec, P.Y.; Etienne, Y. Midterm Benefits of Left Univentricular Pacing in Patients with Congestive Heart Failure. Circulation 2004, 109, 1741–1744. [Google Scholar] [CrossRef] [PubMed]
- Butter, C.; Wellnhofer, E.; Seifert, M.; Schlegl, M.; Hoersch, W.; Goehring, A.; Fleck, E. Time course of left ventricular volumes in severe congestive heart failure patients treated by optimized AV sequential left ventricular pacing alone—A 3-dimensional echocardiographic study. Am. Heart J. 2006, 151, 115–123. [Google Scholar] [CrossRef]
- Gopi, A.; Sundar, G.; Yelagudri, S.; Lalukota, K.; Sridevi, C.; Narasimhan, C. Atrial synchronous left ventricular only pacing with VDD pacemaker system—A cost effective alternative to conventional cardiac resynchronization therapy. Indian Heart J. 2014, 66, 612–616. [Google Scholar] [CrossRef]
- Zhao, L.; Zhao, L.; Pu, L.; Hua, B.; Wang, Y.; Li, S.; Li, Q.; Guo, T. Left Univentricular Pacing by Rate-Adaptive Atrioventricular Delay in Treatment of Chronic Heart Failure. Med. Sci. Monit. 2017, 23, 3971–3980. [Google Scholar] [CrossRef]
- Pu, L.J.; Wang, Y.; Zhao, L.L.; Guo, T.; Li, S.M.; Hua, B.T.; Yang, P.; Yang, J.; Lu, Y.Z.; Yang, L.Q.; et al. Left univentricular pacing for cardiac resynchronization therapy using rate-adaptive atrioventricular delay. J. Geriatr. Cardiol. 2017, 14, 118. [Google Scholar] [CrossRef] [PubMed]
- Cozma, D.; Vacarescu, C.; Petrescu, L.; Mornos, C.; Goanta, E.; Feier, H.; Luca, C.T.; Gusetu, G.; Vatasescu, R. CRT Pacing: Midterm Follow-Up in LV Only Pacing without RV Lead in Patients with Normal AV Conduction. J. Clin. Med. 2018, 7, 531. [Google Scholar] [CrossRef]
- Goanță, E.V.; Luca, C.T.; Vacarescu, C.; Crișan, S.; Petrescu, L.; Vatasescu, R.; Lazăr, M.A.; Gurgu, A.; Turi, V.R.; Cozma, D. Nonischemic Super-Responders in Fusion CRT Pacing with Normal Atrioventricular Conduction. Diagnostics 2022, 12, 2032. [Google Scholar] [CrossRef]
- Gurgu, A.; Luca, C.T.; Vacarescu, C.; Gaiță, D.; Crișan, S.; Faur-Grigori, A.A.; Cozlac, A.R.; Tudoran, C.; Margan, M.M.; Cozma, D. Heart Rate Recovery Index and Improved Diastolic Dyssynchrony in Fusion Pacing Cardiac Resynchronization Therapy. J. Clin. Med. 2024, 13, 6365. [Google Scholar] [CrossRef] [PubMed]
- Văcărescu, C.; Cozma, D.; Crișan, S.; Gaiță, D.; Anutoni, D.D.; Margan, M.M.; Faur-Grigori, A.A.; Roteliuc, R.; Luca, S.A.; Lazăr, M.A.; et al. Left Atrium Reverse Remodeling in Fusion CRT Pacing: Implications in Cardiac Resynchronization Response and Atrial Fibrillation Incidence. J. Clin. Med. 2024, 13, 4814. [Google Scholar] [CrossRef]
- Hunt, S.A.; Abraham, W.T.; Chin, M.H.; Feldman, A.M.; Francis, G.S.; Ganiats, T.G.; Jessup, M.; Konstam, M.A.; Mancini, D.M.; Michl, K.; et al. ACC/AHA 2005 Guideline Update for the Diagnosis and Management of Chronic Heart Failure in the Adult: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Update the 2001 Guidelines for the Evaluation and Management of Heart Failure): Developed in collaboration with the American College of Chest Physicians and the International Society for Heart and Lung Transplantation: Endorsed by the Heart Rhythm Society. Circulation 2005, 112, 12. [Google Scholar] [CrossRef]
- Graham, I.; Atar, D.; Borch-Johnsen, K.; Boysen, G.; Burell, G.; Cifkova, R.; Dallongeville, J.; De Backer, G.; Ebrahim, S.; Gjelsvik, B.; et al. European guidelines on cardiovascular disease prevention in clinical practice: Executive summary: Fourth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (Constituted by representatives of nine societies and by invited experts). Eur. Heart J. 2007, 28, 2375–2414. [Google Scholar] [CrossRef] [PubMed]
- Brignole, M.; Auricchio, A.; Baron-Esquivias, G.; Bordachar, P.; Boriani, G.; Breithardt, O.A.; Cleland, J.; Deharo, J.C.; Delgado, V.; Elliott, P.M.; et al. 2013 ESC Guidelines on cardiac pacing and cardiac resynchronization therapy: The Task Force on cardiac pacing and resynchronization therapy of the European Society of Cardiology (ESC). Developed in collaboration with the European Heart Rhythm Association (EHRA). Eur. Heart J. 2013, 34, 2281–2329. [Google Scholar] [CrossRef]
- Ponikowski, P.; Voors, A.A.; Anker, S.D.; Bueno, H.; Cleland, J.G.; Coats, A.J.; Falk, V.; González-Juanatey, J.R.; Harjola, V.P.; Jankowska, E.A.; et al. ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur. Heart J. 2016, 37, 2129–2200. [Google Scholar] [CrossRef]
- Kurzidim, K.; Reinke, H.; Sperzel, J.; Schneider, H.J.; Danilovic, D.; Siemon, G.; Neumann, T.; Hamm, C.W.; Pitschner, H.F. Invasive optimization of cardiac resynchronization therapy: Role of sequential biventricular and left ventricular pacing. Pacing Clin. Electrophysiol. 2005, 28, 754–761. [Google Scholar] [CrossRef]
- Gold, M.R.; Auricchio, A.; Leclercq, C.; Wold, N.; Stein, K.M.; Ellenbogen, K.A. Atrioventricular optimization improves cardiac resynchronization response in patients with long interventricular electrical delays: A pooled analysis of the SMART-AV and SMART-CRT trials. Heart Rhythm 2024, 21, 1686–1694. [Google Scholar] [CrossRef] [PubMed]
- Vacarescu, C.; Luca, C.T.; Petrescu, L.; Ionac, A.; Mornos, C.; Goanta, E.V.; Crisan, S.; Lazar, M.A.; Cozma, D. AV delay optimisation in LV only CRT: Constant fusion pacing is easier in patients with first degree AV block. Eur. Heart J. 2020, 41 (Suppl. S2), 810. [Google Scholar] [CrossRef]
- Manetti, C.A.; van Osta, N.; Beela, A.S.; Herbots, L.; Prinzen, F.W.; Delhaas, T.; Lumens, J. Impact of myocardial phenotype on optimal atrioventricular delay settings during biventricular and left bundle branch pacing at rest and during exercise: Insights from a virtual patient study. Europace 2025, 27, 5–8. [Google Scholar] [CrossRef]
- Loeb, J.M.; DeTarnowsky, J.M.; Warner, M.R.; Whitson, C.C. Dynamic interactions between heart rate and atrioventricular conduction. Am. J. Physiol. 1985, 249 Pt 2, H505–H511. [Google Scholar] [CrossRef]
- Loeb, J.M.; DeTarnowsky, J.M. Integration of heart rate and sympathetic neural effects on AV conduction. Am. J. Physiol. 1988, 254 Pt 2, H651–H657. [Google Scholar] [CrossRef]
- Niu, H.; Yu, Y.; Ravikumar, V.; Gold, M.R. The impact of chronotropic incompetence on atrioventricular conduction times in heart failure patients. J. Interv. Card. Electrophysiol. 2023, 66, 9. [Google Scholar] [CrossRef]
- Niu, H.; Yu, Y.; Sturdivant, J.L.; An, Q.; Gold, M.R. The effect of posture, exercise, and atrial pacing on atrioventricular conduction in systolic heart failure. J Cardiovasc Electrophysiol. 2019, 30, 2892–2899. [Google Scholar] [CrossRef] [PubMed]
- Vatasescu, R.; Berruezo, A.; Mont, L.; Tamborero, D.; Sitges, M.; Silva, E.; Tolosana, J.M.; Vidal, B.; Andreu, D.; Brugada, J. Midterm ‘super-response’ to cardiac resynchronization therapy by biventricular pacing with fusion: Insights from electro-anatomical mapping. Europace 2009, 11, 1675–1682. [Google Scholar] [CrossRef]
- Aksu, T.; Guler, T.E.; Bozyel, S.; Yalin, K. Potential usage of cardioneuroablation in vagally mediated functional atrioventricular block. SAGE Open Med. 2019, 7, 2050312119836308. [Google Scholar] [CrossRef]
- Gasparini, M.; Mantica, M.; Galimberti, P.; Genovese, L.; Pini, D.; Faletra, F.; Marchesina, U.L.; Mangiavacchi, M.; Klersy, C.; Gronda, E. Is the outcome of cardiac resynchronization therapy related to the underlying etiology? Pacing Clin. Electrophysiol. 2003, 26, 175–180. [Google Scholar] [CrossRef]
- Sutton, M.G.S.J.; Plappert, T.; Hilpisch, K.E.; Abraham, W.T.; Hayes, D.L.; Chinchoy, E. Sustained reverse left ventricular structural remodeling with cardiac resynchronization at one year is a function of etiology: Quantitative Doppler echocardiographic evidence from the Multicenter InSync Randomized Clinical Evaluation (MIRACLE). Circulation 2006, 113, 266–272. [Google Scholar] [CrossRef]
- Wikstrom, G.; Blomström-Lundqvist, C.; Andren, B.; Lönnerholm, S.; Blomström, P.; Freemantle, N.; Remp, T.; Cleland, J.G. The effects of aetiology on outcome in patients treated with cardiac resynchronization therapy in the CARE-HF trial. Eur. Heart J. 2009, 30, 782–788. [Google Scholar] [CrossRef] [PubMed]
- St John Sutton, M.; Ghio, S.; Plappert, T.; Tavazzi, L.; Scelsi, L.; Daubert, C.; Abraham, W.T.; Gold, M.R.; Hassager, C.; Herre, J.M.; et al. Cardiac resynchronization induces major structural and functional reverse remodeling in patients with New York Heart Association class I/II heart failure. Circulation 2009, 120, 1858–1865. [Google Scholar] [CrossRef] [PubMed]
- Barsheshet, A.; Goldenberg, I.; Moss, A.J.; Eldar, M.; Huang, D.T.; McNitt, S.; Klein, H.U.; Hall, W.J.; Brown, M.W.; Goldberger, J.J.; et al. Response to preventive cardiac resynchronization therapy in patients with ischaemic and nonischaemic cardiomyopathy in MADIT-CRT. Eur. Heart J. 2011, 32, 1622–1630. [Google Scholar] [CrossRef]
- Stătescu, C.; Ureche, C.; Enachi, Ș.; Radu, R.; Sascău, R.A. Cardiac Resynchronization Therapy in Non-Ischemic Cardiomyopathy: Role of Multimodality Imaging. Diagnostics 2021, 11, 625. [Google Scholar] [CrossRef]
- Abe, T.A.; Evbayekha, E.O.; Jackson, L.R.; Al-Khatib, S.M.; Lewsey, S.C.; Breathett, K. Evolving Indications, Challenges, and Advances in Cardiac Resynchronization Therapy for Heart Failure. J. Card. Fail. 2025, in press. [Google Scholar] [CrossRef]
- Desai, A.S.; Fang, J.C.; Maisel, W.H.; Baughman, K.L. Implantable defibrillators for the prevention of mortality in patients with nonischemic cardiomyopathy: A meta-analysis of randomized controlled trials. JAMA 2004, 292, 2874–2879. [Google Scholar] [CrossRef] [PubMed]
- Henkens, M.T.H.M.; Weerts, J.; Verdonschot, J.A.J.; Raafs, A.G.; Stroeks, S.; Sikking, M.A.; Amin, H.; Mourmans, S.G.J.; Geraeds, C.B.G.; Sanders-van Wijk, S.; et al. Improving diagnosis and risk stratification across the ejection fraction spectrum: The Maastricht Cardiomyopathy registry. ESC Heart Fail. 2022, 9, 1463–1470. [Google Scholar] [CrossRef] [PubMed]
- Moss, A.J.; Zareba, W.; Hall, W.J.; Klein, H.; Wilber, D.J.; Cannom, D.S.; Daubert, J.P.; Higgins, S.L.; Brown, M.W.; Andrews, M.L. Prophylactic implantation of a defibrillator in patients with myocardial infarction and reduced ejection fraction. N. Engl. J. Med. 2002, 346, 877–883. [Google Scholar] [CrossRef]
- Bardy, G.H.; Lee, K.L.; Mark, D.B.; Poole, J.E.; Packer, D.L.; Boineau, R.; Domanski, M.; Troutman, C.; Anderson, J.; Johnson, G.; et al. Amiodarone or an implantable cardioverter-defibrillator for congestive heart failure. N. Engl. J. Med. 2005, 352, 225–237. [Google Scholar] [CrossRef] [PubMed]
- Køber, L.; Thune, J.J.; Nielsen, J.C.; Haarbo, J.; Videbæk, L.; Korup, E.; Jensen, G.; Hildebrandt, P.; Steffensen, F.H.; Bruun, N.E.; et al. Defibrillator Implantation in Patients with Nonischemic Systolic Heart Failure. N. Engl. J. Med. 2016, 375, 1221–1230. [Google Scholar] [CrossRef]
- Disertori, M.; Masè, M.; Rigoni, M.; Nollo, G.; Arbustini, E.; Ravelli, F. The post-DANISH era in clinical cardiology: Need of a better selection of patients for implantable cardioverter-defibrillator in dilated cardiomyopathy. J. Cardiovasc. Electrophysiol. 2017, 28, E7. [Google Scholar] [CrossRef]
- Perazzolo Marra, M.; De Lazzari, M.; Zorzi, A.; Migliore, F.; Zilio, F.; Calore, C.; Vettor, G.; Tona, F.; Tarantini, G.; Cacciavillani, L.; et al. Impact of the presence and amount of myocardial fibrosis by cardiac magnetic resonance on arrhythmic outcome and sudden cardiac death in nonischemic dilated cardiomyopathy. Heart Rhythm 2023, 7752, 856–863. [Google Scholar] [CrossRef]
- Argentiero, A.; Carella, M.C.; Mandunzio, D.; Greco, G.; Mushtaq, S.; Baggiano, A.; Fazzari, F.; Fusini, L.; Muscogiuri, G.; Basile, P.; et al. Cardiac Magnetic Resonance as Risk Stratification Tool in Non-Ischemic Dilated Cardiomyopathy Referred for Implantable Cardioverter Defibrillator Therapy—State of Art and Perspectives. J. Clin. Med. 2023, 12, 7752. [Google Scholar] [CrossRef]
- Singh, J.P.; Cha, Y.M.; Lunati, M.; Chung, E.S.; Li, S.; Smeets, P.; O’Donnell, D. Real-world behavior of CRT pacing using the AdaptivCRT algorithm on patient outcomes: Effect on mortality and atrial fibrillation incidence. J. Cardiovasc. Electrophysiol. 2020, 31, 825–833. [Google Scholar] [CrossRef]
- Lazăr-Höcher, A.I.; Crișan, S.; Văcărescu, C.; Nistor, S.; Faur-Grigori, A.A.; Cozgarea, A.; Baneu, P.; Cirin, L.; Brăescu, L.; Dăniluc, L.; et al. Analyzing Insights of Super-Response in Cardiac Resynchronization Therapy with Fusion Pacing. Diagnostics 2025, 15, 1118. [Google Scholar] [CrossRef]
- Gatti, P.; Linde, C.; Benson, L.; Thorvaldsen, T.; Normand, C.; Savarese, G.; Dahlström, U.; Maggioni, A.P.; Dickstein, K.; Lund, L.H. What determines who gets cardiac resynchronization therapy in Europe? A comparison between ESC-HF-LT registry, SwedeHF registry, and ESC-CRT Survey II. Eur. Heart J. Qual. Care Clin. Outcomes 2023, 9, 741–748. [Google Scholar] [CrossRef] [PubMed]
- Leyva, F.; Zegard, A.; Okafor, O.; de Bono, J.; McNulty, D.; Ahmed, A.; Marshall, H.; Ray, D.; Qiu, T. Survival after cardiac resynchronization therapy: Results from 50 084 implantations. Europace 2017, 21, 754–762. [Google Scholar] [CrossRef] [PubMed]
- Vijayaraman, P.; Bordachar, P.; Ellenbogen, K.A. The Continued Search for Physiological Pacing: Where Are We Now? J. Am. Coll. Cardiol. 2017, 69, 3099–3114. [Google Scholar] [CrossRef] [PubMed]


| No Specific Inclusion Criteria | Specific Inclusion Criteria | Specific Exclusion Criteria | |
|---|---|---|---|
| [16] | sinus rhythm [13,14,15,17,18,19,20,25] | AF | paroxysmal [17,25] |
| persistent [14,17,20,25] | |||
| permanent [14,17,19,20,25] | |||
| PRi < 250 ms [14] | 2nd- or 3rd-degree AV block [14,19,20,25] | ||
| 2 weeks of VDD mode before randomization [13] | β-blocker therapy for ≤90 days [13] | ||
| LV mechanical dyssynchrony on Echo [18] | pm dependency; pacing for bradycardia [13,14,17,19,20] | ||
| 2–8 weeks of “run-in” test before randomization [14] | patients without hemodynamic improvement during acute LV-based pacing (test response) [16] | ||
| Patient Selection | Common Elements |
|---|---|
| Etiology | patients with predominantly nonischemic etiology |
| QRS morphology and duration | LBBB QRS duration ≥ 130–150 ms |
| AV conduction | preserved intrinsic AV conduction, defined as PRi ≤ 200 ms (a CRT)/1 study used PRi < 250 ms |
| Rhythm selection | all patients were in sinus rhythm; permanent AF was an exclusion criterion in every study |
| Fusion pacing algorithm | most studies used aCRT algorithm |
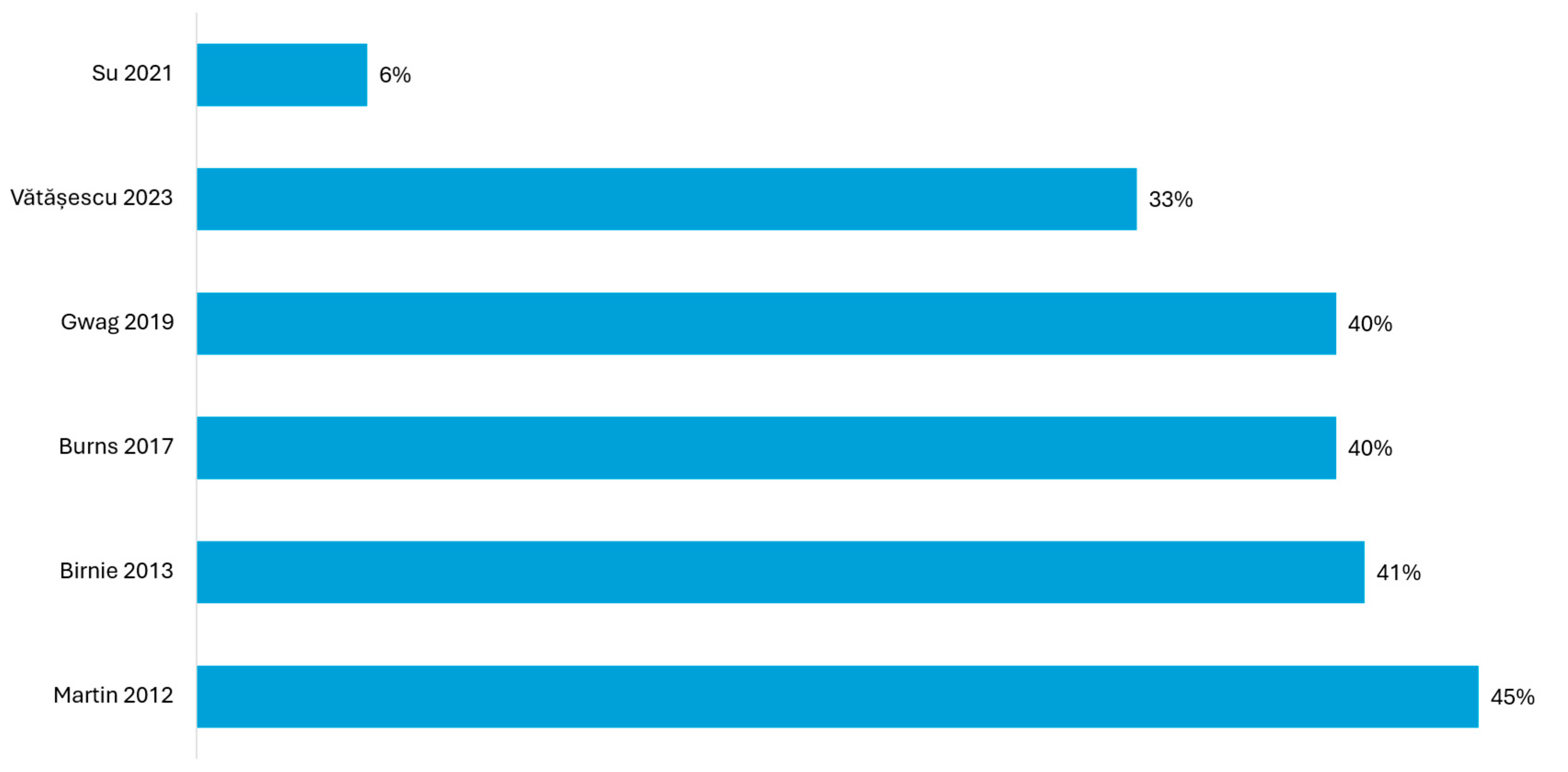
| Study | Definition of LV-Only fCRTp | LV-Only fCRTp (Mean) % | Results |
|---|---|---|---|
| Martin (2012) [22] | LV-only fCRTp ≥ 50% | 64% | significant improvement in functional status and event-free survival (CCS) in 80.7% vs. 68.4% in this subgroup (p = 0.04) |
| Birnie (2013) [23] | LV-only fCRTp ≥ 50% | 68–73% | a significantly lower risk of death or heart failure hospitalization (p = 0.012) in the subgroup with LV fCRTp ≥ 50%; higher rate of improvement in CCS: 80% vs. 62% compared to those with % LV fCRTp < 50% (12 months) (p = 0.0006) |
| Burns (2017) [24] | LV-only fCRTp ≥ 80% | >80% | greater improvements in LVEF (8.5% vs. 5.5%, p = 0.038) and global radial strain (6.3% vs. 4.0%, p = 0.046) in LV-only fCRTp compared to echo-optimized BiV pacing; 77% clinical responders in the LV-only group; (66% responders in BiV group) |
| Gwag (2019) [26] | LV-only fCRTp ≥ 50% | >97% | super-responder rate of 58.3%, compared to 36.3% in the echo-optimized BiV pacing group; no adverse clinical events in LV-only fCRTp |
| Su (2021) [28] | LV-only fCRTp with intentional non-capture RV pacing | 88.7% | super-responder rate of 68.4%, compared to 36.4% in the echo-optimized BiV pacing group |
| Positive Effects | Complications | ||||
|---|---|---|---|---|---|
| Outcome | Range | References | Type | Observation | |
| NYHA class improvement | reported in 8/10 studies | [30,31,32,34,35,36,37,38,39] | AV block requiring upgrade | reported in 1 patient (1.8%) [36], 4 patients (5.5%) [38], and 3 patients (4%) [39] | |
| QRS narrowing | reported in 7/10 studies | [30,31,32,33,34,35,37] | no study reported the need for an upgrade to ICD/CRT-D during follow-up | ||
| LVEF improvement | reported in 10/10 studies | [30,31,32,33,34,35,36,37,38,39] | Mortality | ||
| Reverse remodeling | |||||
| LVESV reduction | reported in 6/10 studies | [31,33,35,36,37,38] | nonischemic cohorts (up to 7%) | [33,36,37,38,39] | |
| reduction in mitral regurgitation | reported in 6/10 studies | [31,33,35,36,37,38] | ischemic patients (up to 23%) | [30,31,32,34,35] | |
| reduction in LA volume and improvement in diastolic dysfunction profile | reported in 4/10 studies | [31,36,38,39] | |||
| economic benefit | cost savings by omitting RV lead; increased device longevity | [33,35] | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Faur-Grigori, A.A.; Văcărescu, C.; Nistor, S.; Luca, S.A.; Liviu, C.; Crișan, S.; Luca, C.-T.; Vătășescu, R.-G.; Cozma, D. Refining Patient Selection Criteria for LV-Only Fusion Pacing in Cardiac Resynchronization Therapy: A Systematic Review. J. Clin. Med. 2025, 14, 4853. https://doi.org/10.3390/jcm14144853
Faur-Grigori AA, Văcărescu C, Nistor S, Luca SA, Liviu C, Crișan S, Luca C-T, Vătășescu R-G, Cozma D. Refining Patient Selection Criteria for LV-Only Fusion Pacing in Cardiac Resynchronization Therapy: A Systematic Review. Journal of Clinical Medicine. 2025; 14(14):4853. https://doi.org/10.3390/jcm14144853
Chicago/Turabian StyleFaur-Grigori, Adelina Andreea, Cristina Văcărescu, Samuel Nistor, Silvia Ana Luca, Cirin Liviu, Simina Crișan, Constantin-Tudor Luca, Radu-Gabriel Vătășescu, and Dragoș Cozma. 2025. "Refining Patient Selection Criteria for LV-Only Fusion Pacing in Cardiac Resynchronization Therapy: A Systematic Review" Journal of Clinical Medicine 14, no. 14: 4853. https://doi.org/10.3390/jcm14144853
APA StyleFaur-Grigori, A. A., Văcărescu, C., Nistor, S., Luca, S. A., Liviu, C., Crișan, S., Luca, C.-T., Vătășescu, R.-G., & Cozma, D. (2025). Refining Patient Selection Criteria for LV-Only Fusion Pacing in Cardiac Resynchronization Therapy: A Systematic Review. Journal of Clinical Medicine, 14(14), 4853. https://doi.org/10.3390/jcm14144853