The Potential Advantages of Remimazolam for Awakening in Deep Brain Stimulation Surgery: A Retrospective Analysis of Cases
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Participants and Data
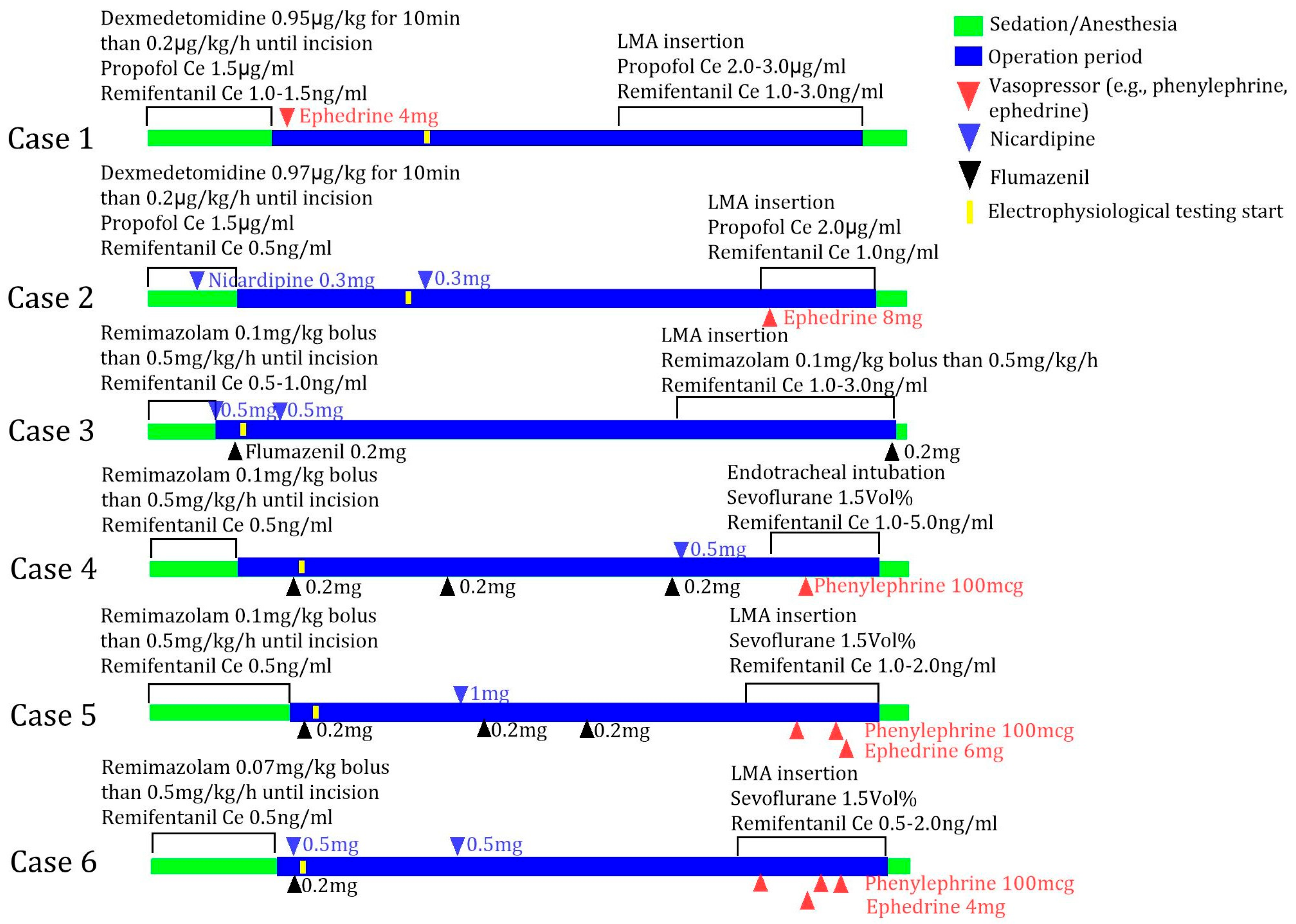
2.3. Standard Deep Brain Stimulation Procedure Under Monitored Anesthetic Care
2.4. Accessing Patient Awakening
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| DBS | Deep brain stimulation |
| BIS | Bispectral index |
| PSI | Patient state index |
| MOAA/S | Modified observer’s assessment of alertness/sedation scale |
| CI | Confidence interval |
References
- Lee, D.J.; Lozano, C.S.; Dallapiazza, R.F.; Lozano, A.M. Current and future directions of deep brain stimulation for neurological and psychiatric disorders. J. Neurosurg. 2019, 131, 333–342. [Google Scholar] [CrossRef] [PubMed]
- Mulroy, E.; Robertson, N.; Macdonald, L.; Bok, A.; Simpson, M. Patients’ perioperative experience of awake deep-brain stimulation for Parkinson disease. World Neurosurg. 2017, 105, 526–528. [Google Scholar] [CrossRef]
- Vinke, R.S.; Geerlings, M.; Selvaraj, A.K.; Georgiev, D.; Bloem, B.R.; Esselink, R.A.J.; Bartels, R.H.M.A. The role of microelectrode recording in deep brain stimulation surgery for Parkinson’s disease: A systematic review and meta-analysis. J. Park. Dis. 2022, 12, 2059–2069. [Google Scholar] [CrossRef]
- Scharpf, D.T.; Sharma, M.; Deogaonkar, M.; Rezai, A.; Bergese, S.D. Practical considerations and nuances in anesthesia for patients undergoing deep brain stimulation implantation surgery. Korean J. Anesthesiol. 2015, 68, 332–339. [Google Scholar] [CrossRef]
- Janssen, M.L.F.; Bos, M.J. Microelectrode assisted deep brain stimulation: Considerations for anesthesia. Deep Brain Stimul. 2024, 4, 13–23. [Google Scholar] [CrossRef]
- Sato, T.; Ando, T.; Ozeki, K.; Asano, I.; Kuwatsuka, Y.; Ando, M.; Motomura, K.; Nishiwaki, K. Prospective randomized controlled trial comparing anesthetic management with remimazolam besylate and flumazenil versus propofol during awake craniotomy following an asleep-awake-asleep method. J. Neurosurg. Anesthesiol. 2025, 37, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.; Kalsotra, S.; Tobias, J.D. The use of remimazolam during awake craniotomy for seizure foci resection in adolescents: A case series. J. Clin. Med. Res. 2024, 16, 319–323. [Google Scholar] [CrossRef]
- Sato, T.; Nishiwaki, K. Two cases of remimazolam anesthesia managed with pharmacokinetic simulations in an awake craniotomy of patients with obesity. Cureus 2024, 16, e69311. [Google Scholar] [CrossRef]
- Moniz-Garcia, D.; Bojaxhi, E.; Borah, B.J.; Dholakia, R.; Kim, H.; Sousa-Pinto, B.; Almeida, J.P.; Mendhi, M.; Freeman, W.D.; Sherman, W.; et al. Awake craniotomy program implementation. JAMA Netw. Open 2024, 7, e2352917. [Google Scholar] [CrossRef]
- Kim, A.; Yang, H.-J.; Kwon, J.-H.; Kim, M.-H.; Lee, J.; Jeon, B. Mortality of deep brain stimulation and risk factors in patients with Parkinson’s disease: A national cohort study in Korea. J. Korean Med. Sci. 2023, 38, e10. [Google Scholar] [CrossRef]
- GlobeNewswire. Global Deep Brain Stimulation Market Forecasts to 2029: Key Trends Include Automated and Closed-Loop Deep Brain Stimulation, Rechargeable Devices with Longer Battery Life and Miniaturized DBS Devices. Available online: https://www.globenewswire.com/news-release/2024/07/03/2908296/28124/en/Global-Deep-Brain-Stimulation-Market-Forecasts-to-2029-Key-Trends-Include-Automated-and-Closed-Loop-Deep-Brain-Stimulation-Rechargeable-Devices-with-Longer-Battery-Life-and-Miniatu.html?utm_source=chatgpt.com (accessed on 15 May 2025).
- Kim, K.M. Remimazolam: Pharmacological characteristics and clinical applications in anesthesiology. Anesth. Pain Med. 2022, 17, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Kramer, D.R.; Halpern, C.H.; Buonacore, D.L.; McGill, K.R.; Hurtig, H.I.; Jaggi, J.L.; Baltuch, G.H. Best surgical practices: A stepwise approach to the University of Pennsylvania deep brain stimulation protocol. Neurosurg. Focus 2010, 29, E3. [Google Scholar] [CrossRef]
- Schnider, T.W.; Minto, C.F.; Gambus, P.L.; Andresen, C.; Goodale, D.B.; Shafer, S.L.; Youngs, E.J. The influence of method of administration and covariates on the pharmacokinetics of propofol in adult volunteers. Anesthesiology 1998, 88, 1170–1182. [Google Scholar] [CrossRef]
- Zhao, T.Y.M.; Chen, D.; Xu, Z.X.; Wang, H.L.; Sun, H. Comparison of bispectral index and patient state index as measures of sedation depth during surgeries using remimazolam tosilate. BMC Anesthesiol. 2023, 23, 208. [Google Scholar] [CrossRef] [PubMed]
- Bae, M.I.; Bae, J.; Song, Y.; Kim, M.; Han, D.W. Comparative analysis of the performance of electroencephalogram parameters for monitoring the depth of sedation during remimazolam target-controlled infusion. Anesth. Analg. 2024, 138, 1295–1303. [Google Scholar] [CrossRef] [PubMed]
- Macbeth, G.; Razumiejczyk, E.; Ledesma, R.D. Cliff’s Delta Calculator: A non-parametric effect size program for two groups of observations. Univ. Psychol. 2011, 10, 545–555. [Google Scholar] [CrossRef]
- Kim, K.N.; Lee, H.J.; Kim, S.Y.; Kim, J.Y. Combined use of dexmedetomidine and propofol in monitored anesthesia care: A randomized controlled study. BMC Anesthesiol. 2017, 17, 34. [Google Scholar] [CrossRef]
- Elbakry, A.E.; Ibrahim, E. Propofol-dexmedetomidine versus propofol-remifentanil conscious sedation for awake craniotomy during epilepsy surgery. Minerva Anestesiol. 2017, 83, 1248–1254. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.J.; Kim, S.H.; So, K.Y.; Jung, K.T. Effects of dexmedetomidine on smooth emergence from anaesthesia in elderly patients undergoing orthopaedic surgery. BMC Anesthesiol. 2015, 15, 139. [Google Scholar] [CrossRef]
- Chen, M.; Sun, Y.; Li, X.; Zhang, C.; Huang, X.; Xu, Y.; Gu, C. Effectiveness of single loading dose of dexmedetomidine combined with propofol for deep sedation of endoscopic retrograde cholangiopancreatography (ERCP) in elderly patients: A prospective randomized study. BMC Anesthesiol. 2022, 22, 85. [Google Scholar] [CrossRef]
- Chae, D.; Kim, H.-C.; Song, Y.; Choi, Y.S.; Han, D.W. Pharmacodynamic analysis of intravenous bolus remimazolam for loss of consciousness in patients undergoing general anaesthesia: A randomised, prospective, double-blind study. Br. J. Anaesth. 2022, 129, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Schüttler, J.; Eisenried, A.; Lerch, M.; Fechner, J.; Jeleazcov, C.; Ihmsen, H. Pharmacokinetics and pharmacodynamics of remimazolam (CNS 7056) after continuous infusion in healthy male volunteers: Part I. Pharmacokinetics and clinical pharmacodynamics. Anesthesiology 2020, 132, 636–651. [Google Scholar] [CrossRef]
- Kim, J.H.; Nam, J.S.; Seo, W.W.; Joung, K.W.; Chin, J.H.; Kim, W.J.; Choi, D.K.; Choi, I.C. Effects of remimazolam versus dexmedetomidine on recovery after transcatheter aortic valve replacement under monitored anesthesia care: A propensity score-matched, non-inferiority study. Korean J. Anesthesiol. 2024, 77, 537–545. [Google Scholar] [CrossRef]
- Wu, Q.; Xu, F.; Wang, J.; Jiang, M. Comparison of remimazolam-flumazenil versus propofol for Recovery from General Anesthesia: A Systematic Review and Meta-Analysis. J. Clin. Med. 2023, 12, 7316. [Google Scholar] [CrossRef]
- Lee, S.; Lee, J.; Hwang, S.Y.; Ju, J.W.; Nam, K.; Ahn, H.J.; Lee, S.R.; Choi, E.K.; Jeon, Y.; Cho, Y.J. Remimazolam-flumazenil provides fast recovery from general anesthesia compared to propofol during radiofrequency catheter ablation of atrial fibrillation. Sci. Rep. 2024, 14, 12660. [Google Scholar] [CrossRef]
- Shen, X.; Zhou, C.; Hua, Q.; Yang, L.; Zhao, W.; Xu, P. Impact of operation duration on short-term and long-term prognosis in patients undergoing radical colorectal surgery. J. Cancer 2022, 13, 1160–1167. [Google Scholar] [CrossRef]
- Marik, P.E.; Varon, J. Perioperative hypertension: A review of current and emerging therapeutic agents. J. Clin. Anesth. 2009, 21, 220–229. [Google Scholar] [CrossRef] [PubMed]
- Kamijo, Y.; Masuda, T.; Nishikawa, T.; Tsuruta, H.; Ohwada, T. Cardiovascular response and stress reaction to flumazenil injection in patients under infusion with midazolam. Crit. Care Med. 2000, 28, 318–323. [Google Scholar] [CrossRef] [PubMed]
- Knudsen, L.; Cold, G.E.; Holdgård, H.O.; Johansen, U.T.; Jensen, S. Effects of flumazenil on cerebral blood flow and oxygen consumption after midazolam anaesthesia for craniotomy. Br. J. Anaesth. 1991, 67, 277–280. [Google Scholar] [CrossRef]
- Kozarek, K.; Sanders, R.D.; Head, D. Perioperative blood pressure in the elderly. Curr. Opin. Anaesthesiol. 2020, 33, 122. [Google Scholar] [CrossRef]
- Spivey, W.H. Flumazenil and seizures: Analysis of 43 cases. Clin. Ther. 1992, 14, 292–305. [Google Scholar] [PubMed]
- Penninga, E.I.; Graudal, N.; Ladekarl, M.B.; Jürgens, G. Adverse events associated with flumazenil treatment for the management of suspected benzodiazepine intoxication–A systematic review with meta-analyses of randomised trials. Basic Clin. Pharmacol. Toxicol. 2016, 118, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, A.; Kurata, S.; Kida, K.; Tsubokawa, T. Anesthetic management for the sleep-awake-sleep technique of awake craniotomy using a novel benzodiazepine remimazolam and its antagonist flumazenil. JA Clin. Rep. 2021, 7, 14. [Google Scholar] [CrossRef] [PubMed]
| Cases | Sedation Drug | Operation Site | Awakening Time (min) | Operation Duration (min) | Postoperative Hospital Stay (day) | Complication |
|---|---|---|---|---|---|---|
| Case 1 (F/70 years) | Propofol and dexmedetomidine | Left | 40 | 190 | 9 | None |
| Case 2 (M/78 years) | Propofol and dexmedetomidine | Bilateral | 60 | 306 | 15 | None |
| Case 3 (F/73 years) | Remimazolam | Left | 15 | 314 | 5 | None |
| Case 4 (F/70 years) | Remimazolam | Bilateral | 25 | 312 | 21 | None |
| Case 5 (F/62 years) | Remimazolam | Bilateral | 15 | 267 | 10 | None |
| Case 6 (F/84 years) | Remimazolam | Left | 19 | 229 | 7 | None |
| Remimazolam (n = 4) | Propofol, Dexmedetomidine (n = 2) | p-Value * | Effect Size # (95% CI) | |
|---|---|---|---|---|
| Awakening time (min) | 17 (15–23) | 50 (40–60) | 0.133 | −1.00 (−1.00, −0.02) |
| Unilateral (n = 3) | Bilateral (n = 3) | p-Value * | Effect Size # (95% CI) | |
|---|---|---|---|---|
| Postoperative hospital stay (day) | 7 (5–9) | 15 (10–21) | 0.100 | −1.00 (−1.00, −0.14) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Byun, S.-H.; Yeo, J.; Lee, S.-H. The Potential Advantages of Remimazolam for Awakening in Deep Brain Stimulation Surgery: A Retrospective Analysis of Cases. J. Clin. Med. 2025, 14, 4724. https://doi.org/10.3390/jcm14134724
Byun S-H, Yeo J, Lee S-H. The Potential Advantages of Remimazolam for Awakening in Deep Brain Stimulation Surgery: A Retrospective Analysis of Cases. Journal of Clinical Medicine. 2025; 14(13):4724. https://doi.org/10.3390/jcm14134724
Chicago/Turabian StyleByun, Sung-Hye, Jinsong Yeo, and Sou-Hyun Lee. 2025. "The Potential Advantages of Remimazolam for Awakening in Deep Brain Stimulation Surgery: A Retrospective Analysis of Cases" Journal of Clinical Medicine 14, no. 13: 4724. https://doi.org/10.3390/jcm14134724
APA StyleByun, S.-H., Yeo, J., & Lee, S.-H. (2025). The Potential Advantages of Remimazolam for Awakening in Deep Brain Stimulation Surgery: A Retrospective Analysis of Cases. Journal of Clinical Medicine, 14(13), 4724. https://doi.org/10.3390/jcm14134724






