Pregestational Diabetes Mellitus and Adverse Perinatal Outcomes: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Eligibility Criteria
2.2. Outcomes
2.3. Search Strategy and Information Sources
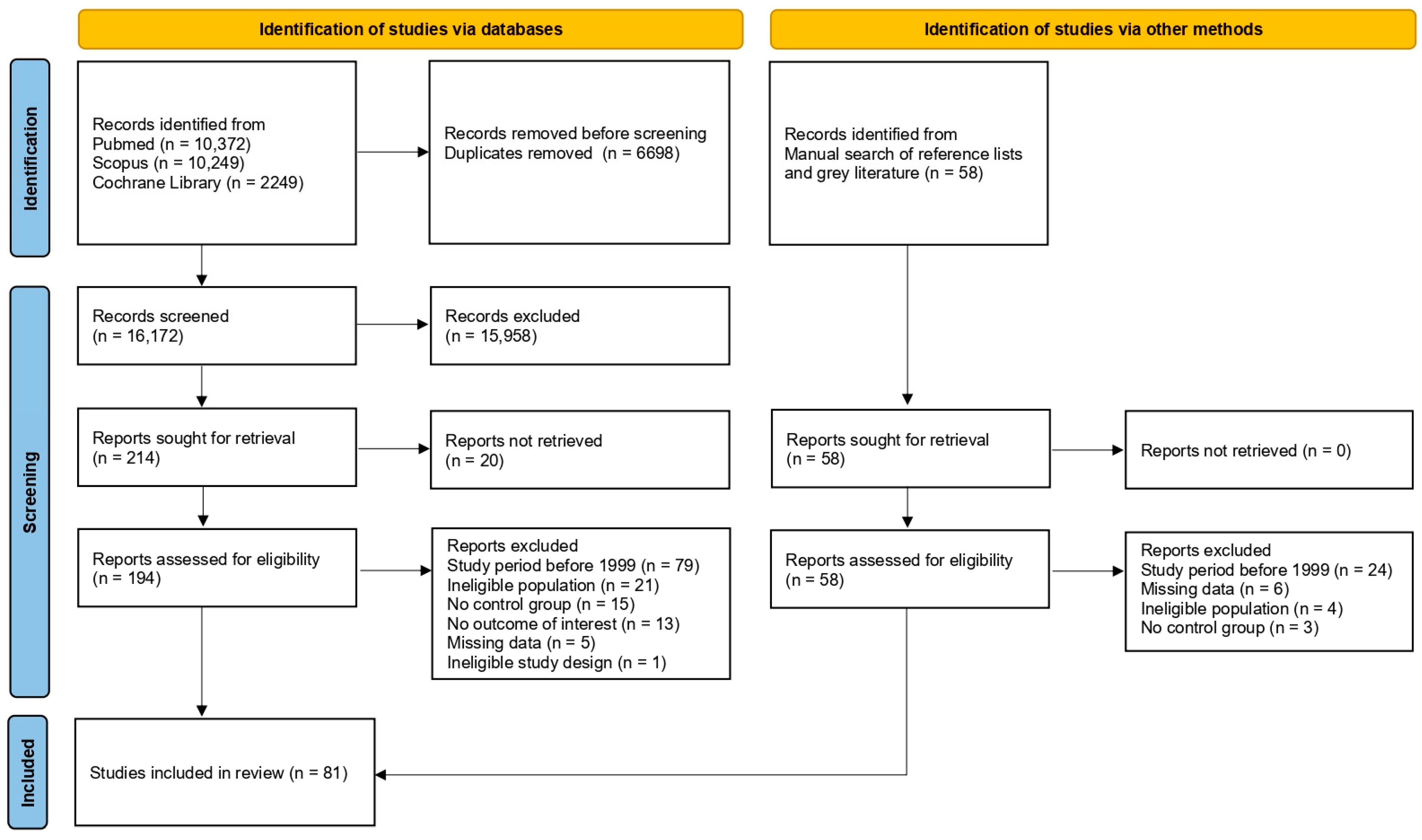
2.4. Study Selection
2.5. Data Extraction
2.6. Risk of Bias Assessment
2.7. Data Synthesis
3. Results
3.1. Study Selection and Study Characteristics
3.2. Risk of Bias Assessment of the Included Studies
3.3. PGDM and Adverse Perinatal Outcomes
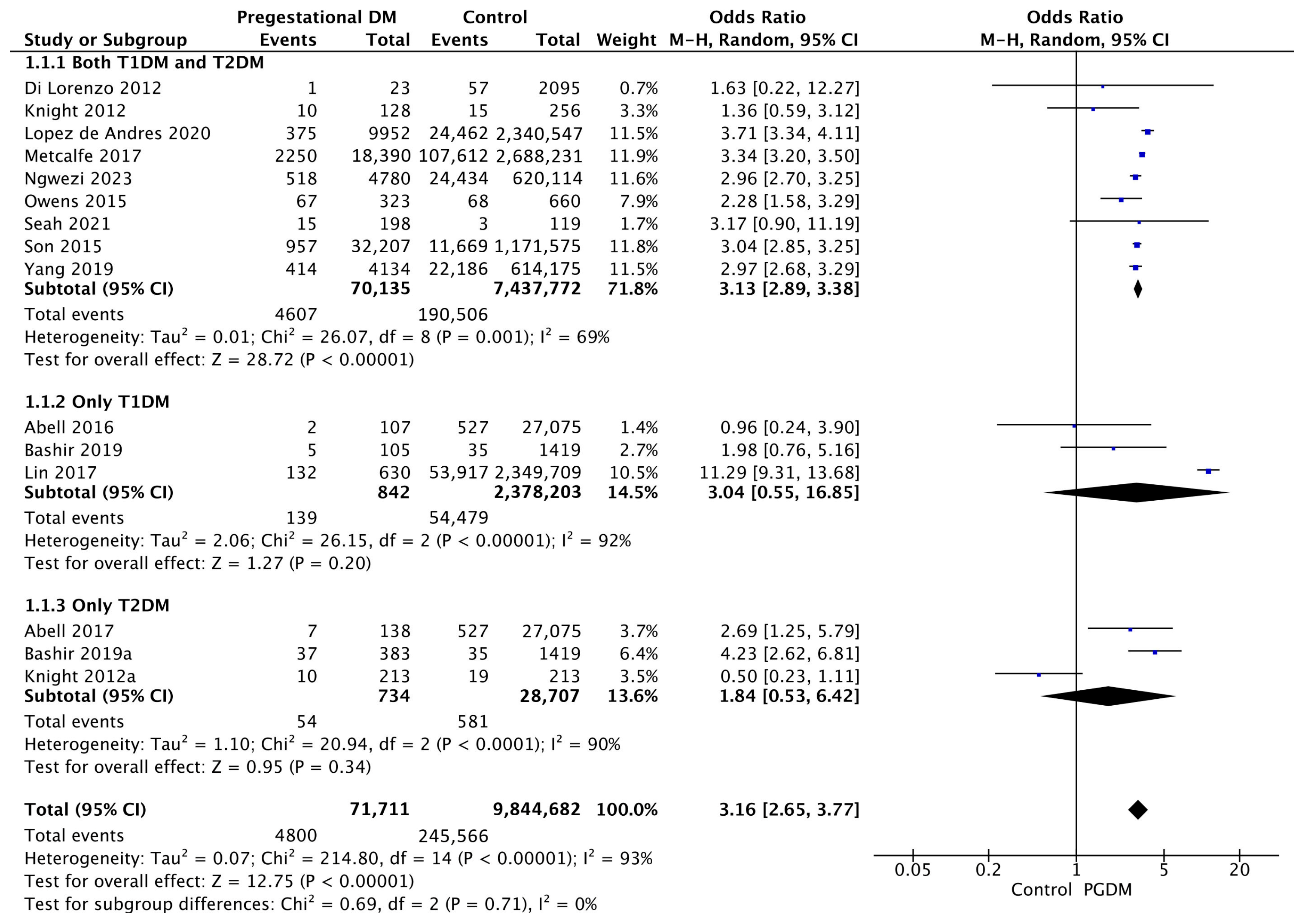
3.3.1. Gestational Hypertension
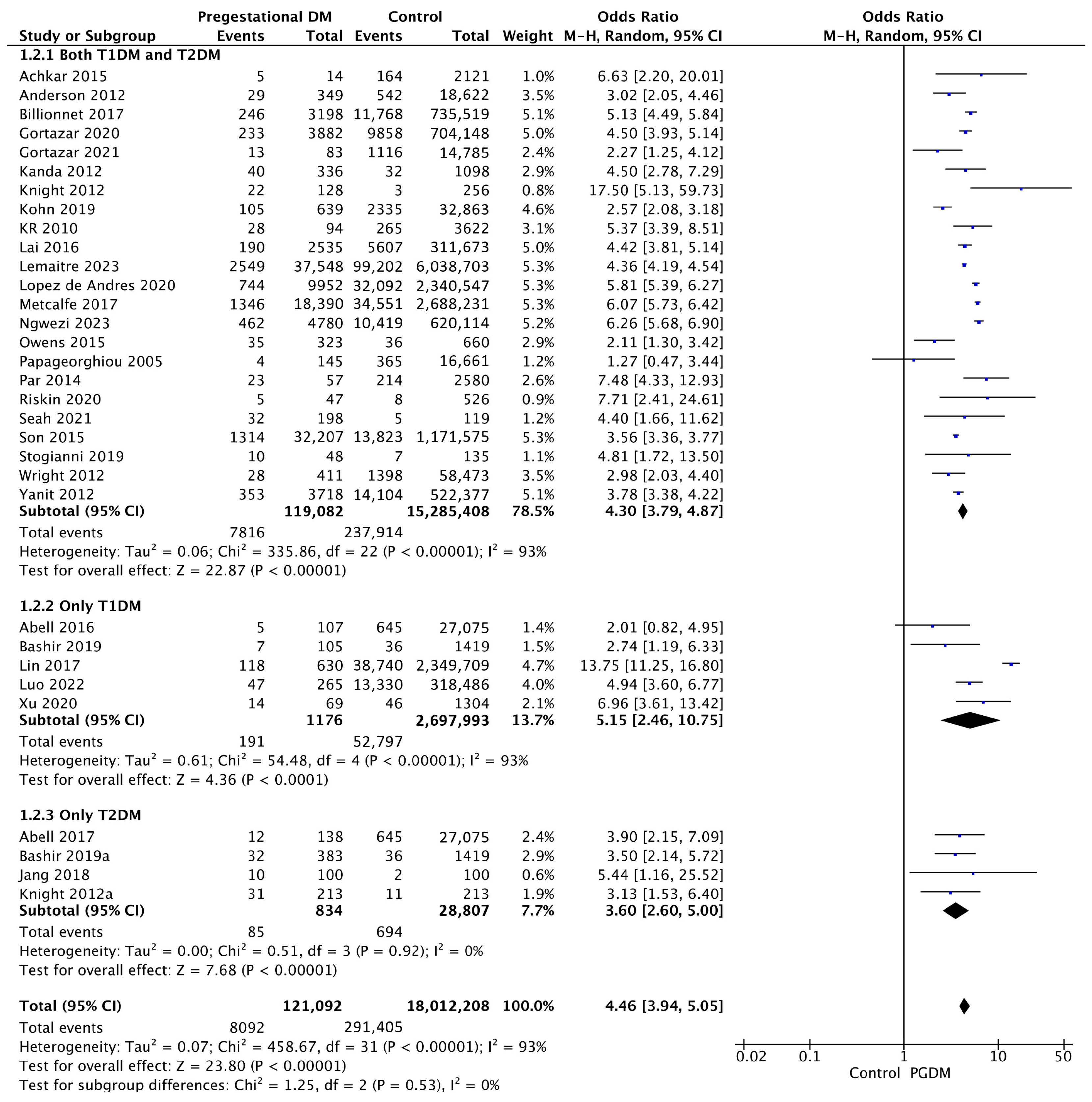
3.3.2. Preeclampsia
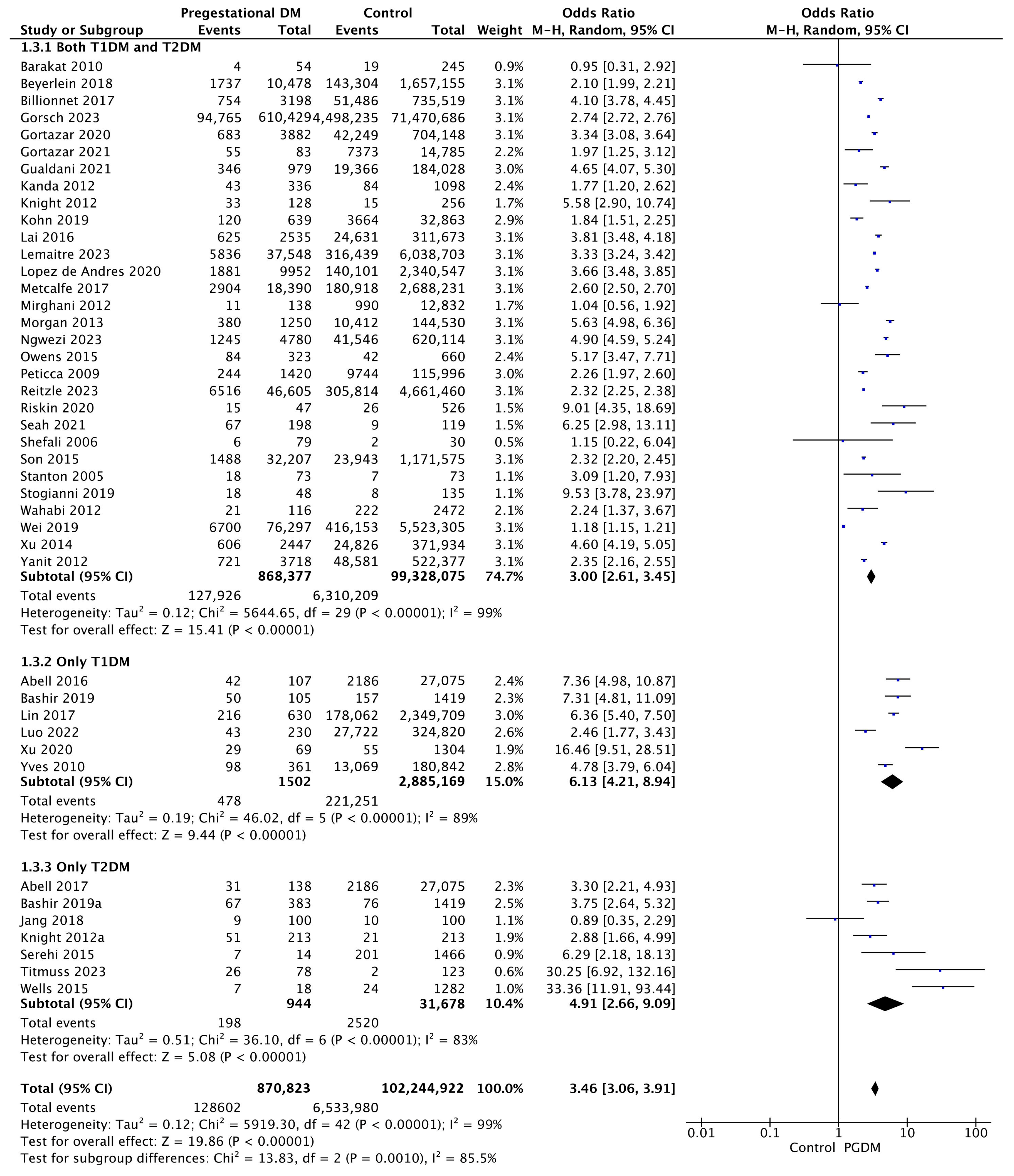
3.3.3. Preterm Delivery
3.3.4. Cesarean Delivery
3.3.5. Induction of Labor
3.3.6. Macrosomia
3.3.7. LGA Neonates
3.3.8. SGA Neonates
3.3.9. Low 5-Min Apgar Score
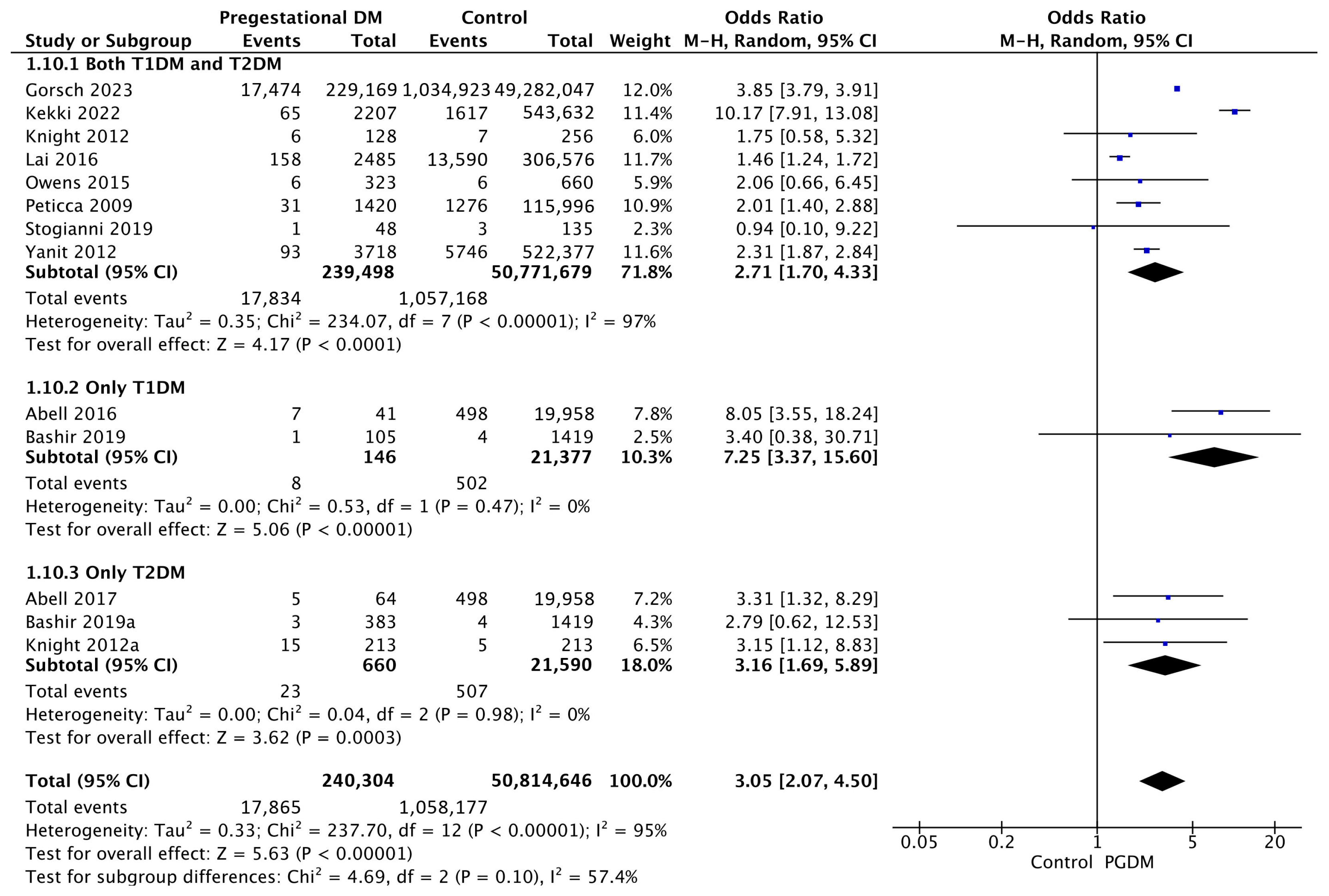
3.3.10. Shoulder Dystocia
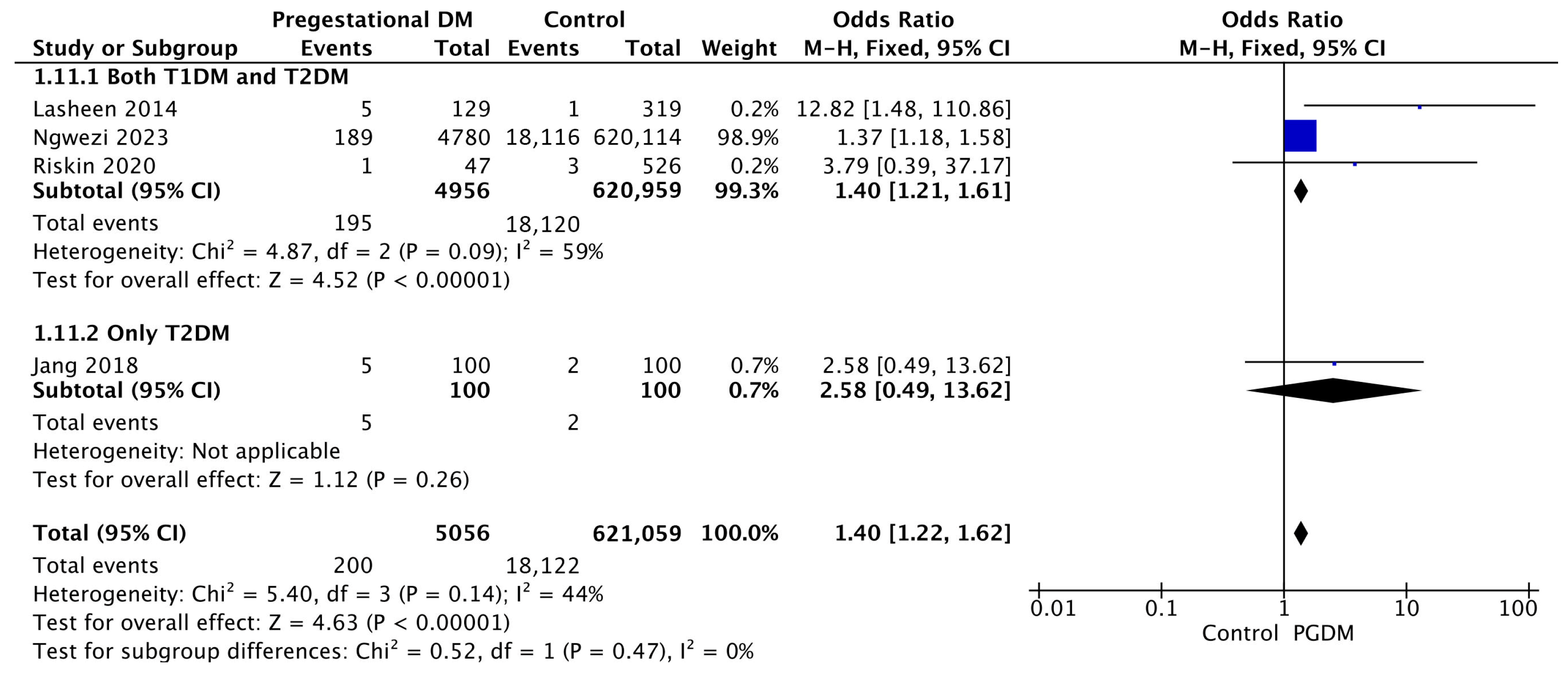
3.3.11. Birth Trauma
3.3.12. Polyhydramnios
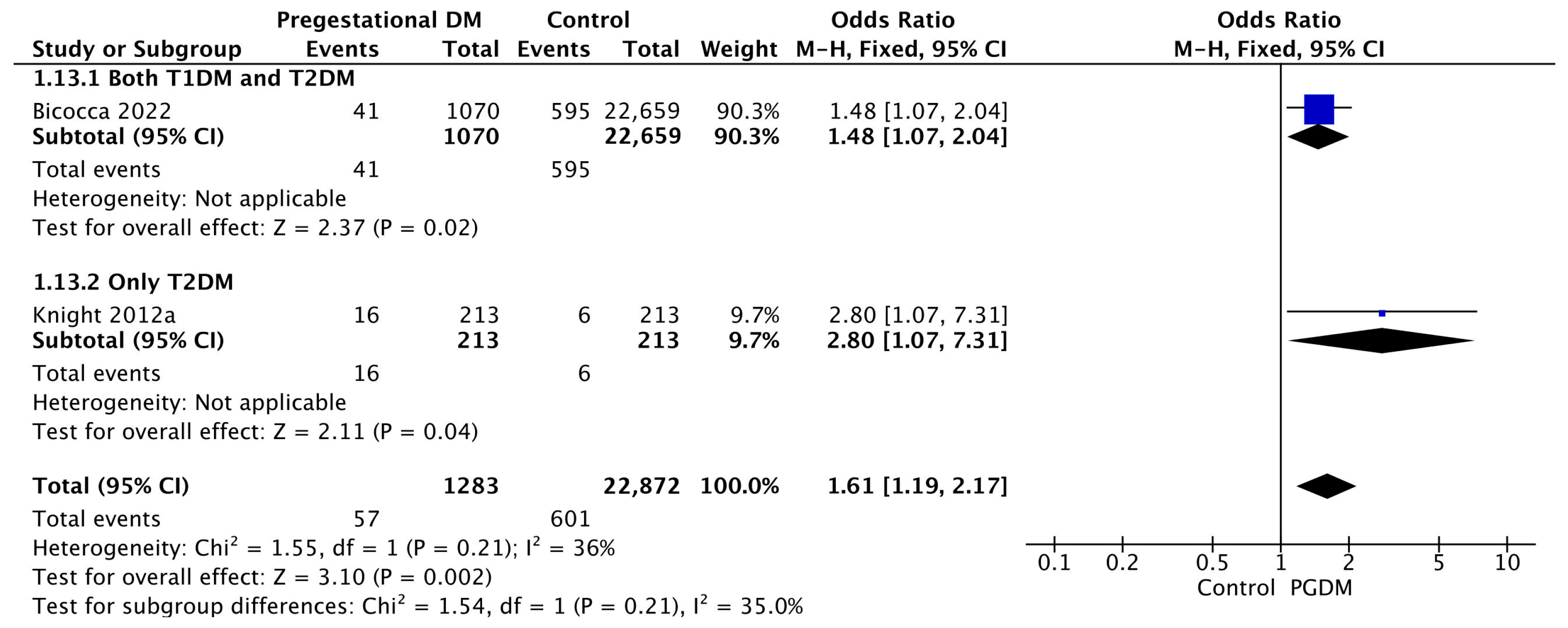
3.3.13. Oligohydramnios
3.3.14. Neonatal Hyperbilirubinemia
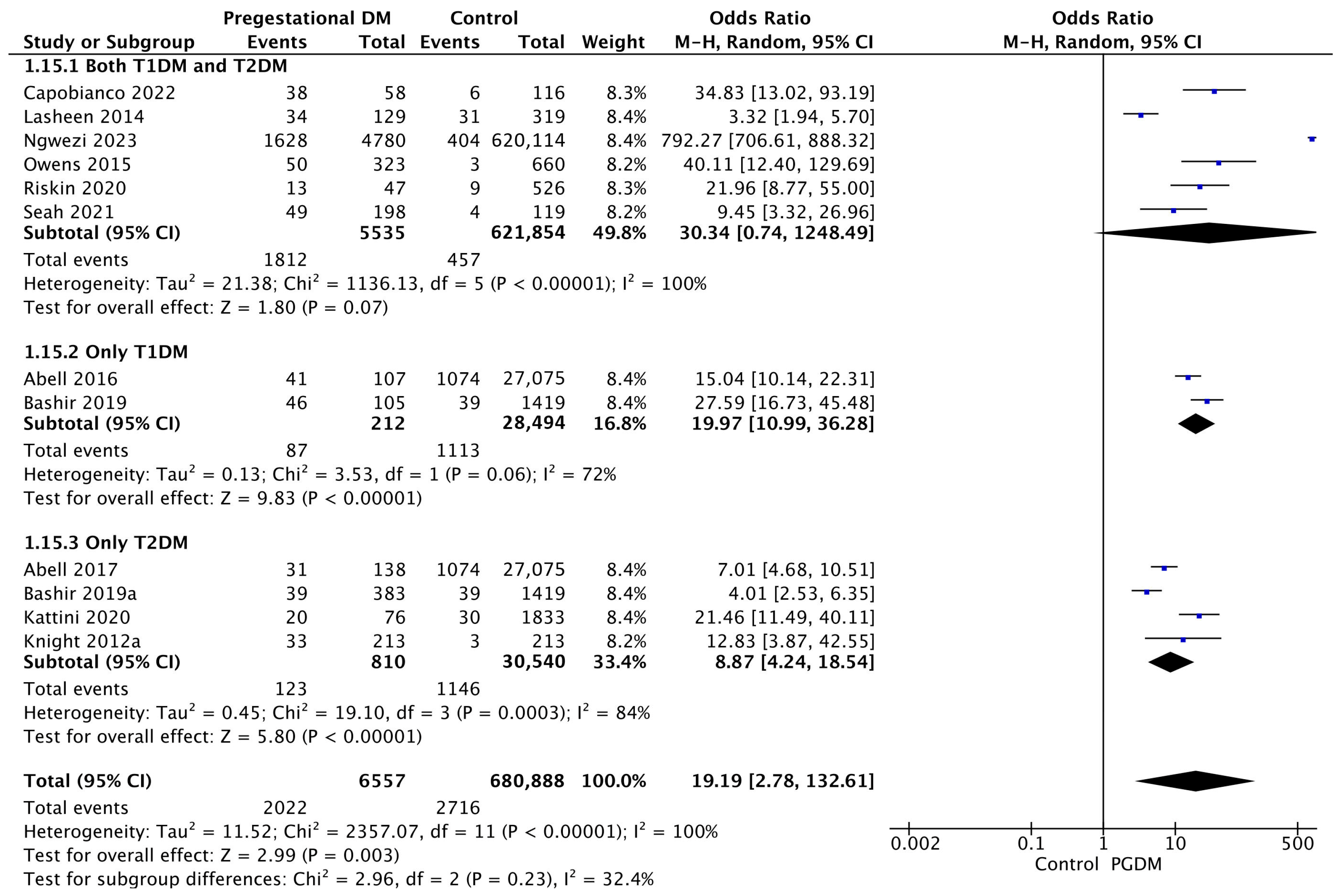
3.3.15. Neonatal Hypoglycemia
3.3.16. NICU Admission
3.3.17. Congenital Malformations
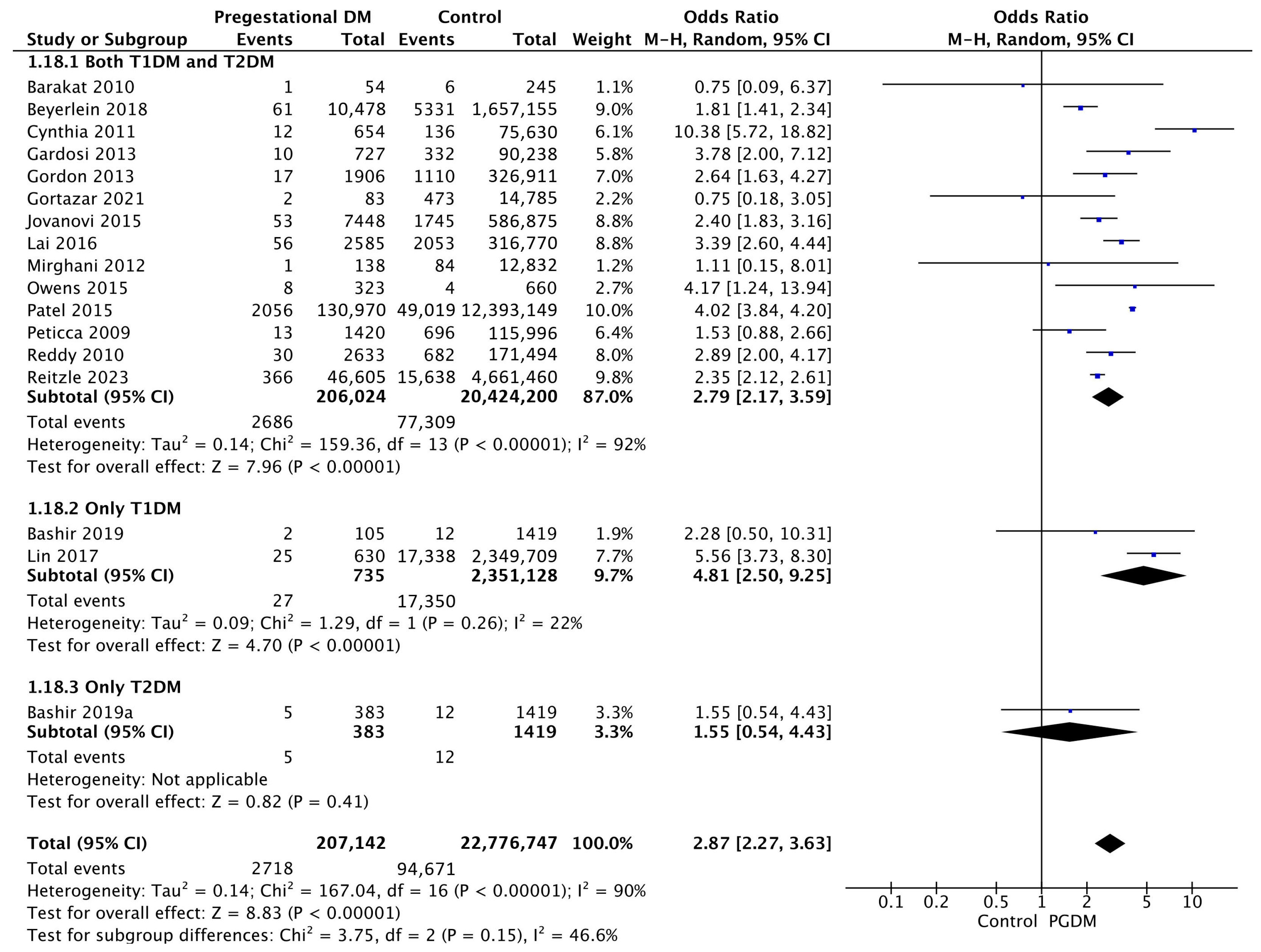
3.3.18. Stillbirth
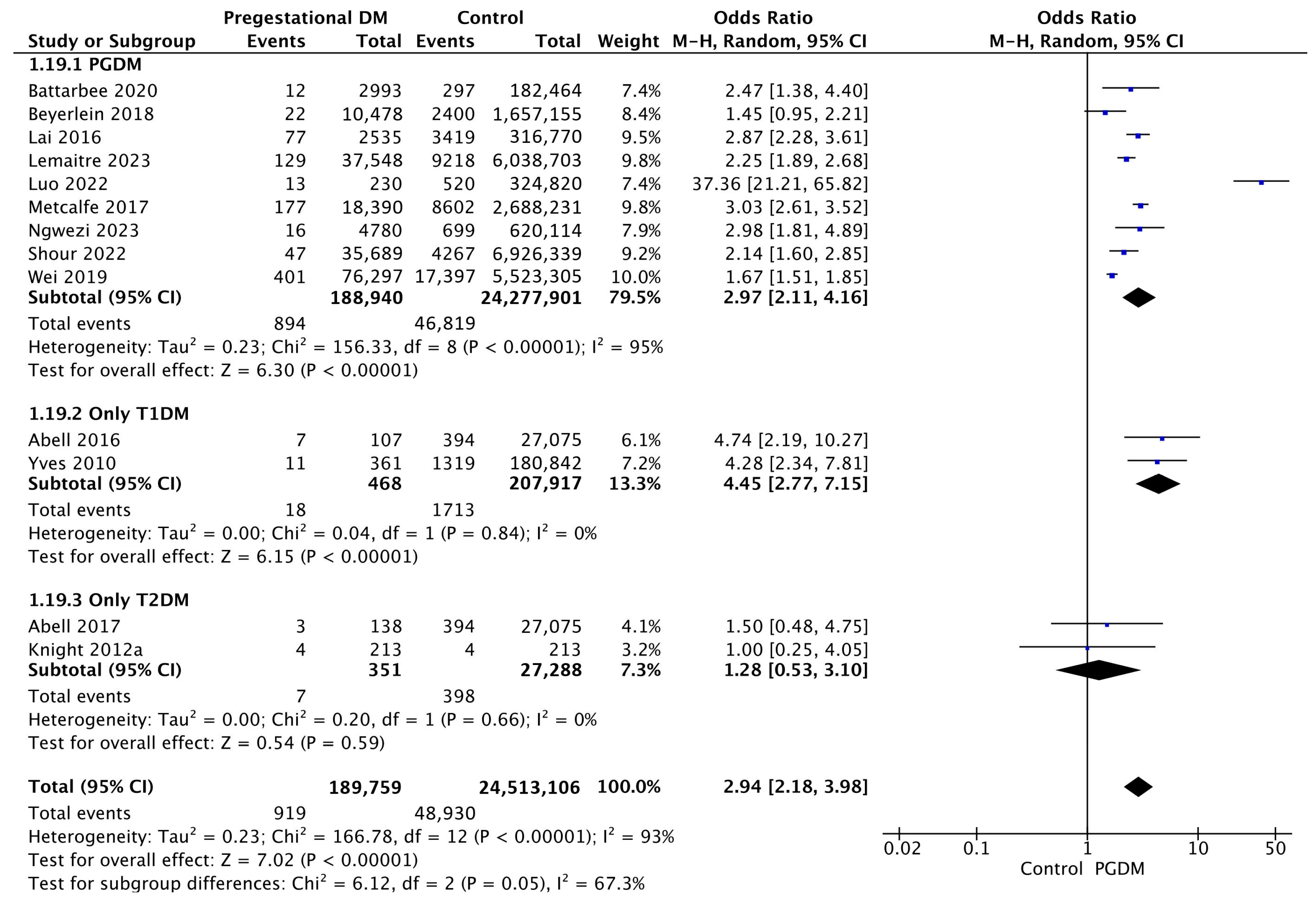
3.3.19. Perinatal Mortality
4. Discussion
4.1. Main Findings
4.2. Comparison with Existing Literature
4.3. Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Appendix A
| Study | Country | Study Design | Types of PGDM Included | PGDM Pregnancies/Controls | Adverse Perinatal Outcomes Studied | Risk of Bias Score (NOS) |
|---|---|---|---|---|---|---|
| Abell 2016 [25] | Australia | Retrospective cohort study | T1DM | 107/27,075 | GH, PE, PD, CD, IoL, LGA, SGA, Low Apgar score, SD, NHB, NHG, NICU admission, CM, Perinatal mortality | 9 |
| Abell 2017 [26] | Australia | Retrospective cohort study | T2DM | 138/27,075 | GH, PE, PD, CD, IoL, LGA, SGA, Low Apgar score, SD, NHB, NHG, NICU admission, CM, Perinatal mortality | 9 |
| Achkar 2015 [27] | Canada | Case-control study | Not specified | 14/2121 | PE | 6 |
| Anderson 2012 [28] | New Zealand | Retrospective cohort study | T1DM, T2DM | 349/18,622 | PE | 8 |
| Barakat 2010 [29] | Oman | Retrospective cohort study | T1DM, T2DM | 54/245 | PD, CD, Macrosomia, CM, Stillbirth | 8 |
| Bashir 2019 [30] | Qatar | Retrospective cohort study | T2DM | 383/1419 | GH, PE, PD, CD, IoL, Macrosomia, LGA, SGA, SD, PH, NHB, NHG, NICU admission, Stillbirth | 7 |
| Bashir 2019 [31] | Qatar | Retrospective cohort study | T1DM | 105/1419 | GH, PE, PD, CD, IoL, Macrosomia, LGA, SGA, SD, PH, NHB, NHG, NICU admission, Stillbirth | 7 |
| Battarbee 2020 [32] | USA | Retrospective cohort study | T1DM, T2DM | 2993/182,464 | CD, LGA, NICU admission, Perinatal mortality | 9 |
| Beyerlein 2018 [33] | Germany | Retrospective cohort study | Not specified | 10,478/1,657,155 | PD, LGA, Low Apgar score, CM, Stillbirth, Perinatal mortality | 9 |
| Bicocca 2022 [34] | USA | Retrospective cohort study | T1DM, T2DM | 1070/22,659 | PH, OH | 7 |
| Billionnet 2017 [35] | France | Retrospective cohort study | T1DM, T2DM | 3198/735,519 | PE, PD, CD, LGA, CM | 8 |
| Capobianco 2022 [36] | Italy | Case-control study | T1DM, T2DM, MODY | 58/116 | CD, NHB, NHG, CM | 7 |
| Chen 2023 [37] | Taiwan | Retrospective cohort study | T1DM, T2DM | 19,957/742,660 | CM | 8 |
| Cynthia 2011 [38] | Australia | Retrospective cohort study | Not specified | 654/75,630 | CD, Macrosomia, Stillbirth | 6 |
| Dalfrà 2011 [39] | Italy | Retrospective cohort study | T1DM | 32/17 | CD | 4 |
| Di Lorenzo 2012 [40] | Italy | Prospective cohort study | Not specified | 23/2095 | GH | 5 |
| Dolk 2020 [41] | UK | Case-control study | Not specified | 8/1200 | CM | 7 |
| Eidem 2010 [42] | Norway | Retrospective cohort study | T1DM | 1583/349,378 | CM | 8 |
| Fang 2023 [43] | Taiwan | Case-control study | Not specified | 103/4516 | CM | 7 |
| Foeller 2015 [44] | USA | Retrospective cohort study | T1DM, T2DM | 2137/258,857 | CD | 8 |
| Gardosi 2013 [45] | UK | Prospective cohort study | Not specified | 727/90,238 | Stillbirth | 9 |
| Giraldo-Grueso 2020 [46] | Colombia | Case-control study | Not specified | 10/3228 | CM | 5 |
| Goetzinger 2010 [47] | USA | Retrospective cohort study | Not specified | 94/3622 | PE | 6 |
| Gordon 2013 [48] | Australia | Prospective cohort study | Not specified | 1906/326,911 | Stillbirth | 8 |
| Gorsch 2023 [49] | USA | Retrospective cohort study | T1DM, T2DM | 610,429/71,470,686 | PD, CD, SD | 8 |
| Gortazar 2020 [50] | Spain | Retrospective cohort study | T1DM, T2DM, other PGDM types | 3882/704,148 | PE, PD, CD, Macrosomia, LGA, SGA | 8 |
| Gortazar 2021 [51] | Spain | Retrospective cohort study | T1DM, T2DM, other PGDM types | 83/14,785 | PE, PD, CD, LGA, SGA, Stillbirth | 8 |
| Gualdani 2021 [52] | Italy | Retrospective cohort study | T1DM, T2DM | 979/184,028 | PD, CD, Macrosomia, LGA, CM | 9 |
| He 2023 [53] | Canada | Retrospective cohort study | Not specified | 7489/550,512 | CM | 9 |
| Hunt 2012 [54] | USA | Retrospective cohort study | Not specified | 4767/198,853 | LGA, SGA | 9 |
| Jang 2018 [55] | Korea | Case-control study | T2DM | 100/100 | PE, PD, CD, IoL, Macrosomia, LGA, SGA, BT, NHB, NICU admission | 6 |
| Jovanovič 2015 [56] | USA | Retrospective cohort study | T1DM, T2DM | 11,261/773,751 | CD, Macrosomia, Stillbirth | 7 |
| Kanda 2012 [57] | Japan | Retrospective cohort study | T1DM, T2DM | 336/1098 | PE, PD, CD, LGA, SGA, CM | 8 |
| Kattini 2020 [58] | Canada | Retrospective cohort study | T2DM | 76/1833 | CD, IoL, Macrosomia, Low Apgar score, NHB, NHG | 7 |
| Kekki 2022 [59] | Finland | Retrospective cohort study | T1DM, T2DM | 2207/543,632 | IoL, LGA, SD | 8 |
| Knight 2012 [60] | USA | Retrospective cohort study | T1DM, T2DM | 128/256 | GH, PE, PD, CD, IoL, LGA, SD, NICU admission | 5 |
| Knight 2012 [61] | USA | Retrospective cohort study | T2DM | 213/213 | GH, PE, PD, CD, IoL, LGA, SGA, SD, PH, OH, NHB, NHG, NICU admission, CM, Perinatal mortality | 9 |
| Kohn 2019 [62] | USA | Retrospective cohort study | Not specified | 639/32,863 | PE, PD, CD, Macrosomia | 8 |
| Kuc 2011 [63] | Netherlands | Case-control study | Not specified | 178/186 | LGA | 6 |
| Lai 2016 [64] | Canada | Retrospective cohort study | T1DM, T2DM | 2535/311,673 | PE, PD, CD, Macrosomia, LGA, SGA, Low Apgar score, SD, NICU admission, CM, Stillbirth, Perinatal mortality | 8 |
| Lasheen 2014 [65] | Saudi Arabia | Prospective cohort study | Not specified | 129/319 | BT, NHB, NHG, CM | 6 |
| Lemaitre 2023 [66] | France | Retrospective cohort study | T1DM, T2DM | 37,548/6,038,703 | PE, PD, CD, LGA, SGA, NICU admission, CM, Perinatal mortality | 8 |
| Lin 2017 [67] | Taiwan | Retrospective cohort study | T1DM | 630/2,349,709 | GH, PE, PD, CD, LGA, SGA, Low Apgar score, Stillbirth | 8 |
| Lindsay 2003 [68] | UK | Case-control study | T1DM | 140/49 | CD | 6 |
| Liu 2013 [69] | Canada | Retrospective cohort study | T1DM, T2DM | 13,673/2,265,165 | CM | 8 |
| Lopez-de-Andres 2020 [70] | Spain | Retrospective cohort study | T1DM, T2DM | 9952/2,340,547 | GH, PE, PD, CD, IoL | 8 |
| Loukovaara 2004 [71] | Finland | Retrospective cohort study | T1DM | 67/62 | CD, LGA | 6 |
| Luo 2022 [72] | China | Retrospective cohort study | T1DM | 265/318,486 | PE, PD, CD, Macrosomia, SGA, NICU admission, CM, Perinatal mortality | 7 |
| Metcalfe 2017 [73] | Canada | Retrospective cohort study | T1DM, T2DM | 18,390/2,688,231 | GH, PE, PD, CD, IoL, Perinatal mortality | 8 |
| Mirghani 2012 [74] | UAE | Prospective cohort study | T1DM, T2DM | 138/12,832 | PD, CD, NICU admission, CM, Stillbirth | 5 |
| Morgan 2013 [75] | UK | Retrospective cohort study | T1DM, T2DM | 1250/144,530 | PD, LGA, SGA | 8 |
| Ngwezi 2023 [76] | Canada | Retrospective cohort study | T1DM, T2DM | 4780/620,114 | GH, PE, PD, CD, IoL, Macrosomia, LGA, SGA, BT, NHB, NHG, NICU admission, Perinatal mortality | 8 |
| Owens 2015 [77] | Ireland | Case-control study | T1DM, T2DM | 323/660 | GH, PE, PD, CD, LGA, SGA, SD, PH, NHB, NHG, NICU admission, CM, Stillbirth | 6 |
| Papageorghiou 2005 [78] | UK | Prospective cohort study | Not specified | 145/16,661 | PE | 6 |
| Paré 2014 [79] | USA | Prospective cohort study | Not specified | 57/2580 | PE | 8 |
| Patel 2015 [80] | USA | Case-control study | Not specified | 130,970/12,393,149 | Stillbirth | 8 |
| Pereda 2020 [81] | Uruguay | Retrospective cohort study | Not specified | 304/33,107 | Macrosomia | 9 |
| Peticca 2009 [82] | Canada | Retrospective cohort study | T1DM, T2DM | 1420/115,996 | PD, CD, IoL, Macrosomia, SD, CM, Stillbirth | 8 |
| Praprotnik 2021 [83] | Croatia | Retrospective cohort study | T1DM | 70/70 | Macrosomia, LGA, SGA | 5 |
| Reddy 2010 [84] | USA | Retrospective cohort study | Not specified | 2633/172,176 | Stillbirth | 8 |
| Reitzle 2023 [85] | Germany | Retrospective cohort study | Not specified | 46,605/4,661,460 | PD, CD, LGA, Stillbirth | 7 |
| Riskin 2020 [86] | Israel | Case-control study | Not specified | 47/526 | PE, PD, CD, LGA, SGA, BT, NHB, NHG, CM | 6 |
| Schraw 2021 [87] | USA | Retrospective cohort study | Not specified | 28,880/6,275,634 | CM | 9 |
| Seah 2021 [88] | Australia | Retrospective cohort study | T1DM, T2DM | 198/119 | GH, PE, PD, LGA, SGA, Low Apgar score, NHB, NHG, NICU admission, CM | 7 |
| Serehi 2015 [89] | Saudi Arabia | Prospective cohort study | T2DM | 14/1466 | PD, CD, IoL, PH, NICU admission | 7 |
| Shefali 2006 [90] | India | Prospective cohort study | T1DM, T2DM | 79/30 | PD | 7 |
| Shour 2022 [91] | USA | Retrospective cohort study | Not specified | 35,689/6,926,339 | CD, Low Apgar score, Perinatal mortality | 9 |
| Son 2015 [92] | Korea | Retrospective cohort study | Not specified | 32,207/1,171,575 | GH, PE, PD, CD, Macrosomia | 8 |
| Stanton 2005 [93] | USA | Retrospective cohort study | Not specified | 73/73 | PD, Macrosomia | 5 |
| Stogianni 2019 [94] | Sweden | Retrospective cohort study | T1DM, T2DM | 48/135 | PE, PD, CD, Macrosomia, LGA, Low Apgar score, SD, CM | 8 |
| Titmuss 2023 [95] | Australia | Prospective cohort study | T2DM | 78/123 | PD, CD | 8 |
| Wahabi 2012 [96] | Saudi Arabia | Retrospective cohort study | T1DM, T2DM | 116/2472 | PD, CD, Macrosomia, Low Apgar score | 8 |
| Wei 2019 [97] | China | Retrospective cohort study | Not specified | 76,297/5,523,305 | PD, Macrosomia, CM, Perinatal mortality | 8 |
| Wells 2015 [98] | Australia | Retrospective cohort study | T2DM | 18/1282 | PD, LGA, SGA | 6 |
| Wright 2012 [99] | UK | Prospective cohort study | T1DM, T2DM | 411/58,473 | PE | 8 |
| Xu 2014 [100] | Australia | Retrospective cohort study | Not specified | 2447/372,954 | PD | 8 |
| Xu 2020 [101] | China | Retrospective cohort study | T1DM | 69/1304 | PE, PD, CD, Macrosomia, LGA, SGA, PH, NHB, NICU admission | 8 |
| Yang 2019 [102] | USA | Retrospective cohort study | Not specified | 4134/614,175 | GH, Macrosomia, CM | 9 |
| Yanit 2012 [103] | USA | Retrospective cohort study | Not specified | 3718/522,377 | PE, PD, LGA, SGA, SD | 8 |
| Yves 2010 [104] | Belgium | Retrospective cohort study | T1DM | 354/177,407 | PD, CD, NICU admission, CM, Perinatal mortality | 7 |
| Zeki 2018 [105] | Australia | Retrospective cohort study | T1DM, T2DM | 5977/938,581 | CD | 8 |
Appendix A.1. MEDLINE/PubMed Search Strategy
- MEDLINE/Pubmed search syntax (Advanced search)
- #1: “pregnancy” [All Fields]
- #2: “pregnant” [All Fields]
- #3: #1 OR #2
- #4: “diabetes” [All Fields]
- #5: #3 AND #4
- #6: “pregestational” [All Fields]
- #7: “pre-gestational” [All Fields]
- #8: “preexisting” [All Fields]
- #9: “pre-existing” [All Fields]
- #10: “type 1 diabetes” [All Fields]
- #11: “diabetes type 1” [All Fields]
- #12: type 1 diabetes mellitus [MesH Terms]
- #13: “type 2 diabetes” [All Fields]
- #14: “diabetes type 2” [All Fields]
- #15: type 2 diabetes mellitus [MesH Terms]
- #16: #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15
- #17: #5 AND #16
- Publication date: 1999 onwards
- MEDLINE/PubMed search string
- (((“pregnancy”) OR (“pregnant”)) AND (“diabetes”)) AND ((((((((((“pregestational”) OR (“pre-gestational”)) OR (“preexisting”)) OR (“pre-existing”)) OR (“type 1 diabetes”)) OR (“diabetes type 1”)) OR (type 1 diabetes mellitus [MeSH Terms])) OR (“type 2 diabetes”)) OR (“diabetes type 2”)) OR (type 2 diabetes mellitus [MeSH Terms]))
- Publication date: 1999 onwards
Appendix A.2. Scopus Search Strategy
- Scopus search syntax (Advanced search)
- #1: “pregnancy”
- #2: “pregnant”
- #3: #1 OR #2
- #4: “diabetes” [All Fields]
- #5: #3 AND #4
- #6: “pregestational”
- #7: “pre-gestational”
- #8: “preexisting”
- #9: “pre-existing”
- #10: “type 1 diabetes”
- #11: “diabetes type 1”
- #12: “diabetes mellitus, type 1”
- #13: “type 2 diabetes”
- #14: “diabetes type 2”
- #15: “diabetes mellitus, type 2”
- #16: #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15
- #17: #5 AND #16
- Limited to Subject Area: Medicine
- Limited to English
- Publication date: 1999 onwards
- Scopus search string
- TITLE-ABS-KEY ((“pregnancy” OR “pregnant”) AND “diabetes” AND (“pregestational” OR “pre-gestational” OR “preexisting” OR “pre-existing” OR “type 1 diabetes” OR “diabetes type 1” OR “diabetes mellitus, type 1” OR “type 2 diabetes” OR “diabetes type 2” OR “diabetes mellitus, type 2”)) AND PUBYEAR > 1998 AND PUBYEAR < 2024 AND (LIMIT-TO (SUBJAREA, “MEDI”)) AND (LIMIT-TO (LANGUAGE, “English”))
Appendix A.3. Cochrane Library Search Strategy
- Cochrane Library search syntax
- #1: “pregnancy”
- #2: “pregnant”
- #3: #1 OR #2
- #4: “diabetes”
- #5: #3 AND #4
- #6: “pregestational”
- #7: “pre-gestational”
- #8: “preexisting”
- #9: “pre-existing”
- #10: “type 1 diabetes”
- #11: “diabetes type 1”
- #12: MeSH descriptor: [Diabetes Mellitus, Type 1] explode all trees
- #13: “type 2 diabetes”
- #14: “diabetes type 2”
- #15: MeSH descriptor: [Diabetes Mellitus, Type 2] explode all trees
- #16: #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15
- #17: #5 AND #16
- Publication date: 1999 onwards
References
- World Health Organisation. Global Report on Diabetes; World Health Organisation: Lyon, France, 2016.
- Sun, H.; Saeedi, P.; Karuranga, S.; Pinkepank, M.; Ogurtsova, K.; Duncan, B.B.; Stein, C.; Basit, A.; Chan, J.C.; Mbanya, J.C.; et al. IDF Diabetes Atlas: Global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res. Clin. Pract. 2022, 183, 109119. [Google Scholar] [CrossRef] [PubMed]
- Ogrotis, I.; Koufakis, T.; Kotsa, K. Changes in the Global Epidemiology of Type 1 Diabetes in an Evolving Landscape of Environmental Factors: Causes, Challenges, and Opportunities. Medicina 2023, 59, 668. [Google Scholar] [CrossRef]
- Basu, S.; Yoffe, P.; Hills, N.; Lustig, R.H.; Wagner, B. The Relationship of Sugar to Population-Level Diabetes Prevalence: An Econometric Analysis of Repeated Cross-Sectional Data. PLoS ONE 2013, 8, e57873. [Google Scholar] [CrossRef] [PubMed]
- HAPO Study Cooperative Research Group; Metzger, B.E.; Lowe, L.P.; Dyer, A.R.; Trimble, E.R.; Chaovarindr, U.; Coustan, D.R.; Hadden, D.R.; McCance, D.R.; Hod, M.; et al. Hyperglycemia and Adverse Pregnancy Outcomes. N. Engl. J. Med. 2008, 358, 1991–2002. [Google Scholar] [CrossRef]
- Sibai, B.M.; Caritis, S.N.; Hauth, J.C.; MacPherson, C.; VanDorsten, J.P.; Klebanoff, M.; Landon, M.; Paul, R.H.; Meis, P.J.; Miodovnik, M.; et al. Preterm delivery in women with pregestational diabetes mellitus or chronic hypertension relative to women with uncomplicated pregnancies. Am. J. Obstet. Gynecol. 2000, 183, 1520–1524. [Google Scholar] [CrossRef]
- Holmes, V.A.; Young, I.S.; Patterson, C.C.; Pearson, D.W.; Walker, J.D.; Maresh, M.J.; McCance, D.R.; For the Diabetes and Pre-eclampsia Intervention Trial Study Group. Optimal Glycemic Control, Pre-eclampsia, and Gestational Hypertension in Women with Type 1 Diabetes in the Diabetes and Pre-eclampsia Intervention Trial. Diabetes Care 2011, 34, 1683–1688. [Google Scholar] [CrossRef] [PubMed]
- Macintosh, M.C.M.; Fleming, K.M.; Bailey, J.A.; Doyle, P.; Modder, J.; Acolet, D.; Golightly, S.; Miller, A. Perinatal mortality and congenital anomalies in babies of women with type 1 or type 2 diabetes in England, Wales, and Northern Ireland: Population based study. BMJ 2006, 333, 177. [Google Scholar] [CrossRef]
- Al-Agha, R.; Firth, R.G.; Byrne, M.; Murray, S.; Daly, S.; Foley, M.; Smith, S.C.; Kinsley, B.T. Outcome of pregnancy in type 1 diabetes mellitus (T1DMP): Results from combined diabetes–obstetrical clinics in Dublin in three university teaching hospitals (1995–2006). Ir. J. Med. Sci. 2012, 181, 105–109. [Google Scholar] [CrossRef]
- Persson, M.; Norman, M.; Hanson, U. Obstetric and Perinatal Outcomes in Type 1 Diabetic Pregnancies. Diabetes Care 2009, 32, 2005–2009. [Google Scholar] [CrossRef]
- Mitanchez, D.; Yzydorczyk, C.; Siddeek, B.; Boubred, F.; Benahmed, M.; Simeoni, U. The offspring of the diabetic mother—Short- and long-term implications. Best Pract. Res. Clin. Obstet. Gynaecol. 2015, 29, 256–269. [Google Scholar] [CrossRef]
- Yu, L.; Zeng, X.-L.; Cheng, M.-L.; Yang, G.-Z.; Wang, B.; Xiao, Z.-W.; Luo, X.; Zhang, B.-F.; Xiao, D.-W.; Zhang, S.; et al. Quantitative assessment of the effect of pre-gestational diabetes and risk of adverse maternal, perinatal and neonatal outcomes. Oncotarget 2017, 8, 61048–61056. [Google Scholar] [CrossRef] [PubMed]
- Greco, E.; Calanducci, M.; Nicolaides, K.H.; Barry, E.V.; Huda, M.S.; Iliodromiti, S. Gestational diabetes mellitus and adverse maternal and perinatal outcomes in twin and singleton pregnancies: A systematic review and meta-analysis. Am. J. Obstet. Gynecol. 2023, 230, 213–225. [Google Scholar] [CrossRef]
- Ye, W.; Luo, C.; Huang, J.; Li, C.; Liu, Z.; Liu, F. Gestational diabetes mellitus and adverse pregnancy outcomes: Systematic review and meta-analysis. BMJ 2022, 377, e067946. [Google Scholar] [CrossRef] [PubMed]
- Mistry, S.K.; Das Gupta, R.; Alam, S.; Kaur, K.; Shamim, A.A.; Puthussery, S. Gestational diabetes mellitus (GDM) and adverse pregnancy outcome in South Asia: A systematic review. Endocrinol. Diabetes Metab. 2021, 4, e00285. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, 71. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Definition, Diagnosis and Classification of Diabetes Mellitus and Its Complications: Report of a WHO Consultation. Part 1: Diagnosis and Classification of Diabetes Mellitus; World Health Organization: Geneva, Switzerland, 1999.
- Acog Practice Bulletin. Gestational Hypertension and Preeclampsia. Obstet. Gynecol. 2020, 135, e237–e260. [Google Scholar] [CrossRef]
- World Health Organization. Preterm Birth. 2018. Available online: https://www.who.int/en/news-room/fact-sheets/detail/preterm-birth (accessed on 10 October 2023).
- ACOG Committee on Practice Bulletins—Obstetrics. Macrosomia: ACOG Practice Bulletin, Number 216. Obstet. Gynecol. 2020, 135, e18–e35. [Google Scholar] [CrossRef]
- Battaglia, F.C.; Lubchenco, L.O. A practical classification of newborn infants by weight and gestational age. J. Pediatr. 1967, 71, 159–163. [Google Scholar] [CrossRef]
- Committee on Obstetric Practice American Academy of Pediatrics—Committee on Fetus and Newborn. Committee Opinion No. 644. Obstet. Gynecol. 2015, 126, e52–e55. [Google Scholar] [CrossRef]
- Tsakiridis, I.; Giouleka, S.; Mamopoulos, A.; Athanasiadis, A.; Dagklis, T. Investigation and management of stillbirth: A descriptive review of major guidelines. J. Perinat. Med. 2022, 50, 796–813. [Google Scholar] [CrossRef]
- Wells, G.A.; Shea, B.; O’Connell, D.; Peterson, J.; Welch, V.; Losos, M.; Tugwell, P. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-Analyses. Available online: www.ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed on 10 October 2023).
- Abell, S.K.; A Boyle, J.; Courten, B.; Knight, M.; Ranasinha, S.; Regan, J.; Soldatos, G.; Wallace, E.M.; Zoungas, S.; Teede, H.J. Contemporary type 1 diabetes pregnancy outcomes: Impact of obesity and glycaemic control. Med. J. Aust. 2016, 205, 162–167. [Google Scholar] [CrossRef] [PubMed]
- Abell, S.K.; Boyle, J.A.; de Courten, B.; Soldatos, G.; Wallace, E.M.; Zoungas, S.; Teede, H.J. Impact of type 2 diabetes, obesity and glycaemic control on pregnancy outcomes. Aust. N. Z. J. Obstet. Gynaecol. 2017, 57, 308–314. [Google Scholar] [CrossRef] [PubMed]
- Achkar, M.; Dodds, L.; Giguère, Y.; Forest, J.-C.; Armson, B.A.; Woolcott, C.; Agellon, S.; Spencer, A.; Weiler, H.A. Vitamin D status in early pregnancy and risk of preeclampsia. Am. J. Obstet. Gynecol. 2015, 212, 511.e1–511.e7. [Google Scholar] [CrossRef]
- Anderson, N.H.; Sadler, L.C.; Stewart, A.W.; Fyfe, E.M.; McCowan, L.M. Ethnicity, body mass index and risk of pre-eclampsia in a multiethnic New Zealand population. Aust. N. Z. J. Obstet. Gynaecol. 2012, 52, 552–558. [Google Scholar] [CrossRef]
- Barakat, M.N.; Youssef, R.M.; Al-Lawati, J.A. Pregnancy outcomes of diabetic women: Charting Oman’s progress towards the goals of the Saint Vincent Declaration. Ann. Saudi Med. 2010, 30, 265–270. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bashir, M.; Dabbous, Z.; Baagar, K.; Elkhatib, F.; Ibrahim, A.; Brich, S.-A.; Abdel-Rahman, M.E.; Konje, J.C.; Abou-Samra, A.-B. Type 2 diabetes mellitus in pregnancy: The impact of maternal weight and early glycaemic control on outcomes. Eur. J. Obstet. Gynecol. Reprod. Biol. 2019, 233, 53–57. [Google Scholar] [CrossRef]
- Bashir, M.; Naem, E.; Taha, F.; Konje, J.C.; Abou-Samra, A.-B. Outcomes of type 1 diabetes mellitus in pregnancy; effect of excessive gestational weight gain and hyperglycaemia on fetal growth. Diabetes Metab. Syndr. Clin. Res. Rev. 2019, 13, 84–88. [Google Scholar] [CrossRef]
- Battarbee, A.N.; Venkatesh, K.K.; Aliaga, S.; Boggess, K.A. The association of pregestational and gestational diabetes with severe neonatal morbidity and mortality. J. Perinatol. 2020, 40, 232–239. [Google Scholar] [CrossRef]
- Beyerlein, A.; Lack, N.; von Kries, R. No further improvement in pregnancy-related outcomes in the offspring of mothers with pre-gestational diabetes in Bavaria, Germany, between 2001 and 2016. Diabet. Med. 2018, 35, 1420–1424. [Google Scholar] [CrossRef]
- Bicocca, M.J.; Qureshey, E.J.; Chauhan, S.P.; Hernandez-Andrade, E.; Sibai, B.M.; Nowlen, C.; Stafford, I. Semiquantitative Assessment of Amniotic Fluid Among Individuals With and Without Diabetes Mellitus. J. Ultrasound Med. 2022, 41, 447–455. [Google Scholar] [CrossRef]
- Billionnet, C.; Mitanchez, D.; Weill, A.; Nizard, J.; Alla, F.; Hartemann, A.; Jacqueminet, S. Gestational diabetes and adverse perinatal outcomes from 716,152 births in France in 2012. Diabetologia 2017, 60, 636–644. [Google Scholar] [CrossRef] [PubMed]
- Capobianco, G.; Gulotta, A.; Tupponi, G.; Dessole, F.; Virdis, G.; Cherchi, C.; De Vita, D.; Petrillo, M.; Olzai, G.; Antonucci, R.; et al. Fetal Growth and Neonatal Outcomes in Pregestational Diabetes Mellitus in a Population with a High Prevalence of Diabetes. J. Pers. Med. 2022, 12, 1320. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.-J.; Chiu, C.-H.; Huang, J.-Y.; Chen, P.-J.; Su, P.-H.; Yang, S.-F.; Chen, J.-Y. Maternal diabetes mellitus and birth defects in Taiwan: A 5-year nationwide population-based cohort study. J. Chin. Med. Assoc. 2023, 86, 589–595. [Google Scholar] [CrossRef]
- Cynthia, P.; Timothy, S.; Isabelle, E. What is the impact of diabetes for Australian Aboriginal women when pregnant? Diabetes Res. Clin. Pract. 2011, 93, e29–e32. [Google Scholar] [CrossRef]
- Dalfrà, M.G.; Sartore, G.; Di Cianni, G.; Mello, G.; Lencioni, C.; Ottanelli, S.; Sposato, J.; Valgimigli, F.; Scuffi, C.; Scalese, M.; et al. Glucose variability in diabetic pregnancy. Diabetes Technol. Ther. 2011, 13, 853–859. [Google Scholar] [CrossRef]
- Di Lorenzo, G.; Ceccarello, M.; Cecotti, V.; Ronfani, L.; Monasta, L.; Brumatti, L.V.; Montico, M.; D’oTtavio, G. First trimester maternal serum PIGF, free β-hCG, PAPP-A, PP-13, uterine artery Doppler and maternal history for the prediction of preeclampsia. Placenta 2012, 33, 495–501. [Google Scholar] [CrossRef] [PubMed]
- Dolk, H.; McCullough, N.; Callaghan, S.; Casey, F.; Craig, B.; Given, J.; Loane, M.; Lagan, B.M.; Bunting, B.; Boyle, B.; et al. Risk Factors for Congenital Heart Disease: The Baby Hearts Study, a Population-Based Case-Control Study. PLoS ONE 2020, 15, e0227908. [Google Scholar] [CrossRef]
- Eidem, I.; Stene, L.C.; Henriksen, T.; Hanssen, K.F.; Vangen, S.; Vollset, S.E.; Joner, G. Congenital anomalies in newborns of women with type 1 diabetes: Nationwide population-based study in Norway, 1999–2004. Acta Obstet. Gynecol. Scand. 2010, 89, 1403–1411. [Google Scholar] [CrossRef]
- Fang, N.-W.; Huang, Y.-S.; Yin, C.-H.; Chen, J.-S.; Chiou, Y.-H. Maternal risk factors in offspring with congenital anomalies of the kidney and urinary tract in Asian women. Pediatr. Nephrol. 2023, 38, 3065–3070. [Google Scholar] [CrossRef]
- E Foeller, M.; Zhao, S.; Szabo, A.; O Cruz, M. Neonatal Outcomes in Twin Pregnancies Complicated by Gestational Diabetes Compared with Non-Diabetic Twins. J. Perinatol. 2015, 35, 1043–1047. [Google Scholar] [CrossRef]
- Gardosi, J.; Madurasinghe, V.; Williams, M.; Malik, A.; Francis, A. Maternal and fetal risk factors for stillbirth: Population based study. BMJ 2013, 346, f108. [Google Scholar] [CrossRef] [PubMed]
- Giraldo-Grueso, M.; Zarante, I.; Mejía-Grueso, A.; Gracia, G. Risk factors for congenital heart disease: A case-control study. Rev. Colomb. Cardiol. 2020, 27, 324–329. [Google Scholar] [CrossRef]
- Goetzinger, K.; Singla, A.; Gerkowicz, S.; Dicke, J.; Gray, D.; Odibo, A. Predicting the risk of pre-eclampsia between 11 and 13 weeks′ gestation by combining maternal characteristics and serum analytes, PAPP-A and free β-hCG. Prenat. Diagn. 2010, 30, 1138–1142. [Google Scholar] [CrossRef]
- Gordon, A.; Raynes-Greenow, C.; McGeechan, K.; Morris, J.; Jeffery, H. Risk factors for antepartum stillbirth and the influence of maternal age in New South Wales Australia: A population based study. BMC Pregnancy Childbirth 2013, 13, 12. [Google Scholar] [CrossRef]
- Gorsch, L.P.; Wen, T.; Lonier, J.Y.; Zork, N.; Mourad, M.; D’aLton, M.E.; Friedman, A.M. Trends in delivery hospitalizations with pregestational and gestational diabetes mellitus and associated outcomes: 2000–2019. Am. J. Obstet. Gynecol. 2023, 229, 63.e1–63.e14. [Google Scholar] [CrossRef] [PubMed]
- Gortazar, L.; Goday, A.; Roux, J.A.F.-L.; Sarsanedas, E.; Payà, A.; Mañé, L.; Pedro-Botet, J.; Benaiges, D. Trends in prevalence of pre-existing diabetes and perinatal outcomes: A large, population-based study in Catalonia, Spain, 2006–2015. BMJ Open Diabetes Res. Care 2020, 8, e001254. [Google Scholar] [CrossRef]
- Gortazar, L.; Roux, J.A.F.-L.; Benaiges, D.; Sarsanedas, E.; Navarro, H.; Payà, A.; Mañé, L.; Pedro-Botet, J.; Goday, A. Trends in Prevalence of Diabetes among Twin Pregnancies and Perinatal Outcomes in Catalonia between 2006 and 2015: The DIAGESTCAT Study. J. Clin. Med. 2021, 10, 1937. [Google Scholar] [CrossRef]
- Gualdani, E.; Di Cianni, G.; Seghieri, M.; Francesconi, P.; Seghieri, G. Pregnancy outcomes and maternal characteristics in women with pregestational and gestational diabetes: A retrospective study on 206,917 singleton live births. Acta Diabetol. 2021, 58, 1169–1176. [Google Scholar] [CrossRef]
- He, R.; Hornberger, L.K.; Kaur, A.; Crawford, S.; Boehme, C.; McBrien, A.; Eckersley, L. Risk of major congenital heart disease in pregestational maternal diabetes is modified by hemoglobin A1c. Ultrasound Obstet. Gynecol. 2023, 63, 378–384. [Google Scholar] [CrossRef]
- Hunt, K.J.; Marlow, N.M.; Gebregziabher, M.; Ellerbe, C.N.; Mauldin, J.; Mayorga, M.E.; Korte, J.E. Impact of maternal diabetes on birthweight is greater in non-Hispanic blacks than in non-Hispanic whites. Diabetologia 2012, 55, 971–980. [Google Scholar] [CrossRef]
- Jang, H.-J.; Kim, H.-S.; Kim, S.-H. Maternal and neonatal outcomes in Korean women with type 2 diabetes. Korean J. Intern. Med. 2018, 33, 1143–1149. [Google Scholar] [CrossRef] [PubMed]
- Jovanovič, L.; Liang, Y.; Weng, W.; Hamilton, M.; Chen, L.; Wintfeld, N. Trends in the incidence of diabetes, its clinical sequelae, and associated costs in pregnancy. Diabetes/Metab. Res. Rev. 2015, 31, 707–716. [Google Scholar] [CrossRef]
- Kanda, E.; Matsuda, Y.; Makino, Y.; Matsui, H. Risk factors associated with altered fetal growth in patients with pregestational diabetes mellitus. J. Matern. Fetal Neonatal Med. 2012, 25, 1390–1394. [Google Scholar] [CrossRef]
- Kattini, R.; Poirier, J.N.; Kelly, L.F.; Madden, S.N.; Ockenden, H.; Dooley, J.P.; Hummelen, R.B. Outcomes of Pregnancies Affected by Gestational Diabetes and Type 2 Diabetes in a Rural First Nations Obstetrical Program in Northwest Ontario. Can. J. Diabetes 2020, 44, 624–627. [Google Scholar] [CrossRef] [PubMed]
- Kekki, M.; Tihtonen, K.; Salonen, A.; Koukkula, T.; Gissler, M.; Laivuori, H.; Huttunen, T.T. Severe birth injuries in neonates and associated risk factors for injury in mothers with different types of diabetes in Finland. Int. J. Gynecol. Obstet. 2022, 159, 195–203. [Google Scholar] [CrossRef] [PubMed]
- Knight, K.M.; Thornburg, L.L.; Pressman, E.K. Pregnancy outcomes in type 2 diabetic patients as compared with type 1 diabetic patients and nondiabetic controls. J. Reprod. Med. 2012, 57, 397–404. [Google Scholar]
- Knight, K.M.; Pressman, E.K.; Hackney, D.N.; Thornburg, L.L. Perinatal outcomes in type 2 diabetic patients compared with non-diabetic patients matched by body mass index. J. Matern. Neonatal Med. 2012, 25, 611–615. [Google Scholar] [CrossRef]
- Kohn, J.R.; Rajan, S.S.; Kushner, J.A.; Fox, K.A. Outcomes, care utilization, and expenditures in adolescent pregnancy complicated by diabetes. Pediatr. Diabetes 2019, 20, 769–777. [Google Scholar] [CrossRef]
- Kuc, S.; Wortelboer, E.; Koster, M.P.H.; de Valk, H.; Schielen, P.; Visser, G. Prediction of macrosomia at birth in type-1 and 2 diabetic pregnancies with biomarkers of early placentation. BJOG 2011, 118, 748–754. [Google Scholar] [CrossRef]
- Lai, F.Y.; Johnson, J.A.; Dover, D.; Kaul, P. Outcomes of singleton and twin pregnancies complicated by pre-existing diabetes and gestational diabetes: A population-based study in Alberta, Canada, 2005–2011. J. Diabetes 2016, 8, 45–55. [Google Scholar] [CrossRef]
- Lasheen, A.E.; Abdelbasit, O.B.; Seidahmed, M.Z.; Hussein, K.A.; Muqdad, A.M.; Al-Zahrani, M.H.; Farid, J.M.; Badr, H.A. Infants of diabetic mothers. A cohort study. Saudi Med. J. 2014, 35, 572–577. [Google Scholar] [PubMed]
- Lemaitre, M.; Bourdon, G.; Bruandet, A.; Lenne, X.; Subtil, D.; Rakza, T.; Vambergue, A. Pre-gestational diabetes and the risk of congenital heart defects in the offspring: A French nationwide study. Diabetes Metab. 2023, 49, 101446. [Google Scholar] [CrossRef]
- Lin, S.-F.; Kuo, C.-F.; Chiou, M.-J.; Chang, S.-H. Maternal and fetal outcomes of pregnant women with type 1 diabetes, a national population study. Oncotarget 2017, 8, 80679–80687. [Google Scholar] [CrossRef]
- Lindsay, R.S.; Walker, J.D.; Halsall, I.; Hales, C.N.; Calder, A.A.; Hamilton, B.A.; Johnstone, F.D. Insulin and insulin propeptides at birth in offspring of diabetic mothers. J. Clin. Endocrinol. Metab. 2003, 88, 1664–1671. [Google Scholar] [CrossRef][Green Version]
- Liu, S.; Joseph, K.; Lisonkova, S.; Rouleau, J.; Hof, M.V.D.; Sauve, R.; Kramer, M.S. Association between maternal chronic conditions and congenital heart defects: A population-based cohort study. Circulation 2013, 128, 583–589. [Google Scholar] [CrossRef] [PubMed]
- López-De-Andrés, A.; Perez-Farinos, N.; Hernández-Barrera, V.; Palomar-Gallego, M.A.; Carabantes-Alarcón, D.; Zamorano-León, J.J.; De Miguel-Diez, J.; Jimenez-Garcia, R. A Population-Based Study of Diabetes during Pregnancy in Spain (2009–2015): Trends in Incidence, Obstetric Interventions, and Pregnancy Outcomes. J. Clin. Med. 2020, 9, 582. [Google Scholar] [CrossRef]
- Loukovaara, M.; Leinonen, P.; Teramo, K.; Koistinen, R. Cord serum glycodelin concentrations in normal pregnancies and pregnancies complicated by diabetes. Arch. Gynecol. Obstet. 2004, 270, 161–164. [Google Scholar] [CrossRef]
- Luo, S.; Ran, X.; Zhang, M.; Hu, J.; Yang, D.; Zhu, D.; Zhao, J.; Xiao, X.; Guo, X.; Yang, T.; et al. Pregnancy outcomes in women with type 1 diabetes in China during 2004 to 2014: A retrospective study (the CARNATION Study). J. Diabetes 2022, 14, 5–14. [Google Scholar] [CrossRef] [PubMed]
- Metcalfe, A.; Sabr, Y.; A Hutcheon, J.; Donovan, L.; Lyons, J.; Burrows, J.; Joseph, K.S. Trends in Obstetric Intervention and Pregnancy Outcomes of Canadian Women with Diabetes in Pregnancy from 2004 to 2015. J. Endocr. Soc. 2017, 1, 1540–1549. [Google Scholar] [CrossRef]
- Mirghani, H.; Begam, M.; Bekdache, G.; Khan, F. Specialised fetal and maternal service: Outcome of pre-gestational diabetes. J. Obstet. Gynaecol. 2012, 32, 426–429. [Google Scholar] [CrossRef]
- Morgan, K.; Rahman, M.; Atkinson, M.; Zhou, S.-M.; Hill, R.; Khanom, A.; Paranjothy, S.; Brophy, S.; Bruce, A. Association of diabetes in pregnancy with child weight at birth, age 12 months and 5 years—A population-based electronic cohort study. PLoS ONE 2013, 8, e79803. [Google Scholar] [CrossRef]
- Ngwezi, D.P.; Savu, A.; Yeung, R.O.; Butalia, S.; Kaul, P. Temporal Trends in Type 1, Type 2, and Gestational Diabetes in Pregnancy: Impact of Rural Residence, Ethnicity, and Material Deprivation. Can. J. Diabetes 2023, 47, 672–679.e3. [Google Scholar] [CrossRef]
- A Owens, L.; Sedar, J.; Carmody, L.; Dunne, F. Comparing type 1 and type 2 diabetes in pregnancy- similar conditions or is a separate approach required? BMC Pregnancy Childbirth 2015, 15, 69. [Google Scholar] [CrossRef]
- Papageorghiou, A.T.; Yu, C.K.; Erasmus, I.E.; Cuckle, H.S.; Nicolaides, K.H. Assessment of risk for the development of pre-eclampsia by maternal characteristics and uterine artery Doppler. BJOG 2005, 112, 703–709. [Google Scholar] [CrossRef]
- Paré, E.; Parry, S.; McElrath, T.F.; Pucci, D.; Newton, A.; Lim, K.-H. Clinical Risk Factors for Preeclampsia in the 21st Century. Obstet. Gynecol. 2014, 124, 763–770. [Google Scholar] [CrossRef] [PubMed]
- Patel, E.M.; Goodnight, W.H.; James, A.H.; Grotegut, C.A. Temporal trends in maternal medical conditions and stillbirth. Am. J. Obstet. Gynecol. 2015, 212, 673.e1–673.e11. [Google Scholar] [CrossRef] [PubMed]
- Pereda, J.; Bove, I.; Pineyro, M.M. Excessive Maternal Weight and Diabetes Are Risk Factors for Macrosomia: A Cross-Sectional Study of 42,663 Pregnancies in Uruguay. Front. Endocrinol. 2020, 11, 588443. [Google Scholar] [CrossRef] [PubMed]
- Peticca, P.; Keely, E.J.; Walker, M.C.; Yang, Q.; Bottomley, J. Pregnancy Outcomes in Diabetes Subtypes: How Do They Compare? A Province-based Study of Ontario, 2005–2006. J. Obstet. Gynaecol. Can. 2009, 31, 487–496. [Google Scholar] [CrossRef]
- Praprotnik, D.; Meštrović, Z.; Vulić, M.; Radman, M.; Roje, D. Diabetes type 1 and pregnancy outcome at University Hospital Center Split—A retrospective study. Med. Flum. 2021, 57, 190–196. [Google Scholar] [CrossRef]
- Reddy, U.M.; Laughon, S.K.; Sun, L.; Troendle, J.; Willinger, M.; Zhang, J. Prepregnancy Risk Factors for Antepartum Stillbirth in the United States. Obstet. Gynecol. 2010, 116, 1119–1126. [Google Scholar] [CrossRef]
- Reitzle, L.; Heidemann, C.; Baumert, J.; Kaltheuner, M.; Adamczewski, H.; Icks, A.; Scheidt-Nave, C. Pregnancy complications in women with pregestational and gestational diabetes mellitus. Dtsch. Aerzteblatt Int. 2023, 120, 81–86. [Google Scholar] [CrossRef]
- Riskin, A.; Itzchaki, O.; Bader, D.; Iofe, A.; Toropine, A.; Riskin-Mashiah, S. Perinatal Outcomes in Infants of Mothers with Diabetes in Pregnancy. Isr. Med. Assoc. J. 2020, 22, 569–575. [Google Scholar] [PubMed]
- Schraw, J.M.; Langlois, P.H.; Lupo, P.J. Comprehensive assessment of the associations between maternal diabetes and structural birth defects in offspring: A phenome-wide association study. Ann. Epidemiol. 2021, 53, 14–20.e8. [Google Scholar] [CrossRef] [PubMed]
- Seah, J.; Kam, N.M.; Wong, L.; Tanner, C.; Shub, A.; Houlihan, C.; Ekinci, E.I. Risk factors for pregnancy outcomes in Type 1 and Type 2 diabetes. Intern. Med. J. 2021, 51, 78–86. [Google Scholar] [CrossRef]
- Al Serehi, A.; Ahmed, A.M.; Shakeel, F.; Alkhatani, K.; El-Bakri, N.K.; Buhari, B.A.M.; Al Mohareb, U.; Aljohani, N. A comparison on the prevalence and outcomes of gestational versus type 2 diabetes mellitus in 1718 Saudi pregnancies. Int. J. Clin. Exp. Med. 2015, 8, 11502–11507. [Google Scholar]
- Shefali, A.K.; Kavitha, M.; Deepa, R.; Mohan, V. Pregnancy outcomes in pre-gestational and gestational diabetic women in comparison to non-diabetic women—A prospective study in Asian Indian mothers (CURES-35). J. Assoc. Physicians India 2006, 54, 613–618. [Google Scholar]
- Shour, A.; Garacci, E.; Palatnik, A.; Dawson, A.Z.; Anguzu, R.; Walker, R.J.; Egede, L. Association between pregestational diabetes and mortality among appropriate-for-gestational age birthweight infants. J. Matern. Neonatal Med. 2022, 35, 5291–5300. [Google Scholar] [CrossRef]
- Son, K.H.; Lim, N.K.; Lee, J.; Cho, M.; Park, H. Comparison of maternal morbidity and medical costs during pregnancy and delivery between patients with gestational diabetes and patients with pre-existing diabetes. Diabet. Med. 2015, 32, 477–486. [Google Scholar] [CrossRef]
- Stanton, S.G.; Ryerson, E.; Moore, S.L.; Sullivan-Mahoney, M.; Couch, S.C. Hearing Screening Outcomes in Infants of Pregestational Diabetic Mothers. Am. J. Audiol. 2005, 14, 86–93. [Google Scholar] [CrossRef]
- Stogianni, A.; Lendahls, L.; Landin-Olsson, M.; Thunander, M. Obstetric and perinatal outcomes in pregnancies complicated by diabetes, and control pregnancies, in Kronoberg, Sweden. BMC Pregnancy Childbirth 2019, 19, 159. [Google Scholar] [CrossRef]
- Titmuss, A.; Barzi, F.; Barr, E.L.M.; Webster, V.; Wood, A.; Kelaart, J.; Kirkwood, M.; Connors, C.; Boyle, J.A.; Moore, E.; et al. Association between maternal hyperglycemia in pregnancy and offspring anthropometry in early childhood: The pandora wave 1 study. Int. J. Obes. 2023, 47, 1120–1131. [Google Scholar] [CrossRef] [PubMed]
- A Wahabi, H.; A Esmaeil, S.; Fayed, A.; Al-Shaikh, G.; Alzeidan, R.A. Pre-existing diabetes mellitus and adverse pregnancy outcomes. BMC Res. Notes 2012, 5, 496. [Google Scholar] [CrossRef]
- Wei, Y.; Xu, Q.; Yang, H.; Yang, Y.; Wang, L.; Chen, H.; Anderson, C.; Liu, X.; Song, G.; Li, Q.; et al. Preconception diabetes mellitus and adverse pregnancy outcomes in over 6.4 million women: A population-based cohort study in China. PLOS Med. 2019, 16, e1002926. [Google Scholar] [CrossRef] [PubMed]
- Wells, G.; Bleicher, K.; Han, X. Maternal Diabetes, Large-for-Gestational-Age Births, and First Trimester Pregnancy-Associated Plasma Protein-A. J. Clin. Endocrinol. Metab. 2015, 100, 2372–2379. [Google Scholar] [CrossRef]
- Wright, D.; Akolekar, R.; Syngelaki, A.; Poon, L.C.; Nicolaides, K.H. A Competing Risks Model in Early Screening for Preeclampsia. Fetal Diagn. Ther. 2012, 32, 171–178. [Google Scholar] [CrossRef]
- Xu, X.K.; A Wang, Y.; Li, Z.; Lui, K.; A Sullivan, E. Risk factors associated with preterm birth among singletons following assisted reproductive technology in Australia 2007–2009–a population-based retrospective study. BMC Pregnancy Childbirth 2014, 14, 406. [Google Scholar] [CrossRef]
- Xu, Q.; Lu, J.; Hu, J.; Ge, Z.; Zhu, D.; Bi, Y. Perinatal outcomes in pregnancies complicated by type 1 diabetes mellitus. Gynecol. Endocrinol. 2020, 36, 879–884. [Google Scholar] [CrossRef]
- Yang, G.-R.; Dye, T.D.; Li, D. Effects of pre-gestational diabetes mellitus and gestational diabetes mellitus on macrosomia and birth defects in Upstate New York. Diabetes Res. Clin. Pract. 2019, 155, 107811. [Google Scholar] [CrossRef] [PubMed]
- Yanit, K.E.; Snowden, J.M.; Cheng, Y.W.; Caughey, A.B. The impact of chronic hypertension and pregestational diabetes on pregnancy outcomes. Am. J. Obstet. Gynecol. 2012, 207, 333.e1–333.e6. [Google Scholar] [CrossRef]
- Yves, J.; Valerie, V.; Katrien, V.H.; Guy, M.; Grobman, W.A. Birth Weight in Type 1 Diabetic Pregnancy. Obstet. Gynecol. Int. 2010, 2010, 397623. [Google Scholar] [CrossRef]
- Zeki, R.; Oats, J.J.; Wang, A.Y.; Li, Z.; Homer, C.S.; Sullivan, E.A. Cesarean section and diabetes during pregnancy: An NSW population study using the Robson classification. J. Obstet. Gynaecol. Res. 2018, 44, 890–898. [Google Scholar] [CrossRef] [PubMed]
- International Diabetes Federation. IDF Diabetes Atlas, 10th ed.; International Diabetes Federation: Brussels, Belgium, 2021. [Google Scholar]
- American Diabetes Association Professional Practice Committee; ElSayed, N.A.; Aleppo, G.; Bannuru, R.R.; Bruemmer, D.; Collins, B.S.; Ekhlaspour, L.; Hilliard, M.E.; Johnson, E.L.; Khunti, K.; et al. 15. Management of Diabetes in Pregnancy: Standards of Care in Diabetes—2024. Diabetes Care 2024, 47 (Suppl. S1), S282–S294. [Google Scholar] [CrossRef]
- Dobrescu, A.; Nussbaumer-Streit, B.; Klerings, I.; Wagner, G.; Persad, E.; Sommer, I.; Herkner, H.; Gartlehner, G. Restricting evidence syntheses of interventions to English-language publications is a viable methodological shortcut for most medical topics: A systematic review. J. Clin. Epidemiol. 2021, 137, 209–217. [Google Scholar] [CrossRef] [PubMed]



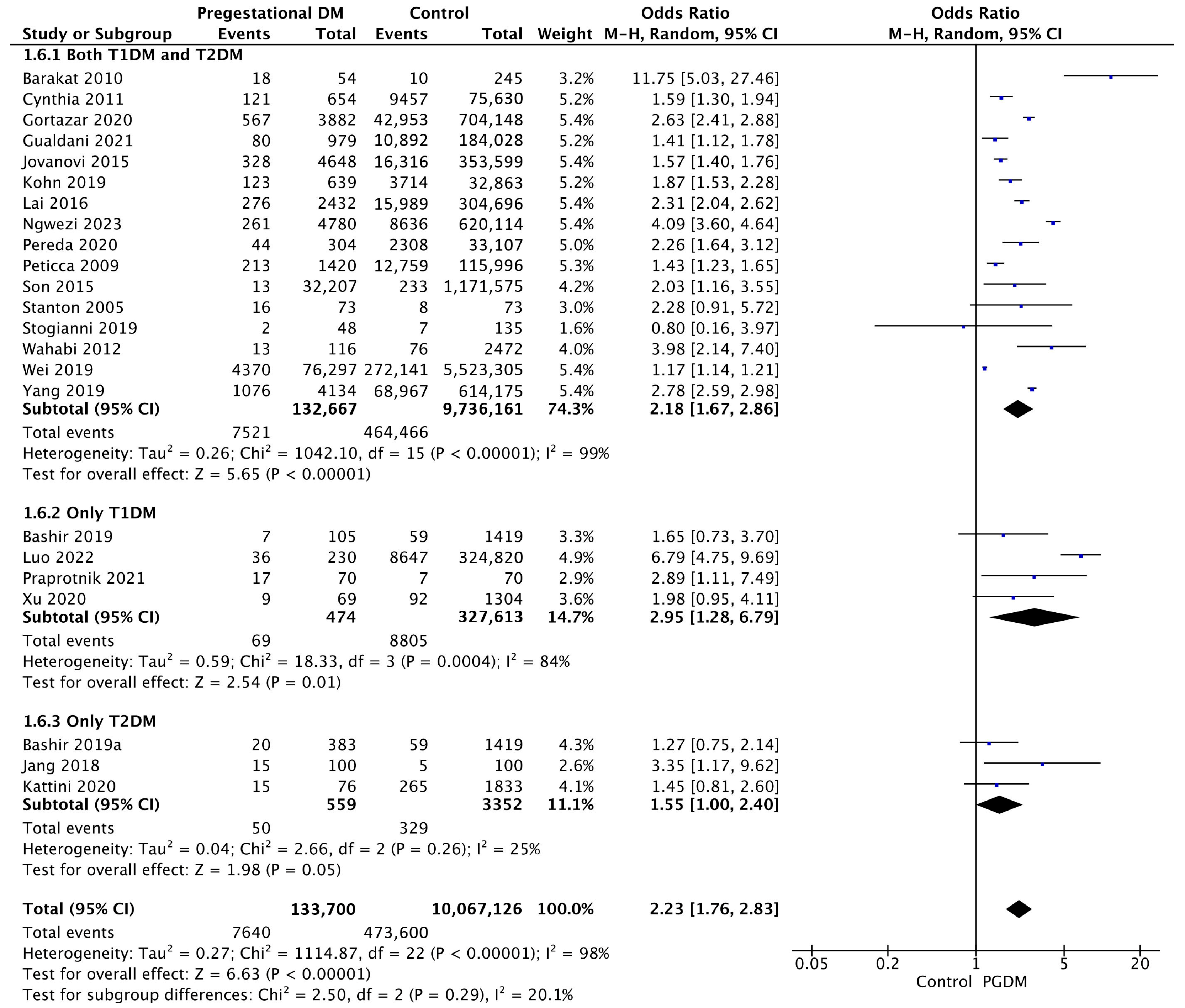
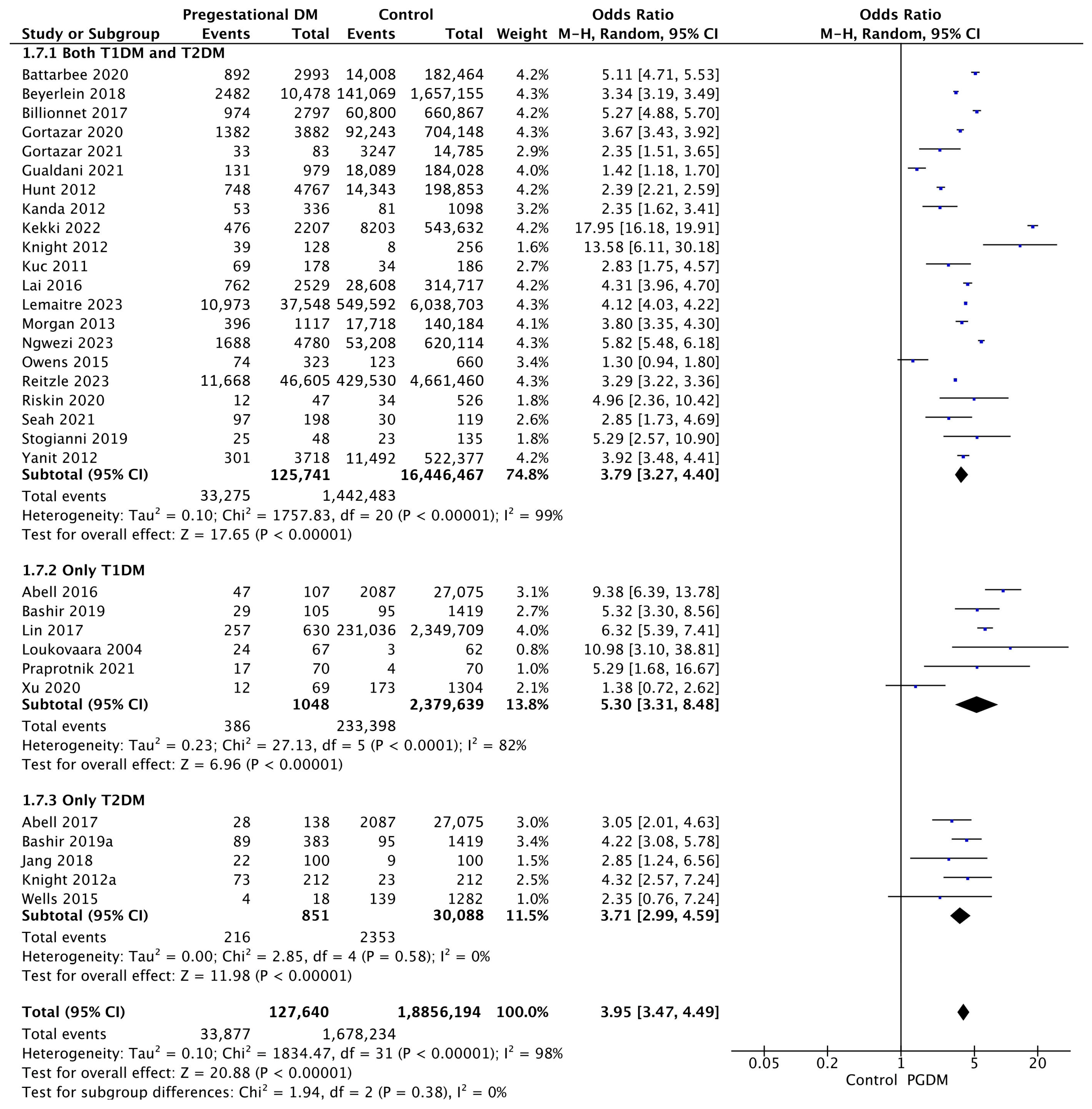
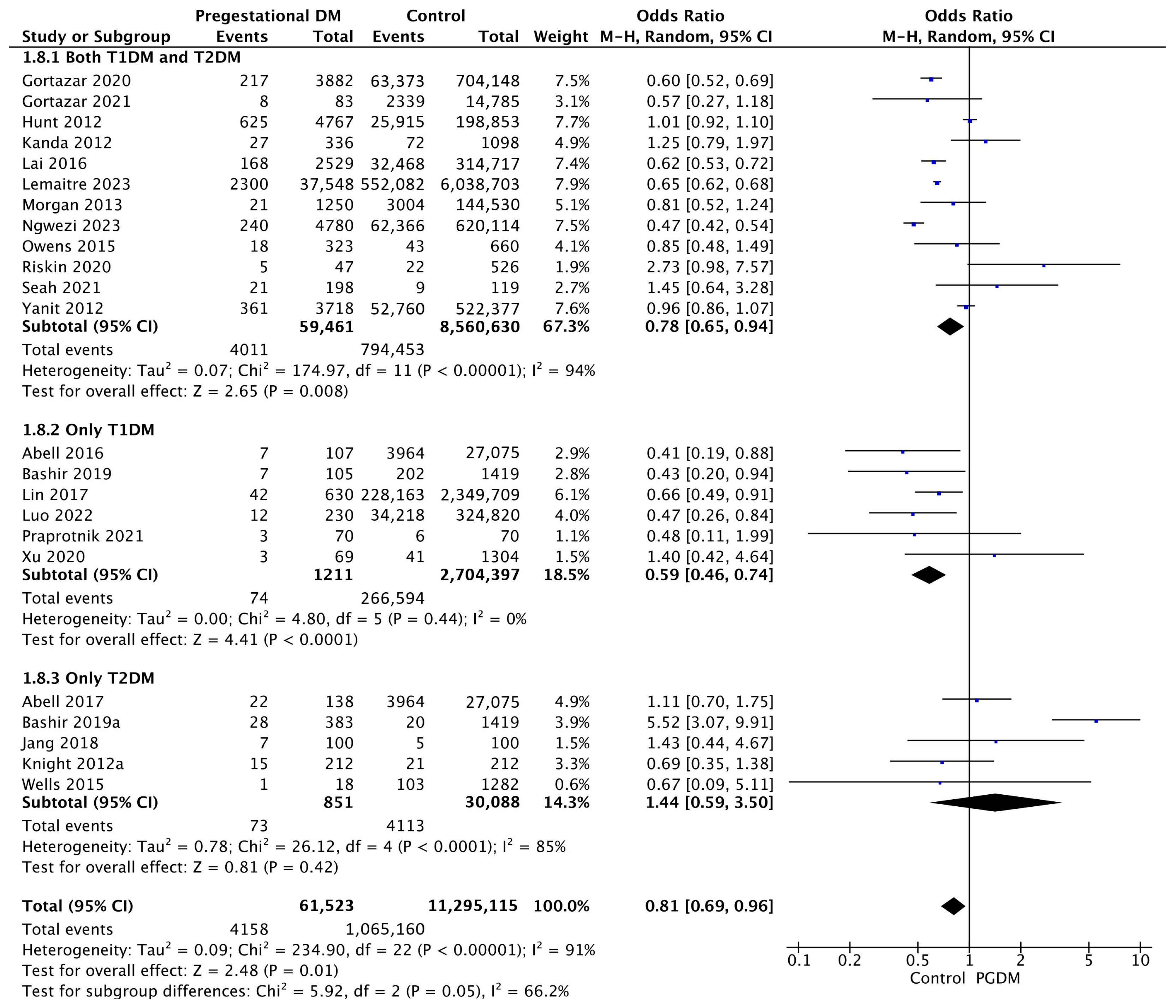
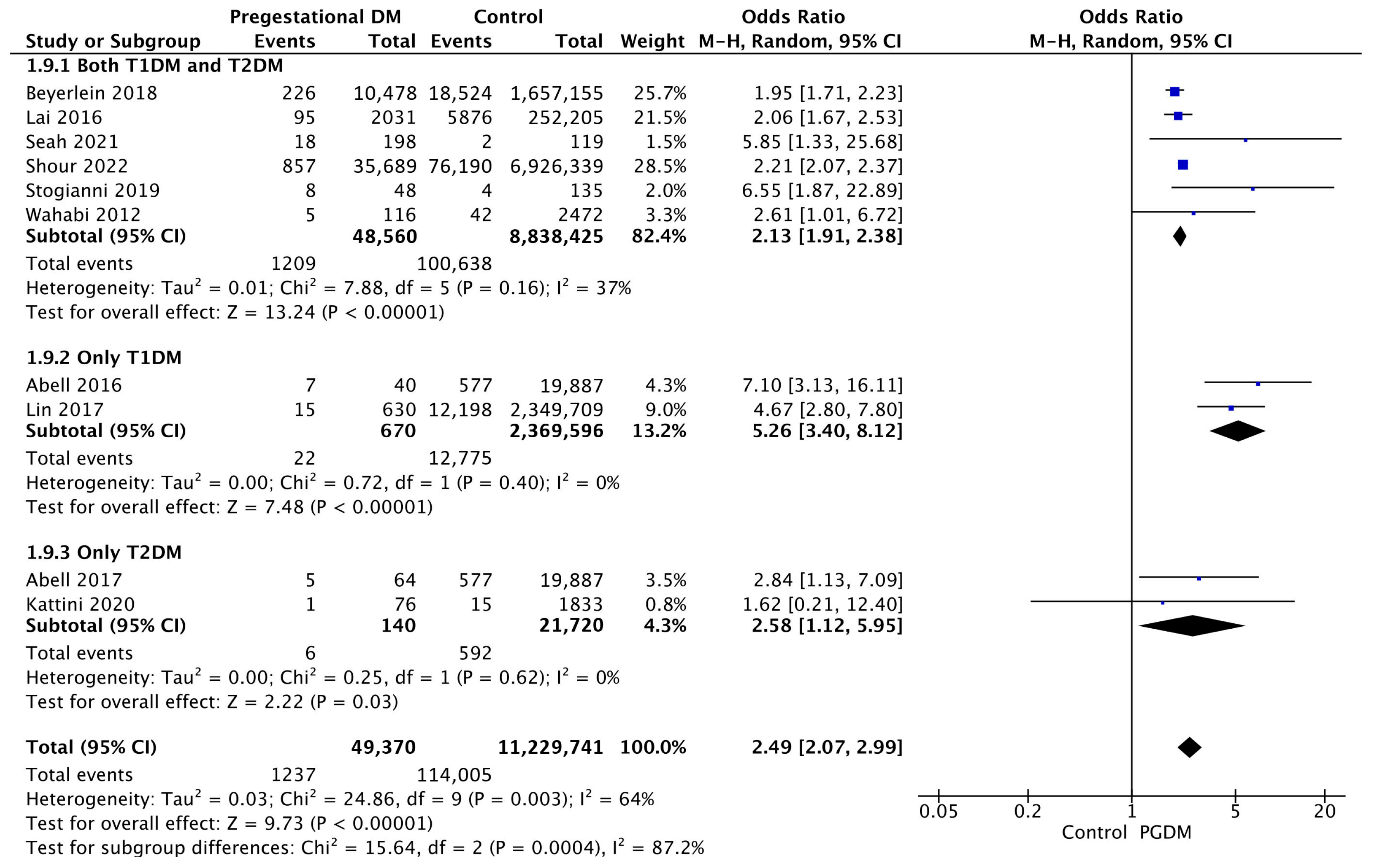




| Adverse Perinatal Outcome | Number of Studies | Odds Ratio [95% CI] | Pregnancies with PGDM | Pregnancies Without PGDM | p-Value | I2 |
|---|---|---|---|---|---|---|
| Gestational hypertension | 15 | 3.16 [2.65, 3.77] | 71,711 | 9,844,682 | p < 10−5 | 93% |
| Preeclampsia | 32 | 4.46 [3.94, 5.05] | 121,092 | 18,012,208 | p < 10−5 | 93% |
| Preterm delivery | 43 | 3.46 [3.06, 3.91] | 870,823 | 102,244,922 | p < 10−5 | 99% |
| Cesarean delivery | 45 | 3.12 [2.81, 3.47] | 831,571 | 102,988,606 | p < 10−5 | 100% |
| Induction of labor | 14 | 2.92 [2.35, 3.63] | 38,013 | 6,369,376 | p < 10−5 | 98% |
| Macrosomia | 23 | 2.23 [1.76, 2.83] | 133,700 | 10,067,126 | p < 10−5 | 98% |
| LGA neonates | 32 | 3.95 [3.47, 4.49] | 127,640 | 18,856,194 | p < 10−5 | 98% |
| SGA neonates | 23 | 0.81 [0.69, 0.96] | 61,523 | 11,295,115 | p = 0.01 | 91% |
| Low 5-min Apgar score | 10 | 2.49 [2.07, 2.99] | 49,370 | 11,229,741 | p < 10−5 | 64% |
| Shoulder dystocia | 13 | 3.05 [2.07, 4.50] | 240,304 | 50,814,646 | p < 10−5 | 95% |
| Birth trauma | 4 | 1.40 [1.22, 1.62] | 5056 | 621,059 | p < 10−5 | 44% |
| Polyhydramnios | 7 | 5.06 [4.33, 5.91] | 2177 | 29,140 | p < 10−5 | 44% |
| Oligohydramnios | 2 | 1.61 [1.19, 2.17] | 1283 | 22,872 | p = 0.002 | 36% |
| Neonatal hyperbilirubinemia | 14 | 3.45 [2.51, 4.74] | 6726 | 682,292 | p < 10−5 | 86% |
| Neonatal hypoglycemia | 12 | 19.19 [2.78, 132.61] | 6557 | 680,888 | p = 0.003 | 100% |
| NICU admission | 18 | 4.54 [3.87, 5.34] | 50,357 | 7,735,598 | p < 10−5 | 94% |
| Congenital malformation | 30 | 2.44 [1.96, 3.04] | 210,265 | 25,877,314 | p < 10−5 | 98% |
| Stillbirth | 17 | 2.87 [2.27, 3.63] | 207,142 | 22,776,747 | p < 10−5 | 90% |
| Perinatal mortality | 13 | 2.94 [2.18, 3.98] | 189,759 | 24,513,106 | p < 10−5 | 93% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gazis, D.; Tranidou, A.; Siargkas, A.; Apostolopoulou, A.; Koutsouki, G.; Goulis, D.G.; Tsakalidis, C.; Tsakiridis, I.; Dagklis, T. Pregestational Diabetes Mellitus and Adverse Perinatal Outcomes: A Systematic Review and Meta-Analysis. J. Clin. Med. 2025, 14, 4789. https://doi.org/10.3390/jcm14134789
Gazis D, Tranidou A, Siargkas A, Apostolopoulou A, Koutsouki G, Goulis DG, Tsakalidis C, Tsakiridis I, Dagklis T. Pregestational Diabetes Mellitus and Adverse Perinatal Outcomes: A Systematic Review and Meta-Analysis. Journal of Clinical Medicine. 2025; 14(13):4789. https://doi.org/10.3390/jcm14134789
Chicago/Turabian StyleGazis, Dionysios, Antigoni Tranidou, Antonios Siargkas, Aikaterini Apostolopoulou, Georgia Koutsouki, Dimitrios G. Goulis, Christos Tsakalidis, Ioannis Tsakiridis, and Themistoklis Dagklis. 2025. "Pregestational Diabetes Mellitus and Adverse Perinatal Outcomes: A Systematic Review and Meta-Analysis" Journal of Clinical Medicine 14, no. 13: 4789. https://doi.org/10.3390/jcm14134789
APA StyleGazis, D., Tranidou, A., Siargkas, A., Apostolopoulou, A., Koutsouki, G., Goulis, D. G., Tsakalidis, C., Tsakiridis, I., & Dagklis, T. (2025). Pregestational Diabetes Mellitus and Adverse Perinatal Outcomes: A Systematic Review and Meta-Analysis. Journal of Clinical Medicine, 14(13), 4789. https://doi.org/10.3390/jcm14134789









