Novel Oral Anticoagulants Versus Antiplatelet Therapy in Post-TAVR Patients: A Single-Center Retrospective Study
Abstract
1. Introduction
2. Methods
2.1. Study Design and Population
2.2. Antithrombotic Groups
2.3. Outcomes
2.4. Statistical Analysis
2.5. Sensitivity and Subgroup Analyses
2.6. Multiple Testing Adjustment
3. Results
3.1. Study Population
3.2. Baseline Characteristics
3.3. Clinical Outcomes: NOAC Versus APT-Only
3.4. Temporal Trends
3.5. Descriptive VKA Outcomes
4. Discussion
4.1. Key Findings and Context
4.2. Methodological Considerations
4.3. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Permission to Reproduce Material from Other Sources
References
- Franke, K.B.; Bhatia, D.; Roberts-Thomson, R.L.; Psaltis, P.J. Aortic valve replacement reduces mortality in moderate aortic stenosis: A systematic review and meta-analysis. J. Geriatr. Cardiol. 2023, 20, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Young, M.N.; Kearing, S.; Malenka, D.; Goodney, P.P.; Skinner, J.; Iribarne, A. Geographic and demographic variability in transcatheter aortic valve replacement dispersion in the United States. J. Am. Heart Assoc. 2021, 10, e019588. [Google Scholar] [CrossRef] [PubMed]
- Blankenberg, S.; Seiffert, M.; Vonthein, R.; Baumgartner, H.; Bleiziffer, S.; Borger, M.A.; Choi, Y.-H.; Clemmensen, P.; Cremer, J.; Czerny, M.; et al. Transcatheter or Surgical Treatment of Aortic-Valve Stenosis. N. Engl. J. Med. 2024, 390, 1572–1583. [Google Scholar] [CrossRef] [PubMed]
- Otto, C.M.; Nishimura, R.A.; Bonow, R.O.; Carabello, B.A.; Erwin, J.P., 3rd; Gentile, F.; Jneid, H.; Krieger, E.V.; Mack, M.; McLeod, C.; et al. 2020 ACC/AHA Guideline for the management of patients with valvular heart disease: A report of the American College of Cardiology/American Heart Association joint committee on clinical practice guidelines. Circulation 2021, 143, e72–e227. [Google Scholar] [PubMed]
- Dangas, G.D.; Tijssen, J.G.P.; Wöhrle, J.; Søndergaard, L.; Gilard, M.; Möllmann, H.; Makkar, R.R.; Herrmann, H.C.; Giustino, G.; Baldus, S.; et al. A Controlled trial of rivaroxaban after transcatheter aortic-valve replacement. N. Engl. J. Med. 2020, 382, 120–129. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, Y.; Howard, J.P.; Madhavan, M.V.; Leon, M.B.; Makkar, R.R. single versus dual antiplatelet therapy after transcatheter aortic valve replacement: A meta-analysis of randomized clinical trials. Cardiovasc. Revasc Med. 2022, 34, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Collet, J.P.; Van Belle, E.; Thiele, H.; Berti, S.; Lhermusier, T.; Manigold, T.; Neumann, F.J.; Gilard, M.; Attias, D.; Beygui, F.; et al. Apixaban vs. standard of care after transcatheter aortic valve implantation: The ATLANTIS trial. Eur. Heart J. 2022, 43, 2783–2797. [Google Scholar] [CrossRef] [PubMed]
- Overtchouk, P.; Guedeney, P.; Rouanet, S.; Verhoye, J.P.; Lefevre, T.; Van Belle, E.; Eltchaninoff, H.; Gilard, M.; Leprince, P.; Iung, B.; et al. Long-term mortality and early valve dysfunction according to anticoagulation use: The FRANCE-TAVI registry. J. Am. Coll. Cardiol. 2019, 73, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Brouwer, J.; Nijenhuis, V.J.; Delewi, R.; Hermanides, R.S.; Holvoet, W.; Dubois, C.L.; Frambach, P.; De Bruyne, B.; van Houwelingen, G.K.; Van Der Heyden, J.A.; et al. Aspirin with or without clopidogrel after transcatheter aortic-valve implantation. N. Engl. J. Med. 2020, 383, 1447–1457. [Google Scholar] [CrossRef] [PubMed]
- Van Mieghem, N.M.; Unverdorben, M.; Hengstenberg, C.; Möllmann, H.; Mehran, R.; López-Otero, D.; Nombela-Franco, L.; Moreno, R.; Nordbeck, P.; Thiele, H.; et al. Edoxaban versus vitamin K antagonist for atrial fibrillation after TAVR. N. Engl. J. Med. 2021, 385, 2150–2160. [Google Scholar] [CrossRef] [PubMed]
- Malik, A.H.; Yandrapalli, S.; Aronow, W.S. Meta-analysis comparing the safety and efficacy of dual versus single antiplatelet therapy following transcatheter aortic valve replacement. Am. J. Cardiol. 2023, 188, 35–43. [Google Scholar]
- Chakravarty, T.; Søndergaard, L.; Friedman, J.; De Backer, O.; Berman, D.; Kofoed, K.F.; Jilaihawi, H.; Shiota, T.; Abramowitz, Y.; Jørgensen, T.H.; et al. Subclinical leaflet thrombosis in surgical and transcatheter bioprosthetic aortic valves: An observational study. Lancet 2017, 389, 2383–2392. [Google Scholar] [CrossRef] [PubMed]
- Vahanian, A.; Beyersdorf, F.; Praz, F.; Milojevic, M.; Baldus, S.; Bauersachs, J.; Capodanno, D.; Conradi, L.; De Bonis, M.; De Paulis, R.; et al. 2021 ESC/EACTS Guidelines for the management of valvular heart disease. Eur. Heart J. 2022, 43, 561–632. [Google Scholar] [CrossRef] [PubMed]
| Year of TAVR procedure |
| Sex |
| Weight |
| Height |
| CHA2DS2-VASc score |
| Indication for oral anticoagulant therapy |
| Chronic obstructive lung disease |
| History of prior cerebrovascular event, myocardial infarction, coronary bypass graft, percutaneous coronary intervention |
| Chronic kidney disease |
| NYHA functional class |
| Malignancy |
| Hemoglobin level at admission |
| Multivessel coronary artery disease |
| Left ventricular ejection fraction |
| Aortic valve area |
| TAVR in the bioprosthesis procedure |
| Implanted prosthesis size |
| Pre-dilation performed |
| Major VARC-2 vascular complications |
| Acute kidney injury Stage II or III |
| Antithrombotic therapy at discharge |
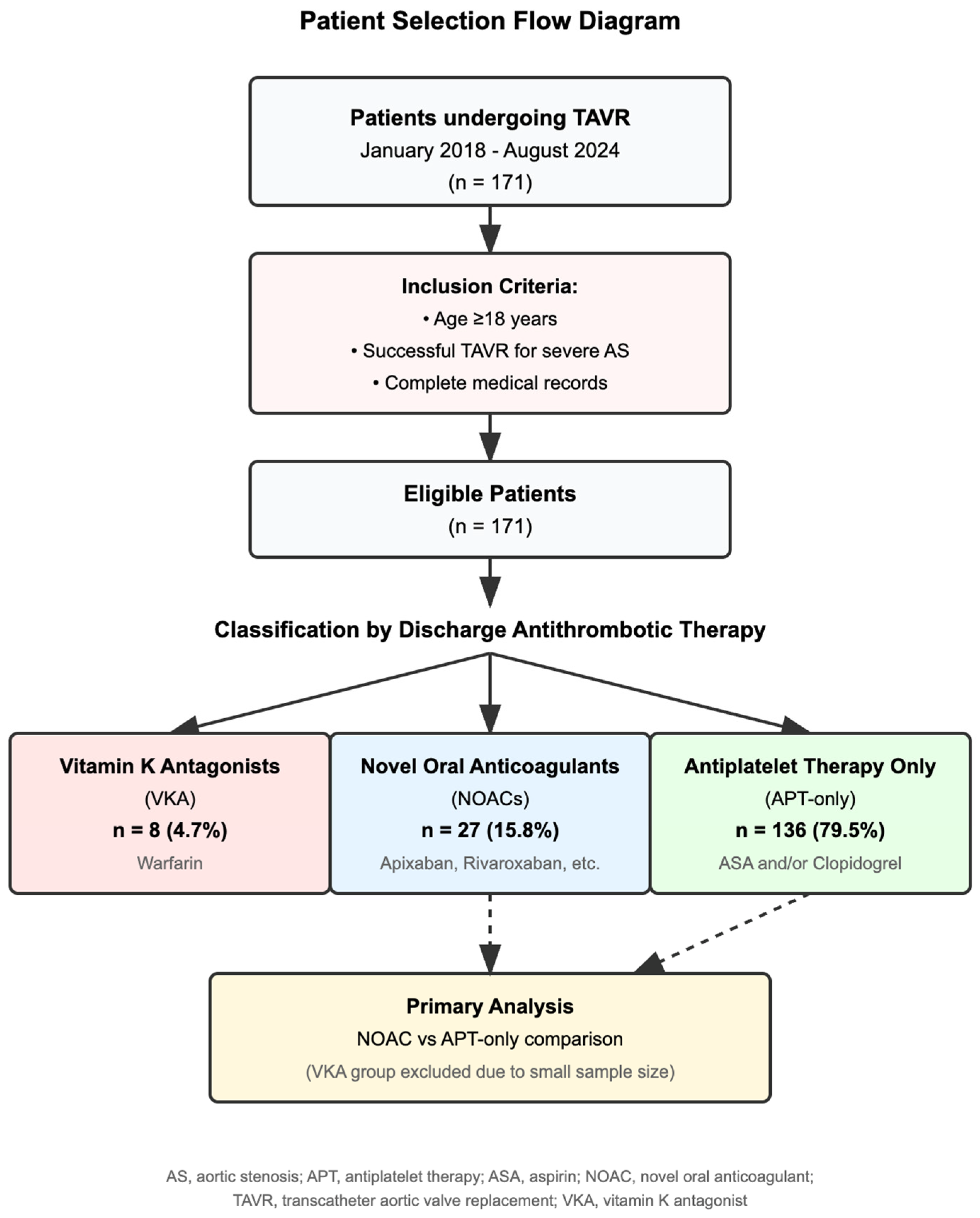
| Antithrombotic Strategy | n | % | Included in Primary Analysis |
|---|---|---|---|
| Antiplatelet therapy only | 136 | 79.5% | Yes |
| - Aspirin monotherapy | 8 | 5.9% * | |
| - Clopidogrel monotherapy | 10 | 7.4% * | |
| - DAPT | 118 | 86.8% * | |
| NOACs | 27 | 15.8% | Yes |
| VKAs | 8 | 4.7% | No (descriptive only) |
| Total | 171 | 100% |
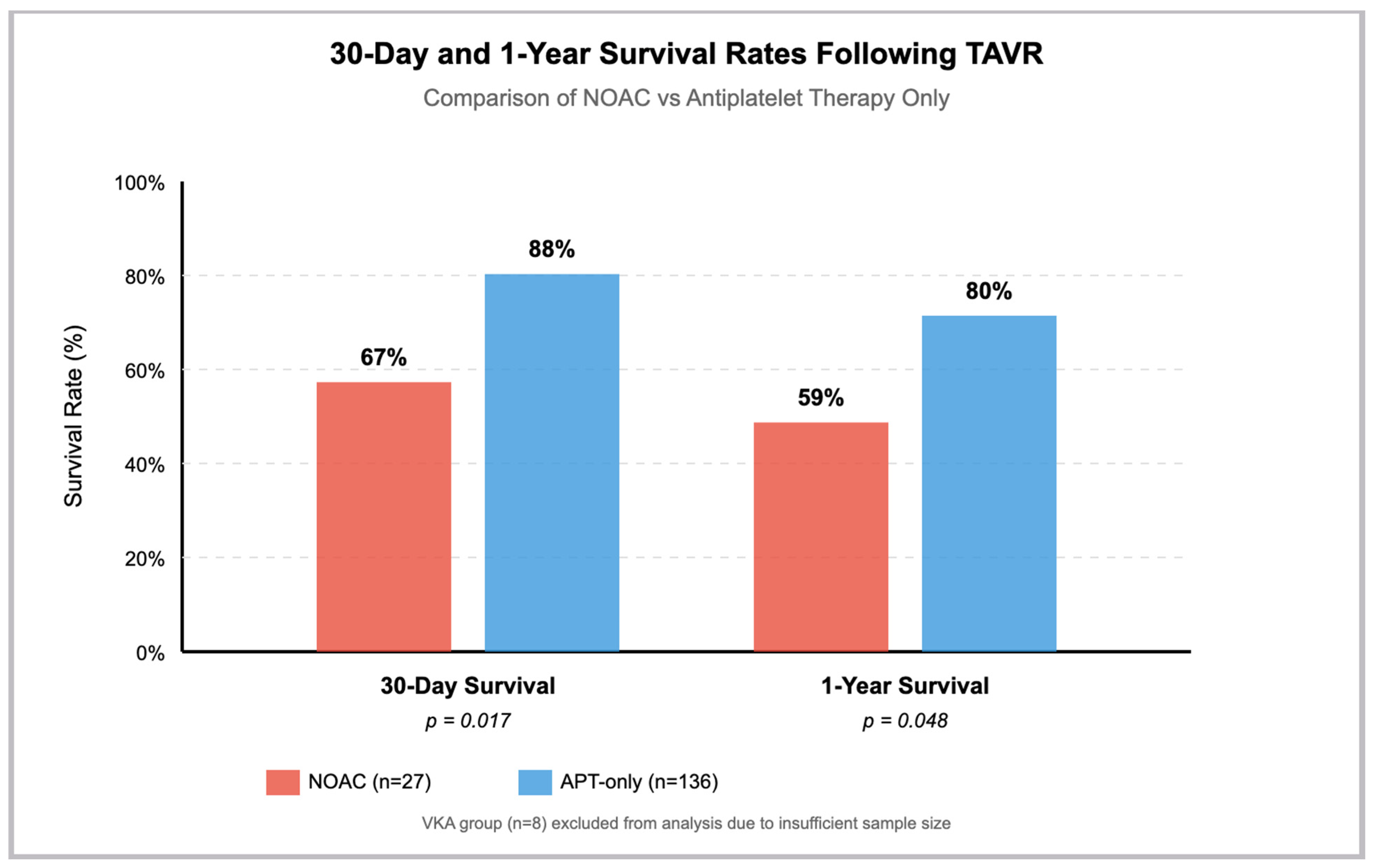
| Outcome | NOACs (n = 27) | APT-Only (n = 136) | Unadjusted | IPTW-Adjusted | ||
|---|---|---|---|---|---|---|
| n (%) | n (%) | OR/HR (95% CI) | p | OR/HR (95% CI) | p | |
| 30-day | ||||||
| Survival | 18 (67%) | 120 (88%) | 0.27 (0.10–0.69) | 0.007 | 0.35 (0.17–0.72) | 0.004 |
| MACCE | 6 (22%) | 11 (8%) | 3.25 (1.08–9.72) | 0.035 | 5.59 (2.56–12.18) | <0.001 |
| 1-year | ||||||
| Survival | 16 (59%) | 109 (80%) | 0.36 (0.15–0.87) | 0.022 | 0.47 (0.25–0.90) | 0.022 |
| MACCE | 9 (34%) | 22 (17%) | 2.58 (1.02–6.53) | 0.045 | 2.40 (1.23–4.68) | 0.010 |
| Non-VKAs (n = 27) | No Therapy, ASA, or Clopidogrel (n = 136) | All (N = 163) | p Value | |
|---|---|---|---|---|
| Age | 76.41 ± 8.04 | 76.28 ± 8.83 | 76.5 ± 8.67 | 0.225 |
| Women | 8 (30%) | 72 (47%) | 87 (51%) | 0.086 |
| Weight | 95.59 ± 25.01 | 87.11 ± 23.14 | 88.08 ± 23.31 | 0.195 |
| Height | 1.73 ± 0.11 | 1.66 ± 0.12 | 1.67 ± 0.12 | 0.055 |
| STS-PROM | 7.87 ± 8.75 | 6.02 ± 8.85 | 6.44 ± 8.66 | 0.056 |
| CHA2DS2-VASc score | 4.7 ± 2.00 | 2.03 ± 2.46 | 2.6 ± 2.61 | p < 0.001 |
| Indication for oral anticoagulant therapy (any) | 25 (93%) | 18 (13%) | 43 (30%) | p < 0.001 |
| Permanent atrial fibrillation | 12 (44%) | 9 (7%) | 21 (14%) | p < 0.001 |
| Paroxysmal atrial fibrillation | 13 (48%) | 8 (6%) | 21 (15%) | |
| Others | 0 (0%) | 1 (1%) | 1 (1%) | |
| Hypertension | 27 (100%) | 128 (94%) | 155 (95%) | 0.340 |
| Diabetes mellitus | 16 (59%) | 72 (53%) | 88 (54%) | 0.746 |
| Peripheral artery disease | 11 (41%) | 65 (48%) | 76(46%) | 0.702 |
| Chronic obstructive lung disease | 10 (38%) | 30 (22%) | 40 (25%) | 0.186 |
| History of cerebrovascular event | 11 (41%) | 13 (10%) | 24 (15%) | p < 0.001 |
| History of myocardial infarction | 9 (33%) | 20 (15%) | 29 (18%) | 0.063 |
| History of aortocoronary bypass graft surgery | 2 (8%) | 5 (10%) | 7 (11%) | 0.047 |
| History of percutaneous coronary intervention | 8 (30%) | 50 (37%) | 58 (37%) | 0.238 |
| Chronic kidney disease | 15 (56%) | 72 (53%) | 82 (53%) | 0.953 |
| NYHA functional class (mean rank comparison) | 2.78 ± 0.75 | 2.43 ± 0.92 | 2.51 ± 0.90 | 0.061 |
| Class I | 2 (7%) | 29 (21%) | 32 (18%) | 0.270 |
| Class II | 5 (19%) | 31 (23%) | 36 (22%) | |
| Class III | 17 (63%) | 65 (48%) | 82 (50%) | |
| Class IV | 3 (11%) | 11 (8%) | 14 (9%) | |
| Malignancy | 5 (19%) | 37 (27%) | 42 (26%) | 0.640 |
| Hemoglobin at admission | 12.18 ± 2.06 | 11.74 ± 1.92 | 11.85 ± 1.94 | 0.255 |
| Congestive heart failure | 15 (56%) | 56 (41%) | 71 (45%) | 0.085 |
| Multivessel coronary artery disease | 14 (52%) | 64 (47%) | 78 (48%) | 0.895 |
| Left ventricular ejection fraction | 51.56 ± 15.37 | 53.31 ± 13.32 | 52.78 ± 13.65 | 0.217 |
| Aortic valve area | 0.7 ± 0.25 | 0.7 ± 0.22 | 0.69 ± 0.23 | 0.571 |
| TAVR in bioprosthesis | 22 (82%) | 83 (61%) | 45 (66%) | 0.014 |
| Implanted prosthesis size | 25.85 (2.74) | 24.71 (2.26) | 24.95 (2.40) | 0.054 |
| Pre-dilation performed | 10 (37%) | 92 (68%) | 104 (61%) | 0.001 |
| Major VARC-2 vascular complications | 2 (7%) | 3 (2%) | 5 (3%) | 0.301 |
| Acute kidney injury stage II or III | 3 (11%) | 5 (4%) | 8 (5%) | 0.201 |
| Antithrombotic therapy at discharge | p < 0.001 | |||
| Aspirin | 2 (7%) | 8 (6%) | 10 (6%) | |
| ADP receptor inhibitors alone | 19 (70%) | 10 (7%) | 29 (20%) | |
| DAPT | 6 (22%) | 118 (87%) | 124 (74%) | |
| 30-days survival | 18 (67%) | 120 (88%) | 138 (84%) | 0.017 |
| 30-days, any BARC bleeding | 2 (7%) | 6 (4%) | 8(5%) | 0.649 |
| No therapy, BARC 0 | 25 (93%) | 130 (96%) | 155 (95%) | 0.712 |
| Minor, BARC 1 | 2 (7%) | 4 (3%) | 6 (4%) | |
| Major or life-threatening, BARC 2 | 0 (0%) | 2 (2%) | 2 (1%) | |
| 30 days, MACCE | 6 (22%) | 11 (8%) | 17 (10%) | 0.051 |
| 30 days, NACE | 6 (22%) | 17 (13%) | 23 (14%) | 0.410 |
| 1-year survival | 16 (59%) | 109 (80%) | 125 (77%) | 0.048 |
| 1-year cardio-cerebrovascular mortality | 1 (4%) | 8 (6%) | 9 (5%) | 0.712 |
| 1-year, any BARC bleeding | 4 (15%) | 14 (10%) | 18 (11%) | 0.478 |
| No therapy, BARC 0 | 23 (85%) | 122 (90%) | 143 (90%) | 0.677 |
| Minor, BARC 1 | 2 (7%) | 4 (3%) | 6 (4%) | |
| Major or life-threatening, BARC 2 | 2 (7%) | 10 (7%) | 12 (7%) | |
| 1 year, Cerebrovascular events | 0.904 | |||
| No therapy | 27 (100%) | 128 (94%) | 163 (95%) | |
| Transient ischemic event | 0 (0%) | 4 (3%) | 4 (2%) | |
| Nondisabling | 0 (0%) | 2 (2%) | 2 (1%) | |
| Disabling | 0 (0%) | 2 (2%) | 2 (1%) | |
| 1 year, MACCE | 7 (26%) | 22 (16%) | 29 (17%) | 0.198 |
| 1 year, NACE | 8 (30%) | 32 (24%) | 41 (24%) | 0.587 |
| Survival time (of those registered to have died), in days | 72 ± 104 | 390 ± 511 | 320 ± 461 | 0.055 |
| Survival time merged, in days * | 642 ± 571 | 956 ± 575 | 892 ± 578 | 0.021 |
| Non-VKAs (n = 62) | ATP (n = 156) | All (N = 181) | p Value | |
|---|---|---|---|---|
| 30-day survival | 44 (71%) | 137 (88%) | 181 (85%) | 0.002 |
| 30-days, any BARC bleeding | 4 (7%) | 7 (5%) | 11 (4%) | 0.254 |
| No therapy, BARC 0 | 58 (94%) | 149 (96%) | 251 (96%) | 0.325 |
| Minor, BARC 1 | 4 (7%) | 5 (3%) | 9 (3%) | |
| Major or life-threatening, BARC 2 | 0 (0%) | 2 (1%) | 2 (1%) | |
| 30 days, MACCE | 21 (34%) | 13 (8%) | 34 (13%) | p < 0.001 |
| 30 days, NACE | 20 (32%) | 19 (12%) | 39 (17%) | 0.002 |
| 1-year survival | 40 (65%) | 124 (80%) | 164 (78%) | 0.002 |
| 1-year cardio-cerebrovascular mortality | 3 (5%) | 9 (6%) | 12 (5%) | 0.269 |
| 1-year, any BARC bleeding | 8 (13%) | 17 (11%) | 25 (10%) | 0.056 |
| No therapy, BARC 0 | 54 (87%) | 140 (90%) | 194 (91%) | 0.042 |
| Minor, BARC 1 | 5 (8%) | 4 (3%) | 9 (3%) | |
| Major or life-threatening, BARC 2 | 3 (5%) | 12 (8%) | 15 (6%) | |
| 1 year, cerebrovascular events | 0.315 | |||
| No therapy | 62 (100%) | 146 (94%) | 208 (96%) | |
| Transient ischemic event | 0 (0%) | 5 (3%) | 5 (2%) | |
| Nondisabling | 0 (0%) | 2 (1%) | 2 (1%) | |
| Disabling | 0 (0%) | 3 (2%) | 3 (1%) | |
| 1 year, MACCE | 21 (34%) | 27 (17%) | 48 (18%) | p < 0.001 |
| 1 year, NACE | 23 (37%) | 39 (25%) | 68 (26%) | 0.031 |
| Survival time, in days * | 65 ± 87 | 376 ± 493 | 283 ± 409 | 0.002 |
| Survival time merged † | 679 ± 550 | 937 ± 576 | 870 ± 563 | 0.033 |
| Cox Regression | Unadjusted | Adjusted, Using Inverse Probability of Treatment Weights | ||
|---|---|---|---|---|
| Group compared with no therapy | Non-VKA oral anticoagulants | Non-VKA oral anticoagulants | ||
| HR (95% CI) | p value | HR (95% CI) | p value | |
| Overall mortality (survival time merged) | 1.737 (0.886–3.408) | 0.108 | 1.440 (0.854–2.427) | 0.171 |
| Overall mortality (survival since baseline) | 2.057 (0.996–4.250) | 0.051 | 2.219 (1.222–4.026) | 0.009 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodriguez Mejia, R.A.; Acker, E.; Dao, V.; Rana, H. Novel Oral Anticoagulants Versus Antiplatelet Therapy in Post-TAVR Patients: A Single-Center Retrospective Study. J. Clin. Med. 2025, 14, 4690. https://doi.org/10.3390/jcm14134690
Rodriguez Mejia RA, Acker E, Dao V, Rana H. Novel Oral Anticoagulants Versus Antiplatelet Therapy in Post-TAVR Patients: A Single-Center Retrospective Study. Journal of Clinical Medicine. 2025; 14(13):4690. https://doi.org/10.3390/jcm14134690
Chicago/Turabian StyleRodriguez Mejia, Ricardo A., Eric Acker, Vinh Dao, and Humza Rana. 2025. "Novel Oral Anticoagulants Versus Antiplatelet Therapy in Post-TAVR Patients: A Single-Center Retrospective Study" Journal of Clinical Medicine 14, no. 13: 4690. https://doi.org/10.3390/jcm14134690
APA StyleRodriguez Mejia, R. A., Acker, E., Dao, V., & Rana, H. (2025). Novel Oral Anticoagulants Versus Antiplatelet Therapy in Post-TAVR Patients: A Single-Center Retrospective Study. Journal of Clinical Medicine, 14(13), 4690. https://doi.org/10.3390/jcm14134690




