Association Between Left Atrial Epicardial Adipose Tissue Attenuation Assessed by Cardiac Computed Tomography and Atrial Fibrillation Recurrence Following Catheter Ablation: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Methods
2.1. Search Strategy
2.2. Inclusion and Exclusion Criteria
- Inclusion Criteria: Original studies (prospective or retrospective) involving adult patients with paroxysmal or persistent AF undergoing CA (radiofrequency or cryoballoon ablation). Studies were required to provide quantitative measurements of LA-EAT attenuation on cardiac CT before ablation, employing established Hounsfield unit (HU) ranges. Included studies were required to provide AF recurrence outcomes throughout a follow-up period of no less than 6 months post-ablation, using a 3-month post-ablation blanking period for the definition of AF recurrence.
- Exclusion Criteria: We eliminated case reports, conference abstracts, review papers, editorials, and research that lacked original patient data. We eliminated studies that did not specifically evaluate LA-EAT attenuation, such as those that simply assessed the EAT volume or thickness without attenuation data. If many publications presented overlapping patient groups, the most complete or latest study was selected to prevent data duplication. We also eliminated research in languages other than English or without a clearly defined AF recurrence outcome post-ablation.
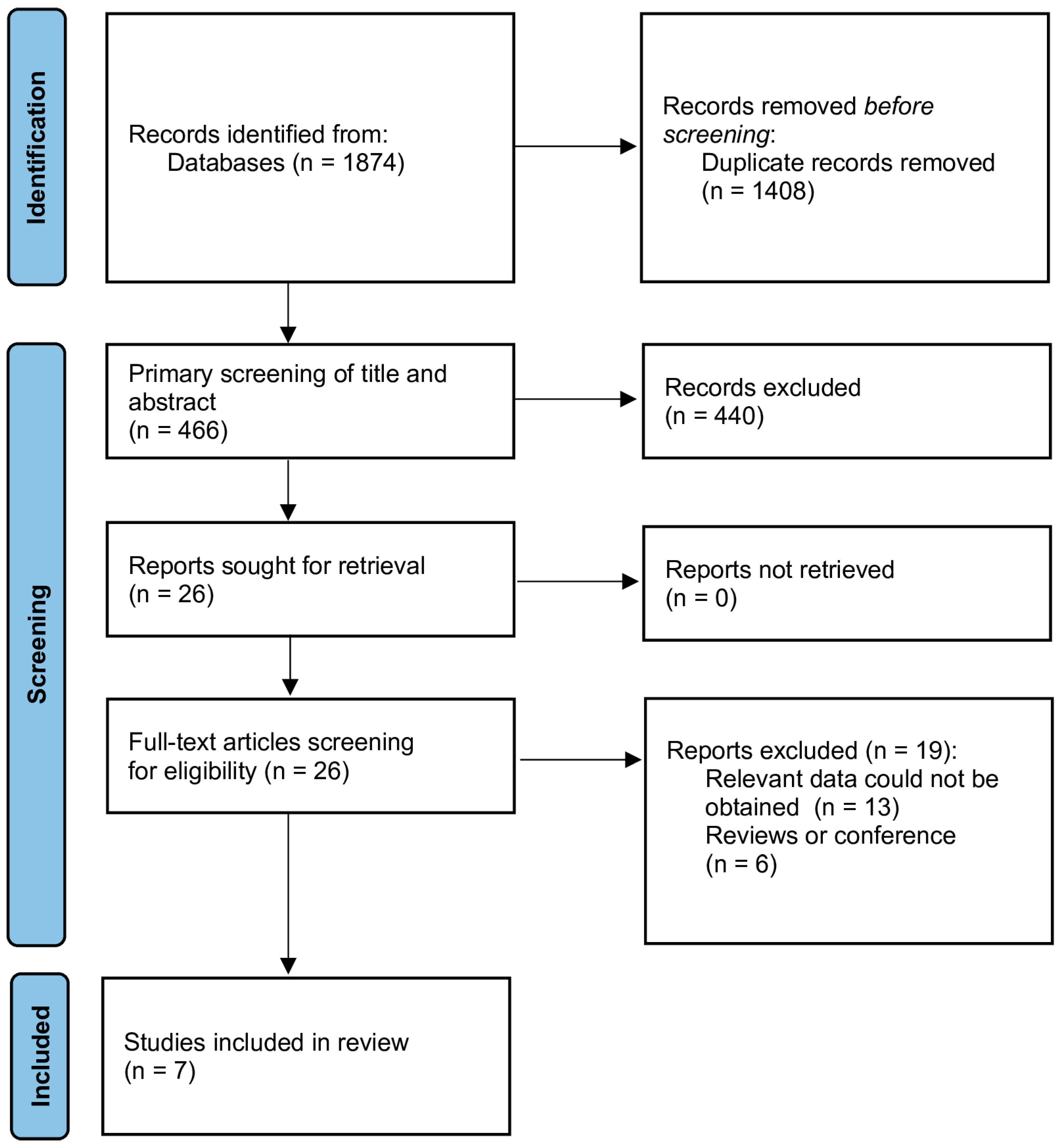
2.3. Study Selection
2.4. Data Extraction
2.5. Risk of Bias Assessment
2.6. Data Synthesis and Analysis
3. Results
3.1. Description of Selected Studies
3.2. Study Characteristics
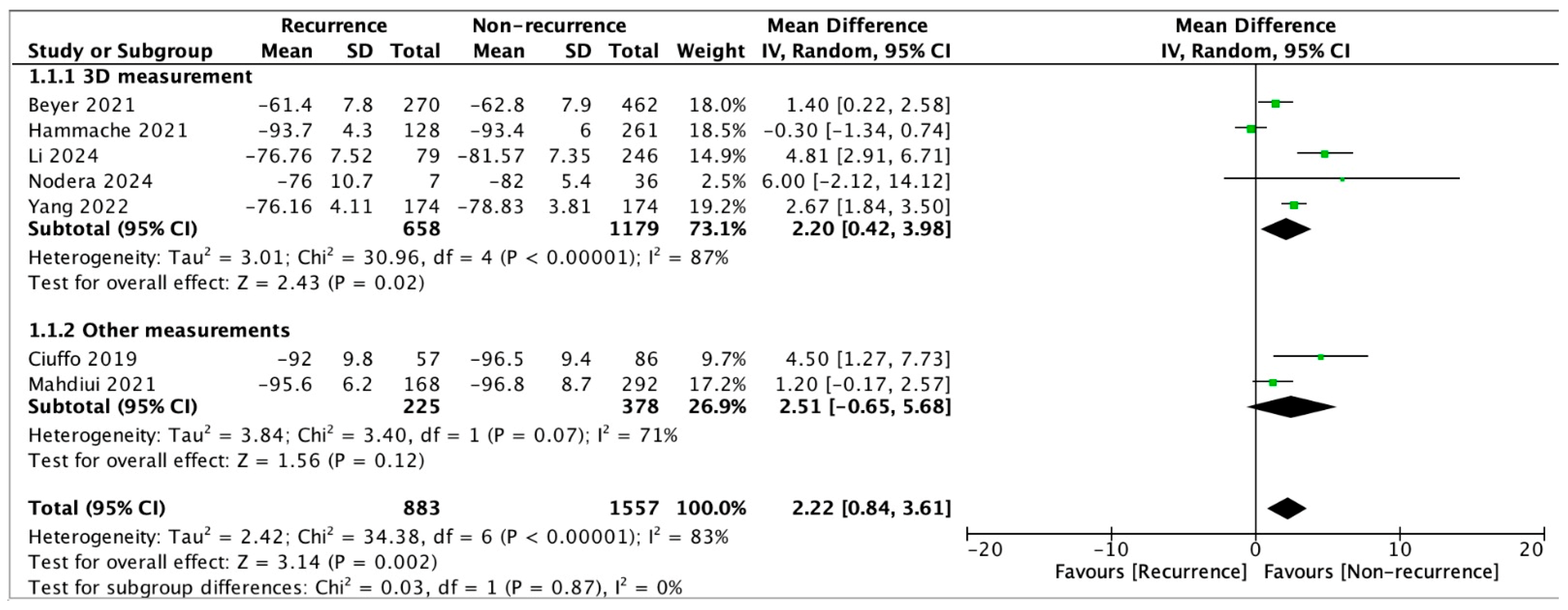
3.3. Results from Meta-Analysis
4. Discussion
Limitations of the Study
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Benjamin, E.J.; Wolf, P.A.; D’Agostino, R.B.; Silbershatz, H.; Kannel, W.B.; Levy, D. Impact of atrial fibrillation on the risk of death: The Framingham Heart Study. Circulation 1998, 98, 946–952. [Google Scholar] [CrossRef] [PubMed]
- Linz, D.; Gawalko, M.; Betz, K.; Hendriks, J.M.; Lip, G.Y.H.; Vinter, N.; Guo, Y.; Johnsen, S. Atrial fibrillation: Epidemiology, screening and digital health. Lancet Reg. Health Eur. 2024, 37, 100786. [Google Scholar] [CrossRef]
- Van Gelder, I.C.; Rienstra, M.; Bunting, K.V.; Casado-Arroyo, R.; Caso, V.; Crijns, H.J.G.M.; De Potter, T.J.R.; Dwight, J.; Guasti, L.; Hanke, T.; et al. 2024 ESC Guidelines for the management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS). Eur. Heart J. 2024, 45, 3314–3414. [Google Scholar] [CrossRef]
- Ngo, L.; Lee, X.W.; Elwashahy, M.; Arumugam, P.; Yang, I.A.; Denman, R.; Haqqani, H.; Ranasinghe, I. Freedom from atrial arrhythmia and other clinical outcomes at 5 years and beyond after catheter ablation of atrial fibrillation: A systematic review and meta-analysis. Eur. Heart J. Qual. Care Clin. Outcomes 2023, 9, 447–458. [Google Scholar] [CrossRef]
- Iacobellis, G. Epicardial adipose tissue in contemporary cardiology. Nat. Rev. Cardiol. 2022, 19, 593–606. [Google Scholar] [CrossRef]
- Iacobellis, G.; Corradi, D.; Sharma, A.M. Epicardial adipose tissue: Anatomic, biomolecular and clinical relationships with the heart. Nat. Clin. Pract. Cardiovasc. Med. 2005, 2, 536–543. [Google Scholar] [CrossRef] [PubMed]
- Iacobellis, G.; Barbaro, G. The double role of epicardial adipose tissue as pro- and anti-inflammatory organ. Horm. Metab. Res. 2008, 40, 442–445. [Google Scholar] [CrossRef]
- Iacobellis, G. Aging Effects on Epicardial Adipose Tissue. Front. Aging 2021, 2, 666260. [Google Scholar] [CrossRef] [PubMed]
- Ernault, A.C.; Meijborg, V.M.F.; Coronel, R. Modulation of Cardiac Arrhythmogenesis by Epicardial Adipose Tissue: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2021, 78, 1730–1745. [Google Scholar] [CrossRef]
- Shamloo, A.S.; Dagres, N.; Dinov, B.; Sommer, P.; Husser-Bollmann, D.; Bollmann, A.; Hindricks, G.; Arya, A. Is epicardial fat tissue associated with atrial fibrillation recurrence after ablation? A systematic review and meta-analysis. Int. J. Cardiol. Heart Vasc. 2019, 22, 132–138. [Google Scholar] [CrossRef]
- Chen, J.; Mei, Z.; Yang, Y.; Dai, C.; Wang, Y.; Zeng, R.; Liu, Q. Epicardial adipose tissue is associated with higher recurrence risk after catheter ablation in atrial fibrillation patients: A systematic review and meta-analysis. BMC Cardiovasc. Disord. 2022, 22, 264. [Google Scholar] [CrossRef] [PubMed]
- Anagnostopoulos, I.; Kousta, M.; Kossyvakis, C.; Paraskevaidis, N.T.; Vrachatis, D.; Deftereos, S.; Giannopoulos, G. Epicardial Adipose Tissue and Atrial Fibrillation Recurrence following Catheter Ablation: A Systematic Review and Meta-Analysis. J. Clin. Med. 2023, 12, 6369. [Google Scholar] [CrossRef] [PubMed]
- Goeller, M.; Achenbach, S.; Cadet, S.; Kwan, A.C.; Commandeur, F.; Slomka, P.J.; Gransar, H.; Albrecht, M.H.; Tamarappoo, B.K.; Berman, D.S.; et al. Pericoronary Adipose Tissue Computed Tomography Attenuation and High-Risk Plaque Characteristics in Acute Coronary Syndrome Compared With Stable Coronary Artery Disease. JAMA Cardiol. 2018, 3, 858–863. [Google Scholar] [CrossRef]
- Antonopoulos, A.S.; Sanna, F.; Sabharwal, N.; Thomas, S.; Oikonomou, E.K.; Herdman, L.; Margaritis, M.; Shirodaria, C.; Kampoli, A.-M.; Akoumianakis, I.; et al. Detecting human coronary inflammation by imaging perivascular fat. Sci. Transl. Med. 2017, 9, eaal2658. [Google Scholar] [CrossRef]
- El Mahdiui, M.; Simon, J.; Smit, J.M.; Kuneman, J.H.; van Rosendael, A.R.; Steyerberg, E.W.; van der Geest, R.J.; Szaraz, L.; Herczeg, S.; Szegedi, N.; et al. Posterior Left Atrial Adipose Tissue Attenuation Assessed by Computed Tomography and Recurrence of Atrial Fibrillation After Catheter Ablation. Circ. Arrhythm. Electrophysiol. 2021, 14, e009135. [Google Scholar] [CrossRef]
- Tuttolomondo, D.; Martini, C.; Nicolini, F.; Formica, F.; Pini, A.; Secchi, F.; Volpi, R.; De Filippo, M.; Gaibazzi, N. Perivascular Adipose Tissue Attenuation on Computed Tomography beyond the Coronary Arteries. A Syst. Review. Diagn. 2021, 11, 1495. [Google Scholar] [CrossRef] [PubMed]
- Oikonomou, E.K.; Marwan, M.; Desai, M.Y.; Mancio, J.; Alashi, A.; Centeno, E.H.; Thomas, S.; Herdman, L.; Kotanidis, C.P.; Thomas, K.E.; et al. Non-invasive detection of coronary inflammation using computed tomography and prediction of residual cardiovascular risk (the CRISP CT study): A post-hoc analysis of prospective outcome data. Lancet 2018, 392, 929–939. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Stang, A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur. J. Epidemiol. 2010, 25, 603–605. [Google Scholar] [CrossRef]
- Hozo, S.P.; Djulbegovic, B.; Hozo, I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med. Res. Methodol. 2005, 5, 13. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Altman, D.G.; Gøtzsche, P.C.; Jüni, P.; Moher, D.; Oxman, A.D.; Savović, J.; Schulz, K.F.; Weeks, L.; Sterne, J.A.C.; et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011, 343, d5928. [Google Scholar] [CrossRef]
- Beyer, C.; Tokarska, L.; Stühlinger, M.; Feuchtner, G.; Hintringer, F.; Honold, S.; Fiedler, L.; Schönbauer, M.-S.; Schönbauer, R.; Plank, F. Structural Cardiac Remodeling in Atrial Fibrillation. JACC Cardiovasc. Imaging 2021, 14, 2199–2208. [Google Scholar] [CrossRef] [PubMed]
- Hammache, N.; Pegorer-Sfes, H.; Benali, K.; Poull, I.M.; Olivier, A.; Echivard, M.; Pace, N.; Minois, D.; Sadoul, N.; Mandry, D.; et al. Is There an Association between Epicardial Adipose Tissue and Outcomes after Paroxysmal Atrial Fibrillation Catheter Ablation? J. Clin. Med. 2021, 10, 3037. [Google Scholar] [CrossRef]
- Li, X.; Wang, Z.; Wang, S.; Chen, W.; Li, C.; Zhang, Y.; Sun, A.; Xie, L.; Hu, C. Combining computed tomography features of left atrial epicardial and pericoronary adipose tissue with the triglyceride-glucose index to predict the recurrence of atrial fibrillation after radiofrequency catheter ablation: A machine learning study. Quant. Imaging Med. Surg. 2024, 14, 9306–9322. [Google Scholar] [CrossRef] [PubMed]
- Nodera, M.; Ishida, T.; Hasegawa, K.; Kakehashi, S.; Mukai, M.; Aoyama, D.; Miyazaki, S.; Uzui, H.; Tada, H. Epicardial adipose tissue density predicts the presence of atrial fibrillation and its recurrence after catheter ablation: Three-dimensional reconstructed image analysis. Heart Vessels 2024, 39, 696–705. [Google Scholar] [CrossRef]
- Yang, M.; Bao, W.; Xu, Z.; Qin, L.; Zhang, N.; Yan, F.; Yang, W. Association between epicardial adipose tissue and recurrence of atrial fibrillation after ablation: A propensity score-matched analysis. Int. J. Cardiovasc. Imaging 2022, 38, 1865–1872. [Google Scholar] [CrossRef] [PubMed]
- Ciuffo, L.; Nguyen, H.; Marques, M.D.; Aronis, K.N.; Sivasambu, B.; de Vasconcelos, H.D.; Tao, S.; Spragg, D.D.; Marine, J.E.; Berger, R.D.; et al. Periatrial Fat Quality Predicts Atrial Fibrillation Ablation Outcome. Circ. Cardiovasc. Imaging 2019, 12, e008764. [Google Scholar] [CrossRef]
- Verma, A.; Wazni, O.M.; Marrouche, N.F.; Martin, D.O.; Kilicaslan, F.; Minor, S.; Schweikert, R.A.; Saliba, W.; Cummings, J.; Burkhardt, J.D.; et al. Pre-existent left atrial scarring in patients undergoing pulmonary vein antrum isolation: An independent predictor of procedural failure. J. Am. Coll. Cardiol. 2005, 45, 285–292. [Google Scholar] [CrossRef]
- Iacobellis, G. Local and systemic effects of the multifaceted epicardial adipose tissue depot. Nat. Rev. Endocrinol. 2015, 11, 363–371. [Google Scholar] [CrossRef]
- Abe, I.; Teshima, Y.; Kondo, H.; Kaku, H.; Kira, S.; Ikebe, Y.; Saito, S.; Fukui, A.; Shinohara, T.; Yufu, K.; et al. Association of fibrotic remodeling and cytokines/chemokines content in epicardial adipose tissue with atrial myocardial fibrosis in patients with atrial fibrillation. Heart Rhythm. 2018, 15, 1717–1727. [Google Scholar] [CrossRef]
- Klein, C.; Brunereau, J.; Lacroix, D.; Ninni, S.; Brigadeau, F.; Klug, D.; Longere, B.; Montaigne, D.; Pontana, F.; Coisne, A. Left atrial epicardial adipose tissue radiodensity is associated with electrophysiological properties of atrial myocardium in patients with atrial fibrillation. Eur. Radiol. 2019, 29, 3027–3035. [Google Scholar] [CrossRef]
- Nuzzi, V.; Raafs, A.; Manca, P.; Henkens, M.T.; Gregorio, C.; Boscutti, A.; Verdonschot, J.; Hazebroek, M.; Knackstedt, C.; Merlo, M.; et al. Left Atrial Reverse Remodeling in Dilated Cardiomyopathy. J. Am. Soc. Echocardiogr. 2023, 36, 154–162. [Google Scholar] [CrossRef]
- Zhao, L.; Harrop, D.L.; Ng, A.C.T.; Wang, W.Y.S. Epicardial Adipose Tissue Is Associated With Left Atrial Dysfunction in People Without Obstructive Coronary Artery Disease or Atrial Fibrillation. Can. J. Cardiol. 2018, 34, 1019–1025. [Google Scholar] [CrossRef] [PubMed]
- Myasoedova, V.A.; Parisi, V.; Moschetta, D.; Valerio, V.; Conte, M.; Massaiu, I.; Bozzi, M.; Celeste, F.; Leosco, D.; Iaccarino, G.; et al. Efficacy of cardiometabolic drugs in reduction of epicardial adipose tissue: A systematic review and meta-analysis. Cardiovasc. Diabetol. 2023, 22, 23. [Google Scholar] [CrossRef]
- Ziyrek, M.; Kahraman, S.; Ozdemir, E.; Dogan, A. Metformin monotherapy significantly decreases epicardial adipose tissue thickness in newly diagnosed type 2 diabetes patients. Rev. Port. Cardiol. 2019, 38, 419–423. [Google Scholar] [CrossRef]
- Iacobellis, G.; Mohseni, M.; Bianco, S.D.; Banga, P.K. Liraglutide causes large and rapid epicardial fat reduction. Obesity 2017, 25, 311–316. [Google Scholar] [CrossRef] [PubMed]
- Christensen, R.H.; Wedell-Neergaard, A.-S.; Lehrskov, L.L.; Legaard, G.E.; Dorph, E.; Larsen, M.K.; Launbo, N.; Fagerlind, S.R.; Seide, S.K.; Nymand, S.; et al. Effect of Aerobic and Resistance Exercise on Cardiac Adipose Tissues: Secondary Analyses From a Randomized Clinical Trial. JAMA Cardiol. 2019, 4, 778–787. [Google Scholar] [CrossRef] [PubMed]
- Iacobellis, G.; Singh, N.; Wharton, S.; Sharma, A.M. Substantial changes in epicardial fat thickness after weight loss in severely obese subjects. Obesity 2008, 16, 1693–1697. [Google Scholar] [CrossRef]
- Mine, T.; Shintaku, Y.; Terao, S.; Sugitani, M.; Kogame, T.; Ishihara, M. Sodium-glucose cotransporter 2 inhibitors are more effective than dipeptidyl peptidase 4 inhibitors in preventing mid-term recurrence after atrial fibrillation ablation. EP Europace 2025, 27, euaf085.323. [Google Scholar] [CrossRef]
- Karakasis, P.; Fragakis, N.; Patoulias, D.; Theofilis, P.; Kassimis, G.; Karamitsos, T.; El-Tanani, M.; Rizzo, M. Effects of Glucagon-Like Peptide 1 Receptor Agonists on Atrial Fibrillation Recurrence After Catheter Ablation: A Systematic Review and Meta-analysis. Adv. Ther. 2024, 41, 3749–3756. [Google Scholar] [CrossRef]
- Mir, J.; Haider, M.Z.; Hamza, M.; Ishaq, S.; Al-Ahmad, M.; Khalil, A.; Weiss, R. Impact of SGLT2 Inhibitors on Atrial Fibrillation Recurrence Following Catheter Ablation: A Meta-Analysis. JACC 2025, 85, 227. [Google Scholar] [CrossRef]
- Cruz, I.; Fernandes, S.L.; Diaz, S.O.; Saraiva, F.; Barros, A.S.; Primo, J.; Sampaio, F.; Ladeiras-Lopes, R.; Fontes-Carvalho, R. Epicardial adipose tissue volume is not an independent predictor of atrial fibrillation recurrence after catheter ablation. Rev. Esp. Cardiol. 2023, 76, 539–547. [Google Scholar] [CrossRef]
- Huber, A.T.; Fankhauser, S.; Chollet, L.; Wittmer, S.; Lam, A.; Baldinger, S.; Madaffari, A.; Seiler, J.; Servatius, H.; Haeberlin, A.; et al. The Relationship between Enhancing Left Atrial Adipose Tissue at CT and Recurrent Atrial Fibrillation. Radiology 2022, 305, 56–65. [Google Scholar] [CrossRef]
- Huber, A.T.; Fankhauser, S.; Wittmer, S.; Chollet, L.; Lam, A.; Maurhofer, J.; Madaffari, A.; Seiler, J.; Servatius, H.; Haeberlin, A.; et al. Epicardial adipose tissue dispersion at CT and recurrent atrial fibrillation after pulmonary vein isolation. Eur. Radiol. 2024, 34, 4928–4938. [Google Scholar] [CrossRef] [PubMed]
- Sang, C.; Hu, X.; Zhang, D.; Shao, Y.; Qiu, B.; Li, C.; Li, F.; Zhang, C.; Wang, Z.; Chen, M. The predictive value of left atrium epicardial adipose tissue on recurrence after catheter ablation in patients with different types of atrial fibrillation. Int. J. Cardiol. 2023, 379, 33–39. [Google Scholar] [CrossRef]
- Feng, L.; Li, L.; Bai, L.; Tang, L.; Zhao, Y.; Zhao, X. The association between features of epicardial adipose tissue and the risks of early recurrence after catheter ablation in patients with atrial fibrillation. Front. Cardiovasc. Med. 2025, 12, 1480473. [Google Scholar] [CrossRef]
| Author, Year | Region | Study Type | N | Males (%) | Age (y) | BMI | Paroxysmal AF (%) | Follow-Up (m) | EAT Assessment Methods | CA Method | Additional Ablation Lesions | CA Success Rate (%) | NOS Score |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Beyer, 2021 [22] | Innsbruck, Austria | Retrospective, OA | 732 | 73% | 57.5 | 26.9 | 88.4% | 31 m | 3D; EAT surrounding LA | RFA 36.9% CBA 63.1% | 0% | 63.1% | 8 |
| Hammache, 2021 [23] | Vandœuvre-lès-Nancy, France | Retrospective, OA | 389 | 65.8% | 58.1 | 27.1 | 100% | 12 m | 3D; EAT surrounding LA | RFA 100% | 0% | 67.1% | 7 |
| Li, 2024 [24] | Xuzhou, China | Retrospective, OA | 325 | 60.9% | 60.6 | 25.5 | 59.1% | 11.5 m | 3D; EAT surrounding LA | RFA 100% | No data | 75.7% | 8 |
| Nodera, 2024 [25] | Fukui, Japan | Retrospective, OA | 43 | 65% | 68.2 | 24.0 | 53% | 20 m | 3D; EAT surrounding LA | RFA 20.9% CBA 79.1% | 25.6% | 83.8% | 7 |
| Yang, 2022 [26] | Shanghai, China | Retrospective, OA | 348 | 63.5% | Non-recurrence: 63.0; Recurrence: 64.0 (medians) | Non-recurrence: 24.49; Recurrence: 24.68 (medians) | 100% | 12 m | 3D; EAT surrounding LA | CBA 100% | No data | 74.4% | 8 |
| Ciuffo, 2019 [27] | Baltimore, USA | Retrospective, OA | 143 | 63.6% | 62.2 | 32.4 | 59.4% | at least 12 m | 2D; EAT surrounding LA | RFA 74.1% CBA 25.9% | 11.9% | 60.1% | 8 |
| Mahdiui, 2021 [15] | Budapest, Hungary | Retrospective, OA | 460 | 65.7% | 61 | 29 | 77.0% | 18 m (median) | 3D; EAT posterior to LA | RFA 100% | No data | 63.5% | 8 |
| Covariate | Coefficient (β) | p-Value | 95% CI Lower | 95% CI Upper |
|---|---|---|---|---|
| Intercept | 14.51 | 0.630 | −266.16 | 295.19 |
| Age | −0.23 | 0.551 | −3.61 | 3.16 |
| BMI | 0.27 | 0.391 | −2.16 | 2.70 |
| % Paroxysmal AF | −0.03 | 0.534 | −0.51 | 0.44 |
| Follow-up (months) | −0.09 | 0.439 | −1.09 | 0.90 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Momot, K.; Krauz, K.; Pruc, M.; Szarpak, L.; Rodkiewicz, D.; Mamcarz, A. Association Between Left Atrial Epicardial Adipose Tissue Attenuation Assessed by Cardiac Computed Tomography and Atrial Fibrillation Recurrence Following Catheter Ablation: A Systematic Review and Meta-Analysis. J. Clin. Med. 2025, 14, 4771. https://doi.org/10.3390/jcm14134771
Momot K, Krauz K, Pruc M, Szarpak L, Rodkiewicz D, Mamcarz A. Association Between Left Atrial Epicardial Adipose Tissue Attenuation Assessed by Cardiac Computed Tomography and Atrial Fibrillation Recurrence Following Catheter Ablation: A Systematic Review and Meta-Analysis. Journal of Clinical Medicine. 2025; 14(13):4771. https://doi.org/10.3390/jcm14134771
Chicago/Turabian StyleMomot, Karol, Kamil Krauz, Michal Pruc, Lukasz Szarpak, Dariusz Rodkiewicz, and Artur Mamcarz. 2025. "Association Between Left Atrial Epicardial Adipose Tissue Attenuation Assessed by Cardiac Computed Tomography and Atrial Fibrillation Recurrence Following Catheter Ablation: A Systematic Review and Meta-Analysis" Journal of Clinical Medicine 14, no. 13: 4771. https://doi.org/10.3390/jcm14134771
APA StyleMomot, K., Krauz, K., Pruc, M., Szarpak, L., Rodkiewicz, D., & Mamcarz, A. (2025). Association Between Left Atrial Epicardial Adipose Tissue Attenuation Assessed by Cardiac Computed Tomography and Atrial Fibrillation Recurrence Following Catheter Ablation: A Systematic Review and Meta-Analysis. Journal of Clinical Medicine, 14(13), 4771. https://doi.org/10.3390/jcm14134771







