A Comprehensive Analysis of the Abdominal Aortic Aneurysm Growth Rate in the Spanish Population
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Inclusion
2.2. Data Cleaning
2.3. Statistical Modeling and Selection of Main Factors Associated with Aortic Diameter
2.4. Statistical Modeling and Selection of Main Factors Associated with Aortic Diameter Growth
3. Results
3.1. Cohort Description
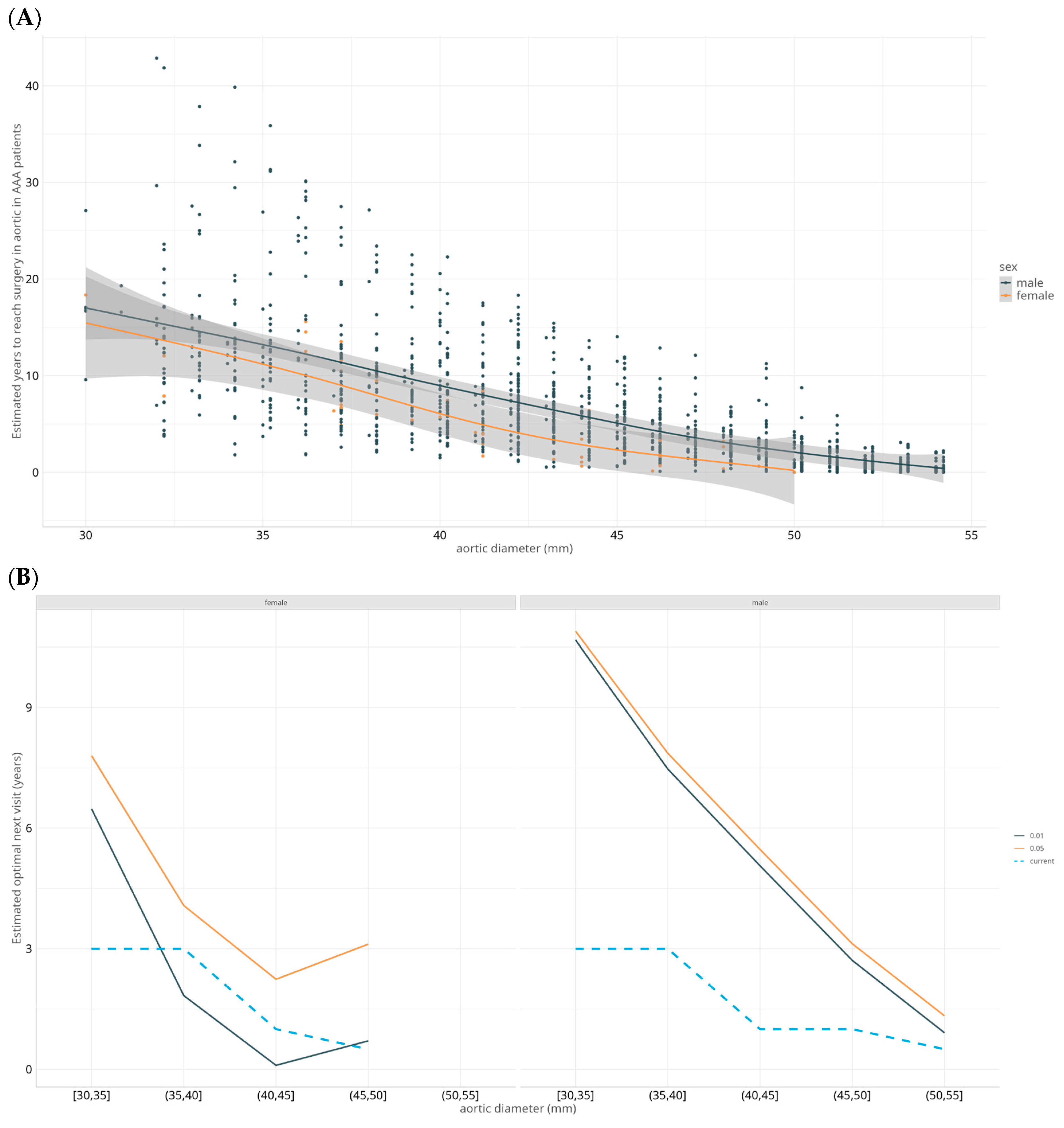
3.2. Growth Rate by Aortic Diameter Intervals
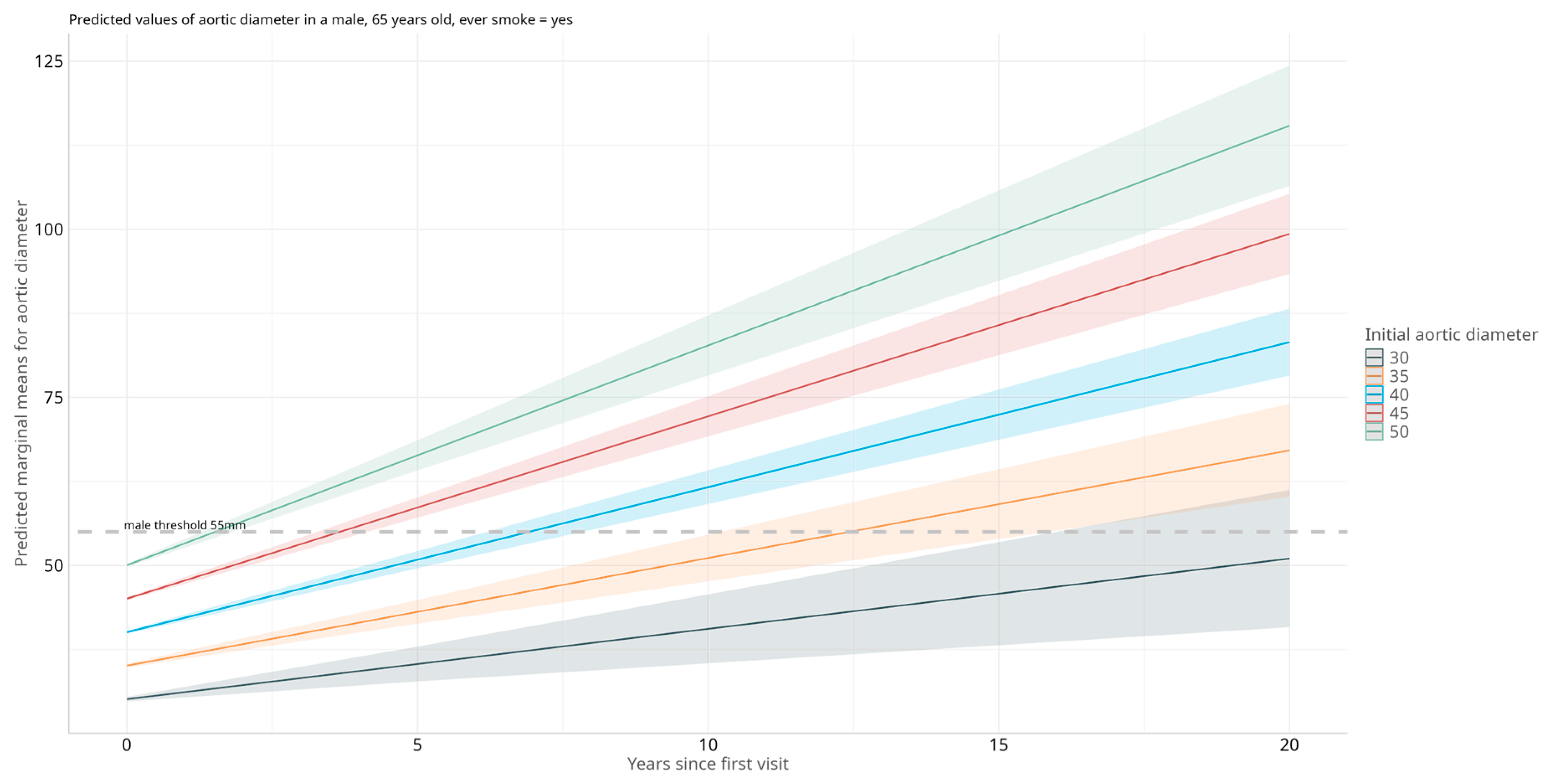
3.3. Selection of Best Variables Influencing AAA Diameter
3.4. Selection of Best Variables Influencing AAA Growth Rate
3.5. Models to Predict Estimated Time to Surgery
4. Discussion
4.1. Clinical Determinants of Aortic Diameter in Individuals with AAA
4.2. AAA Expansion Rates per Diameter Interval and Clinical Determinants of Expansion
4.3. Optimization of Surveillance Intervals
4.4. Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AAA | Abdominal Aortic Aneurysm |
| BMI | Body mass index |
| CIs | Confidence Intervals |
| CV | Other cardiovascular |
| CVE | Cerebrovascular event |
| COPD | Chronic obstructive pulmonary disease |
| DM | Diabetes mellitus |
| ESVS | European Society for Vascular Surgery |
| PAD | Peripheral artery disease |
| TABS | Triple-A Barcelona Study |
References
- Boddy, A.M.; Lenk, G.M.; Lillvis, J.H.; Nischan, J.; Kyo, Y.; Kuivaniemi, H. Basic research studies to understand aneurysm disease. Drug News Perspect. 2008, 21, 142–148. [Google Scholar]
- Brown, L.C.; Powell, J.T. Risk factors for aneurysm rupture in patients kept under ultrasound surveillance. UK Small Aneurysm Trial Participants. Ann. Surg. 1999, 230, 289–296, discussion 296–297. [Google Scholar] [CrossRef] [PubMed]
- Brown, P.M.; Zelt, D.T.; Sobolev, B. The risk of rupture in untreated aneurysms: The impact of size, gender, and expansion rate. J. Vasc. Surg. 2003, 37, 280–284. [Google Scholar] [CrossRef]
- Cronenwett, J.L.; Murphy, T.F.; Zelenock, G.B.; Whitehouse, W.M.; Lindenauer, S.M.; Graham, L.M.; Quint, L.E.; Silver, T.M.; Stanley, J.C. Actuarial analysis of variables associated with rupture of small abdominal aortic aneurysms. Surgery 1985, 98, 472–483. [Google Scholar] [PubMed]
- Norman, P.E.; Powell, J.T. Abdominal aortic aneurysm: The prognosis in women is worse than in men. Circulation 2007, 115, 2865–2869. [Google Scholar] [CrossRef] [PubMed]
- Reed, W.W.; Hallett, J.W.; Damiano, M.A.; Ballard, D.J. Learning from the last ultrasound. A population-based study of patients with abdominal aortic aneurysm. Arch. Intern. Med. 1997, 157, 2064–2068. [Google Scholar] [CrossRef]
- Scott, R.A.; Tisi, P.V.; Ashton, H.A.; Allen, D.R. Abdominal aortic aneurysm rupture rates: A 7-year follow-up of the entire abdominal aortic aneurysm population detected by screening. J. Vasc. Surg. 1998, 28, 124–128. [Google Scholar] [CrossRef]
- Conway, K.P.; Byrne, J.; Townsend, M.; Lane, I.F. Prognosis of patients turned down for conventional abdominal aortic aneurysm repair in the endovascular and sonographic era: Szilagyi revisited? J. Vasc. Surg. 2001, 33, 752–757. [Google Scholar] [CrossRef]
- Lederle, F.A.; Johnson, G.R.; Wilson, S.E.; Ballard, D.J.; Jordan, W.D.; Blebea, J.; Littooy, F.N.; Freischlag, J.A.; Bandyk, D.; Rapp, J.H.; et al. Rupture rate of large abdominal aortic aneurysms in patients refusing or unfit for elective repair. JAMA 2002, 287, 2968–2972. [Google Scholar] [CrossRef]
- Thompson, A.R.; Cooper, J.A.; Ashton, H.A.; Hafez, H. Growth rates of small abdominal aortic aneurysms correlate with clinical events. Br. J. Surg. 2010, 97, 37–44. [Google Scholar] [CrossRef]
- Stonebridge, P.A.; Draper, T.; Kelman, J.; Howlett, J.; Allan, P.L.; Prescott, R.; Ruckley, C. Growth rate of infrarenal aortic aneurysms. Eur. J. Vasc. Endovasc. Surg. 1996, 11, 70–73. [Google Scholar] [CrossRef] [PubMed]
- Sakalihasan, N.; Michel, J.-B.; Katsargyris, A.; Kuivaniemi, H.; Defraigne, J.-O.; Nchimi, A.; Powell, J.T.; Yoshimura, K.; Hultgren, R. Abdominal aortic aneurysms. Nat. Rev. Dis. Primers 2018, 4, 34. [Google Scholar] [CrossRef]
- Wanhainen, A.; Van Herzeele, I.; Bastos Goncalves, F.; Bellmunt Montoya, S.; Berard, X.; Boyle, J.R.; D’oRia, M.; Prendes, C.F.; Karkos, C.D.; Kazimierczak, A.; et al. Editor’s Choice—European Society for Vascular Surgery (ESVS) 2024 Clinical Practice Guidelines on the Management of Abdominal Aorto-Iliac Artery Aneurysms. Eur. J. Vasc. Endovasc. Surg. 2024, 67, 192–331. [Google Scholar] [CrossRef]
- Owens, D.K.; Davidson, K.W.; Krist, A.H.; Barry, M.J.; Cabana, M.; Caughey, A.B.; Doubeni, C.A.; Epling, J.W.; Kubik, M.; Landefeld, C.S.; et al. Screening for Abdominal Aortic Aneurysm: US Preventive Services Task Force Recommendation Statement. JAMA 2019, 322, 2211–2218. [Google Scholar] [CrossRef]
- Sisó-Almirall, A.; Kostov, B.; Navarro González, M.; Cararach Salami, D.; Pérez Jiménez, A.; Gilabert Solé, R.; Saumell, C.B.; Bach, L.D.; Martí, M.V.; Paz, L.G.-D.; et al. Abdominal aortic aneurysm screening program using hand-held ultrasound in primary healthcare. PLoS ONE 2017, 12, e0176877. [Google Scholar] [CrossRef] [PubMed]
- Salvador-González, B.; Martín-Baranera, M.; Borque-Ortega, Á.; Sáez-Sáez, R.M.; de Albert-Delas Vigo, M.; Carreño-García, E.; Tarín-Masriera, L.; Badia-Millán, P.; Martínez-Gil, M.; Torrabadella-Fàbrega, J. Prevalence of Abdominal Aortic Aneurysm in Men Aged 65–74 Years in a Metropolitan Area in North-East Spain. Eur. J. Vasc. Endovasc. Surg. 2016, 52, 75–81. [Google Scholar] [CrossRef]
- Bravo-Merino, L.; González-Lozano, N.; Maroto-Salmón, R.; Meijide-Santos, G.; Suárez-Gil, P.; Fañanás-Mastral, A. Validez de la ecografía abdominal en Atención Primaria para detección de aneurisma de aorta abdominal en varones de entre 65 y 75 años. Aten. Primaria 2019, 51, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Yu, G.; Wang, L.-G.; Han, Y.; He, Q.-Y. clusterProfiler: An R Package for Comparing Biological Themes Among Gene Clusters. OMICS 2012, 16, 284–287. [Google Scholar] [CrossRef]
- Subramanian, A.; Tamayo, P.; Mootha, V.K.; Mukherjee, S.; Ebert, B.L.; Gillette, M.A.; Paulovich, A.; Pomeroy, S.L.; Golub, T.R.; Lander, E.S.; et al. Gene set enrichment analysis: A knowledge-based approach for interpreting genome-wide expression profiles. Proc. Natl. Acad. Sci. USA 2005, 102, 15545–15550. [Google Scholar] [CrossRef]
- Fité, J.; Gimenez, E.; Soto, B.; Artigas, V.; Escudero, J.R.; Bellmunt-Montoya, S.; Espallargues, M. Systematic review on abdominal aortic aneurysm screening cost-efficiency and methodological quality assessment. Int. Angiol. 2021, 40, 67–76. [Google Scholar] [CrossRef]
- Sweeting, M.J.; Thompson, S.G. Joint modelling of longitudinal and time-to-event data with application to predicting abdominal aortic aneurysm growth and rupture. Biom. J. 2011, 53, 750–763. [Google Scholar] [CrossRef]
- Ehlers, L.; Sørensen, J.; Jensen, L.G.; Bech, M.; Kjølby, M. Is population screening for abdominal aortic aneurysm cost-effective? BMC Cardiovasc. Disord. 2008, 8, 32. [Google Scholar] [CrossRef]
- Thompson, S.G.; Ashton, H.A.; Gao, L.; Scott, R.A.P. Screening men for abdominal aortic aneurysm: 10 year mortality and cost effectiveness results from the randomised Multicentre Aneurysm Screening Study. BMJ 2009, 338, b2307. [Google Scholar] [CrossRef]
- Hager, J.; Henriksson, M.; Carlsson, P.; Länne, T.; Lundgren, F. Revisiting the cost-effectiveness of screening 65-year-old men for abdominal aortic aneurysm based on data from an implemented screening program. Int. Angiol. 2017, 36, 517–525. [Google Scholar] [CrossRef] [PubMed]
- Guirguis-Blake, J.M.; Beil, T.L.; Senger, C.A.; Coppola, E.L. Primary Care Screening for Abdominal Aortic Aneurysm. JAMA 2019, 322, 2219. [Google Scholar] [CrossRef] [PubMed]
- Roychowdhury, T.; Klarin, D.; Levin, M.G.; Spin, J.M.; Rhee, Y.H.; Deng, A.; Headley, C.A.; Tsao, N.L.; Gellatly, C.; Zuber, V.; et al. Genome-wide association meta-analysis identifies risk loci for abdominal aortic aneurysm and highlights PCSK9 as a therapeutic target. Nat. Genet. 2023, 55, 1831–1842. [Google Scholar] [CrossRef] [PubMed]
- Portilla-Fernandez, E.; Klarin, D.; Hwang, S.-J.; Biggs, M.L.; Bis, J.C.; Weiss, S.; Rospleszcz, S.; Natarajan, P.; Hoffmann, U.; Rogers, I.S.; et al. Genetic and clinical determinants of abdominal aortic diameter: Genome-wide association studies, exome array data and Mendelian randomization study. Hum. Mol. Genet. 2022, 31, 3566–3579. [Google Scholar] [CrossRef]
- Brady, A.R.; Thompson, S.G.; Fowkes, F.G.R.; Greenhalgh, R.M.; Powell, J.T. Abdominal Aortic Aneurysm Expansion. Circulation 2004, 110, 16–21. [Google Scholar] [CrossRef]
- Welsh, P.; Welsh, C.E.; Jhund, P.S.; Woodward, M.; Brown, R.; Lewsey, J.; Celis-Morales, C.A.; Ho, F.K.; MacKay, D.F.; Gill, J.M.; et al. Derivation and Validation of a 10-Year Risk Score for Symptomatic Abdominal Aortic Aneurysm: Cohort Study of Nearly 500 000 Individuals. Circulation 2021, 144, 604–614. [Google Scholar] [CrossRef]
- Prendes, C.F.; E Melo, R.G.; Caldeira, D.; D’Oria, M.; Tsilimparis, N.; Koelemay, M.; Van Herzeele, I.; Wanhainen, A. Editor’s Choice—Systematic Review and Meta-Analysis of Contemporary Abdominal Aortic Aneurysm Growth Rates. Eur. J. Vasc. Endovasc. Surg. 2024, 67, 132–145. [Google Scholar] [CrossRef]
- RESCAN Collaborators; Bown, M.J.; Sweeting, M.J.; Brown, L.C.; Powell, J.T.; Thompson, S.G. Surveillance intervals for small abdominal aortic aneurysms: A meta-analysis. JAMA 2013, 309, 806–813. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Oliver-Williams, C.; Sweeting, M.J.; Turton, G.; Parkin, D.; Cooper, D.; Rodd, C.; Thompson, S.G.; Earnshaw, J.J. Lessons learned about prevalence and growth rates of abdominal aortic aneurysms from a 25-year ultrasound population screening programme. Br. J. Surg. 2018, 105, 68–74. [Google Scholar] [CrossRef]
- Thorbjørnsen, K.; Svensjö, S.; Gilgen, N.P.; Wanhainen, A. Long Term Outcome of Screen Detected Sub-Aneurysmal Aortas in 65 Year Old Men: A Single Scan After Five Years Identifies Those at Risk of Needing AAA Repair. Eur. J. Vasc. Endovasc. Surg. 2021, 62, 380–386. [Google Scholar] [CrossRef] [PubMed]
- Rockley, M.; Radonjic, A.; LeBlanc, D.; Jetty, P. The futility of surveillance for old and small aneurysms. J. Vasc. Surg. 2020, 72, 162–170.e1. [Google Scholar] [CrossRef]
- Fite, J.; Gayarre-Aguado, R.; Puig, T.; Zamora, S.; Escudero, J.R.; Solà Roca, J.; Bellmunt-Montoya, S. Feasibility and Efficiency Study of a Population-Based Abdominal Aortic Aneurysm Screening Program in Men and Women in Spain. Ann. Vasc. Surg. 2021, 73, 429–437. [Google Scholar] [CrossRef]
- Salcedo Jódar, L.; Alcázar Carmona, P.; Tenías Burillo, J.M.; García Tejada, R. Prevalencia del aneurisma de aorta abdominal en varones de 65–80 años de una población rural. SEMERGEN—Med. De Fam. 2014, 40, 425–430. [Google Scholar] [CrossRef] [PubMed]
- Sisó-Almirall, A.; Gilabert Solé, R.; Bru Saumell, C.; Kostov, B.; Mas Heredia, M.; González-de Paz, L.; Montal, L.S.; Àreu, J.B. Utilidad de la ecografía portátil en el cribado del aneurisma de aorta abdominal y de la ateromatosis de aorta abdominal. Med. Clin. 2013, 141, 417–422. [Google Scholar] [CrossRef]
- Barba, Á.; Vega de Céniga, M.; Estallo, L.; de la Fuente, N.; Viviens, B.; Izagirre, M. Prevalence of Abdominal Aortic Aneurysm is Still High in Certain Areas of Southern Europe. Ann. Vasc. Surg. 2013, 27, 1068–1073. [Google Scholar] [CrossRef]
- Kim, L.G.; Thompson, S.G.; Briggs, A.H.; Buxton, M.J.; Campbell, H.E. How cost-effective is screening for abdominal aortic aneurysms? J. Med. Screen. 2007, 14, 46–52. [Google Scholar] [CrossRef]
- Lindholt, J.S.; Juul, S.; Fasting, H.; Henneberg, E.W. Cost-effectiveness Analysis of Screening for Abdominal Aortic Aneurysms Based on Five Year Results from a Randomised Hospital Based Mass Screening Trial. Eur. J. Vasc. Endovasc. Surg. 2006, 32, 9–15. [Google Scholar] [CrossRef]
- Ministerio de Sanidad. Informe Anual del Sistema Nacional de Salud 2023. Available online: https://www.sanidad.gob.es/estadEstudios/estadisticas/sisInfSanSNS/tablasEstadisticas/InfAnualSNS2023/INFORME_ANUAL_2023.pdf (accessed on 20 June 2025).

| Variable | [30,40] (n = 121) | (40,50] (n = 161) | (50,100] (n = 187) | Total (n = 469) |
|---|---|---|---|---|
| sex [female], %, n | 4.1, 5 | 5.6, 9 | 1.6, 3 | 3.6, 17 |
| mean age (SD), n | 69.54 (7.24), 121 | 72.10 (7.43), 161 | 73.89 (8.10), 187 | 72.15 (7.83), 469 |
| mean height (SD), n | 171.55 (6.97), 118 | 170.37 (7.10), 157 | 169.90 (6.92), 148 | 170.54 (7.01), 423 |
| mean weight (SD), n | 79.91 (10.61), 118 | 80.57 (13.36), 156 | 78.95 (12.84), 149 | 79.81 (12.45), 423 |
| smoking [never], %, n | 14.0, 17 | 8.1, 13 | 12.8, 24 | 11.5, 54 |
| smoking [before 6 months], %, n | 48.8, 59 | 51.9, 83 | 55.1, 103 | 52.4, 245 |
| smoking [current smoker], %, n | 37.2, 45 | 40.0, 64 | 32.1, 60 | 36.1, 169 |
| hypertension [yes], %, n | 71.9, 87 | 80.0, 128 | 79.0, 147 | 77.5, 362 |
| dyslipidemia [yes], %, n | 69.4, 84 | 74.4, 119 | 62.6, 117 | 68.4, 320 |
| diabetes [yes], %, n | 25.6, 31 | 22.5, 36 | 26.2, 49 | 24.8, 116 |
| PAD [yes], %, n | 15.7, 19 | 15.8, 25 | 9.5, 17 | 13.3, 61 |
| other_aneurysms [yes], %, n | 18.1, 21 | 14.6, 23 | 18.1, 32 | 16.9, 76 |
| cve [yes], %, n | 7.4, 9 | 8.8, 14 | 7.0, 13 | 7.7, 36 |
| cvd [yes], %, n | 18.2, 22 | 24.1, 38 | 36.3, 65 | 27.3, 125 |
| copd [yes], %, n | 16.5, 20 | 26.6, 42 | 26.8, 48 | 24.0, 110 |
| kidney_disease [yes], %, n | 12.4, 15 | 15.8, 25 | 17.9, 32 | 15.7, 72 |
| antihypertensives [yes], %, n | 66.7, 60 | 79.6, 82 | 76.7, 125 | 75.0, 267 |
| aines_aspirin [yes], %, n | 56.7, 51 | 55.3, 57 | 65.0, 106 | 60.1, 214 |
| glucocorticoids [yes], %, n | 3.3, 3 | 3.0, 3 | 3.2, 5 | 3.2, 11 |
| statins [yes], %, n | 75.6, 68 | 77.5, 79 | 77.3, 126 | 76.9, 273 |
| antidiabetics [yes], %, n | 23.3, 21 | 15.5, 16 | 20.2, 33 | 19.7, 70 |
| anticoagulants [yes], %, n | 7.8, 7 | 14.6, 15 | 17.8, 29 | 14.3, 51 |
| surgery [no], %, n | 94.1, 111 | 92.3, 131 | 23.8, 40 | 65.9, 282 |
| surgery [yes(EVAR, endovascular repair)], %, n | 3.4, 4 | 6.3, 9 | 55.4, 93 | 24.8, 106 |
| surgery [yes(open surgery)], %, n | 2.5, 3 | 1.4, 2 | 20.8, 35 | 9.3, 40 |
| mean BMI (SD), n | 27.18 (3.35), 117 | 27.71 (3.93), 156 | 27.28 (3.75), 148 | 27.41 (3.71), 421 |
| mean aortic_diameter (SD), n | 35.75 (3.11), 121 | 44.92 (2.99), 161 | 62.25 (11.39), 187 | 49.46 (13.35), 469 |
| mean number_visits (SD), n | 6.01 (3.91), 121 | 4.58 (3.32), 161 | 1.53 (1.42), 187 | 3.74 (3.47), 469 |
| mean years_follow_up (SD), n | 5.03 (3.61), 121 | 2.73 (2.63), 161 | 0.27 (0.69), 187 | 2.34 (3.09), 469 |
| Male | ||||||
|---|---|---|---|---|---|---|
| Years to Next Visit at 5% | Years to Next Visit at 1% | Current Years to Next Visit | Number of Visits at 5% | Number of Visits at 1% |
Current Number of Visits Until
55 mm | |
| [30,35] | 10.9 | 10.7 | 3.0 | 4.5 | 5.2 | 15.6 |
| (35,40] | 7.9 | 7.5 | 3.0 | 3.8 | 4.5 | 13.1 |
| (40,45] | 5.5 | 5.1 | 1.0 | 2.9 | 3.5 | 10.7 |
| (45,50] | 3.1 | 2.7 | 0.5 | 2.1 | 2.7 | 6.5 |
| (50,55] | 1.3 | 0.9 | 0.5 | 0.8 | 1.2 | 2.4 |
| Female | ||||||
| Years to Next Visit at 5% | Years to Next Visit at 1% | Current Years to Next Visit | Number of Visits at 5% | Number of Visits at 1% |
Current Number of Visits Until
50 mm | |
| [30,35] | 7.8 | 6.5 | 3.0 | 5.9 | 52.5 | 13.2 |
| (35,40] | 4.1 | 1.8 | 1.0 | 5 | 51.4 | 10.7 |
| (40,45] | 2.2 | 0.1 | 1.0 | 3.2 | 47.4 | 8.3 |
| (45,50] | 3.1 | 0.7 | 0.5 | 1.3 | 5.9 | 4.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peypoch, O.; Calsina Juscafresa, L.; Vega-Méndez, A.; Lobato-Delgado, B.; Fité, J.; Soto, B.; Nieto, L.; de la Rosa Estadella, M.; Uribezubia, A.; Romero, J.-M.; et al. A Comprehensive Analysis of the Abdominal Aortic Aneurysm Growth Rate in the Spanish Population. J. Clin. Med. 2025, 14, 4720. https://doi.org/10.3390/jcm14134720
Peypoch O, Calsina Juscafresa L, Vega-Méndez A, Lobato-Delgado B, Fité J, Soto B, Nieto L, de la Rosa Estadella M, Uribezubia A, Romero J-M, et al. A Comprehensive Analysis of the Abdominal Aortic Aneurysm Growth Rate in the Spanish Population. Journal of Clinical Medicine. 2025; 14(13):4720. https://doi.org/10.3390/jcm14134720
Chicago/Turabian StylePeypoch, Olga, Laura Calsina Juscafresa, Antón Vega-Méndez, Bárbara Lobato-Delgado, Joan Fité, Begoña Soto, Lluis Nieto, Mireia de la Rosa Estadella, Ager Uribezubia, Jose-María Romero, and et al. 2025. "A Comprehensive Analysis of the Abdominal Aortic Aneurysm Growth Rate in the Spanish Population" Journal of Clinical Medicine 14, no. 13: 4720. https://doi.org/10.3390/jcm14134720
APA StylePeypoch, O., Calsina Juscafresa, L., Vega-Méndez, A., Lobato-Delgado, B., Fité, J., Soto, B., Nieto, L., de la Rosa Estadella, M., Uribezubia, A., Romero, J.-M., Plana, E., Miralles, M., Clarà, A., Dilmé, J., Soria, J. M., Camacho, M., Martinez-Perez, A., & Sabater-Lleal, M. (2025). A Comprehensive Analysis of the Abdominal Aortic Aneurysm Growth Rate in the Spanish Population. Journal of Clinical Medicine, 14(13), 4720. https://doi.org/10.3390/jcm14134720







