An Iterative Design Approach to Development of an Ex Situ Normothermic Multivisceral Perfusion Platform
Abstract
1. Introduction
2. Materials and Methods
2.1. Rationale for Iterative Design Process
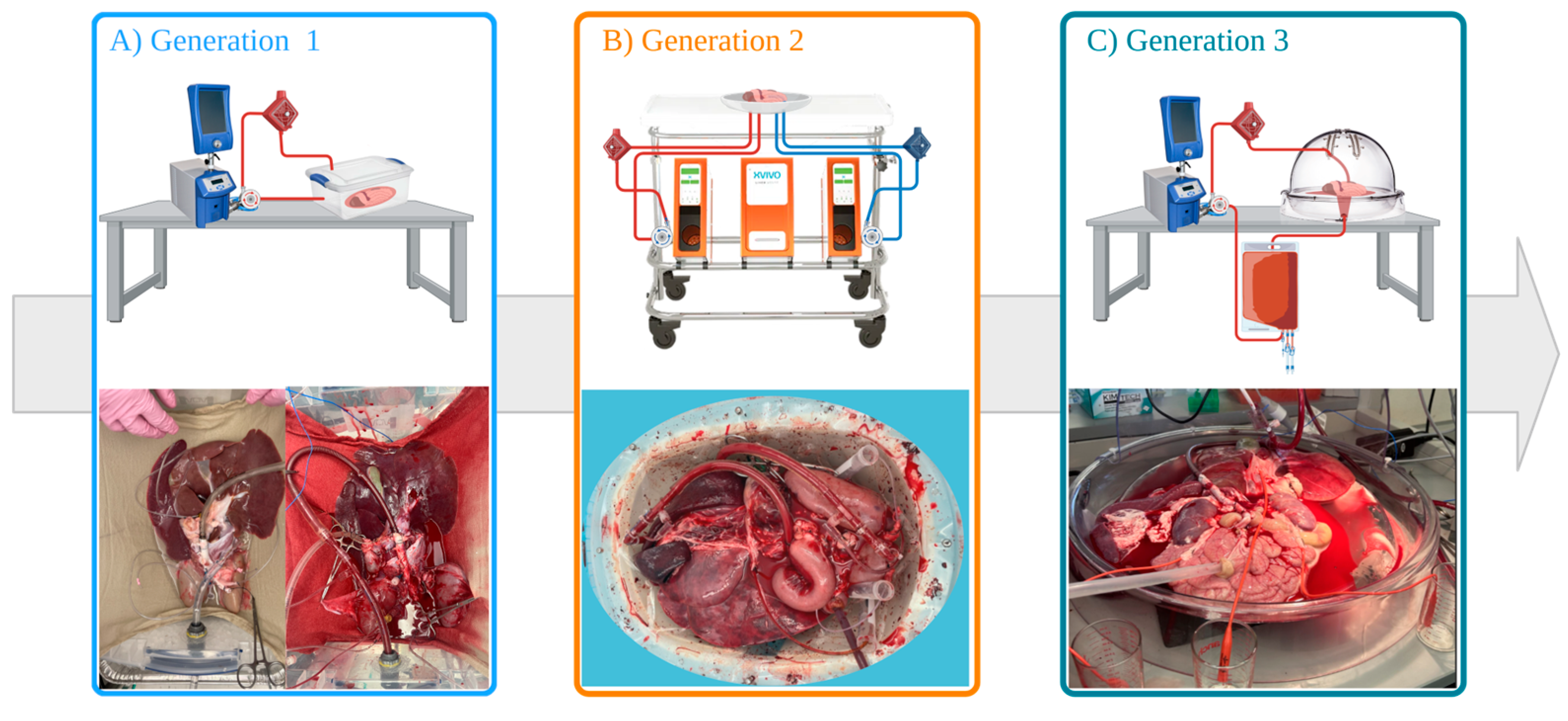
2.2. Generation 1 Perfusion Set-Up (Gen 1)
2.3. Generation 2 Perfusion Set-Up (Gen 2)
2.4. Generation 3 Perfusion Set-Up (Gen 3)
2.5. Logging of Perfusion Parameters and Viability Assessment
- Hb: hemoglobin content (mmol/L).
- pO2: partial oxygen pressure (kPa).
- K: solubility constant of oxygen in H2O at 37 °C (0.0225 mlO2 per kPa).
- SO2: hemoglobin saturation (%).
- Q: blood flow (L/min).
2.6. Clinical Chemistry
2.7. Histological Analysis
2.8. Data and Statistical Analysis
3. Results
3.1. Preservation Characteristics
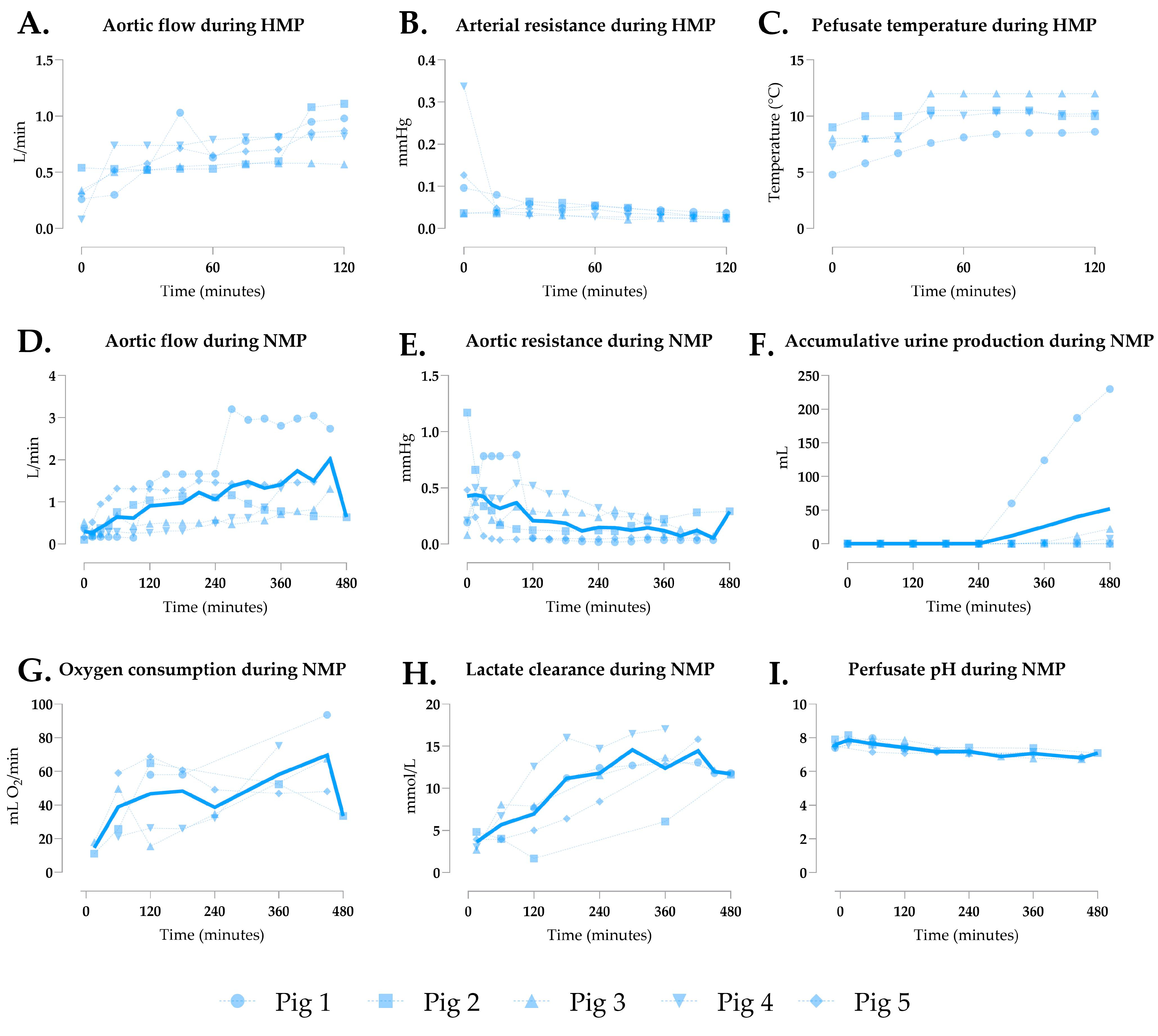
3.2. Generation 1 Perfusion
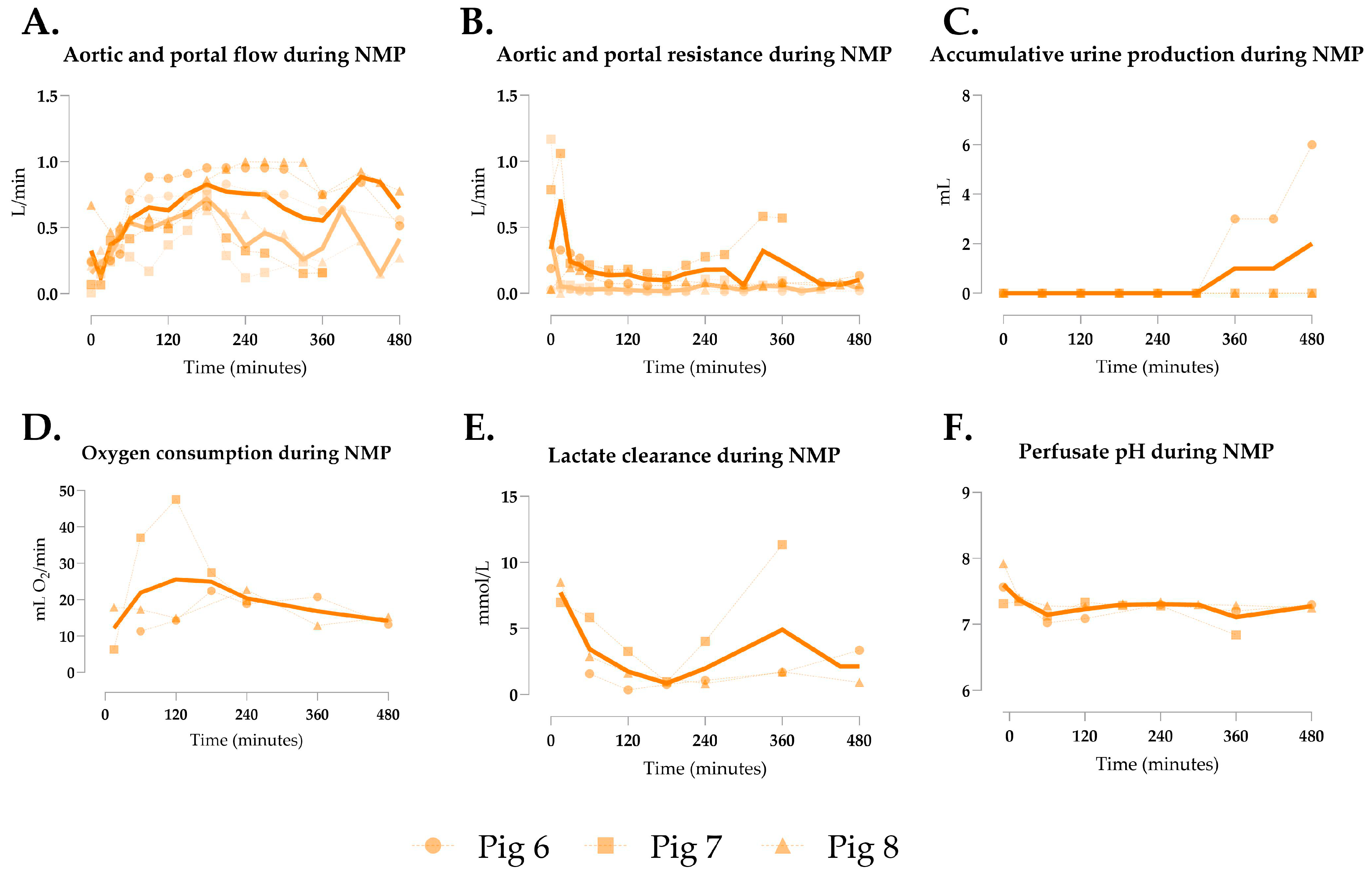
3.3. Generation 2 Perfusion
3.4. Generation 3 Perfusion
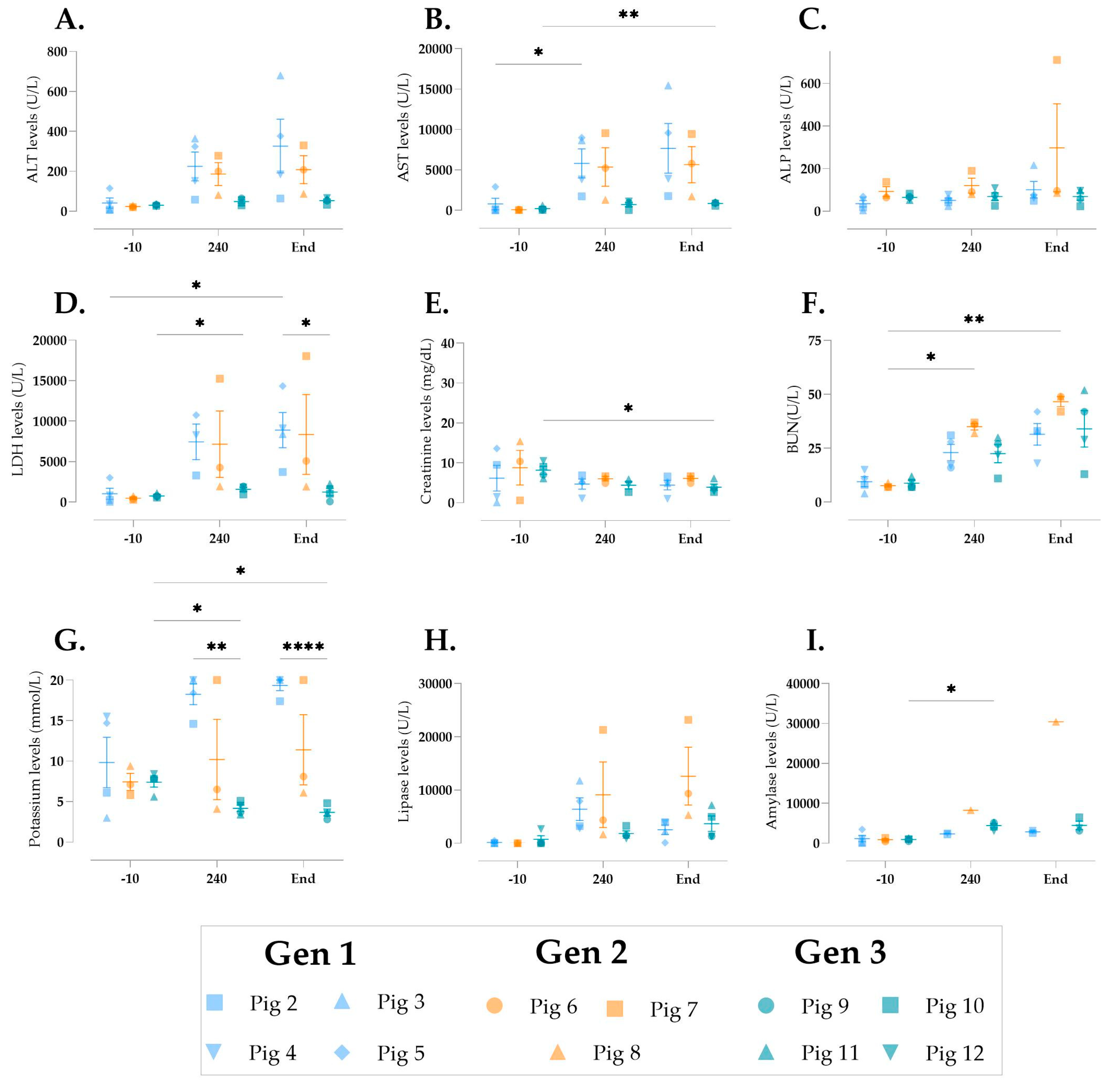
3.5. Cellular Injury During Multivisceral Perfusion
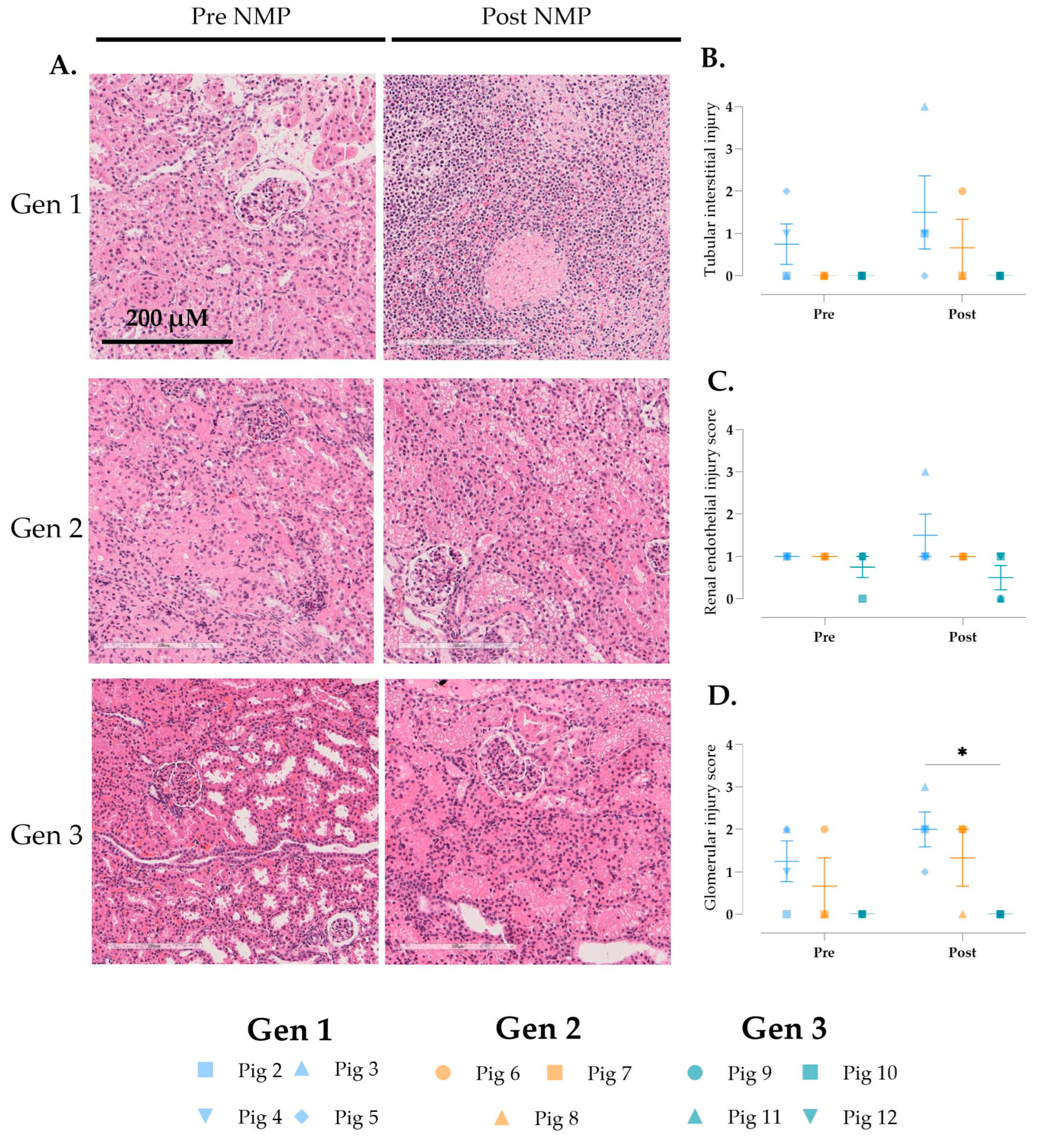
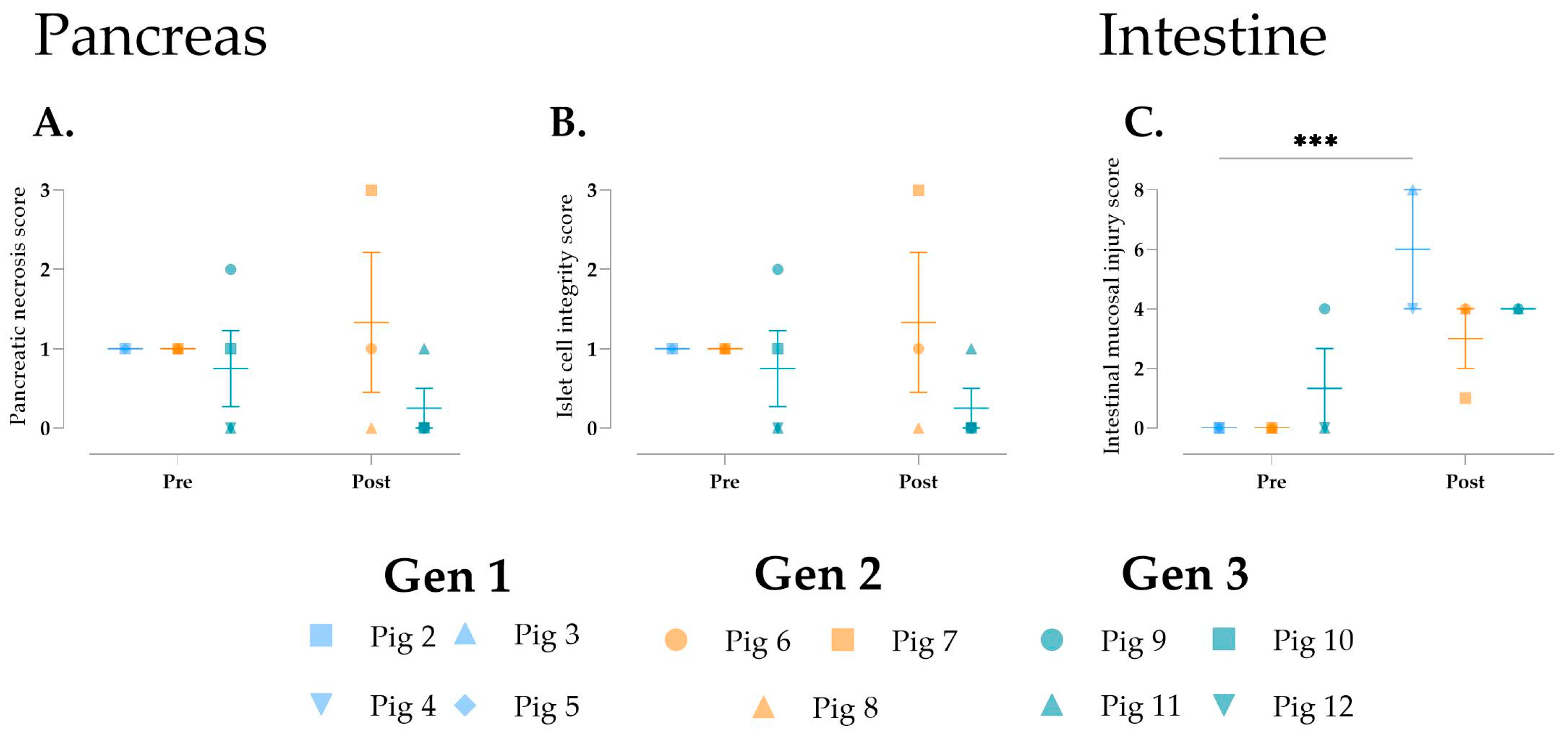
3.6. Histological Changes over Time
4. Discussion
4.1. Study Strengths and Key Findings
4.2. Preclinical Evidence in Multivisceral Organ Perfusion Research
4.3. Experimental Considerations, Study Limitations, and Future Directions
4.4. Clinical Perspectives of Multivisceral Perfusion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ALP | Alkaline phosphatase |
| ALT | Alanine aminotransferase |
| ANOVA | Analysis of variance |
| AST | Aspartate aminotransferase |
| BUN | Blood urea nitrogen |
| DCD | Donation after circulatory death |
| H&E | Hematoxylin and eosin |
| HMP | Hypothermic machine perfusion |
| IRI | Ischemia-reperfusion injury |
| IVC | Inferior vena cava |
| LDH | Lactate dehydrogenase |
| MAP | Mean arterial pressure |
| NMP | Normothermic machine perfusion |
| NRP | Normothermic regional perfusion |
| WIT | Warm ischemia time |
Appendix A
Surgical Protocol at the In-House Animal Facility
| Pig 1 | Pig 2 | Pig 3 | Pig 4 | Pig 5 | Pig 6 | Pig 7 | Pig 8 | Pig 9 | Pig 10 | Pig 11 | Pig 12 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Generation set-up | 1 | 1 | 1 | 1 | 1 | 2 | 2 | 2 | 3 | 3 | 3 | 3 |
| Pig type | Butcher | Butcher | Butcher | Butcher | Butcher | Butcher | Butcher | Laboratory | Laboratory | Laboratory | Laboratory | Laboratory |
| Pig age (months) | 6 | 6 | 8 | 6 | 6 | 6 | 5 | 3 | 3.5 | 3 | 3 | 3 |
| Pig sex | Female | Female | Female | Male | Female | Male | Male | Male | Male | Male | Male | Male |
| Pig weight (Kg) | NR | NR | NR | NR | NR | NR | NR | 46.4 | 50.9 | 33.8 | 37 | 39.9 |
| Flush type | 3L NaCl + 4L HTK | 3L NaCl + 4L Servator B™ Safe | 3L NaCl + 4L HTK | 3L NaCl + 4L HTK | 3L NaCl + 4L HTK | 6L NaCl | 3L NaCl + 4L HTK | 3L NaCl + 4L HTK | 3L NaCl + 4L HTK | 3L NaCl + 4L HTK | 6L HTK | 6L HTK |
| CIT (hours) | 5:55 | 5:45 | 5:57 | 5:21 | 7:45 | 4:58 | 5:04 | 3:14 | 2:52 | 3:29 | 3:33 | 2:25 |
| Bowel length (m) | 0.03 | 0.03 | 0.02 | 0.1–0.3 | 0.1–0.3 | 0.03 | 0.03 | 0.03 | 2.25 | 2.25 | 2.25 | 2.25 |
| Graft weight pre NMP (g) | NR | 1013 | 1453 | NR | NR | NR | NR | 1900 | 1800 | 2550 | 1800 | 2000 |
| Perfusate composition NMP | ||||||||||||
| Whole blood (mL) | 1000 | 1000 | 1000 | 1600 | 1900 | 1250 | 1300 | 1800 | 1775 | 2100 | 1550 | 1670 |
| Cefuroxim in 20 mL NaCl (g) | 1.5 | 1.5 | 1.5 | 1.5 | 1.5 | 1.5 | 1.5 | 1.5 | 1.5 | 1.5 | 1.5 | 1.5 |
| Calcium gluconate (mL) | 10 | 20 | 20 | 20 | 10 | 20 | 30 | 20 | 20 | 20 | 20 | 20 |
| TPN (mL) | 30 | 30 | 30 | 30 | 70 | 30 | 36 | 38 | 30 | 30 | 30 | 30 |
| Albumin 5% (mL) | 250 | 250 | 250 | 250 | 1000 | 700 | 750 | 250 | 250 | 250 | 750 | 500 |
| Albumin 25% (mL) | - | - | - | - | - | - | - | - | 200 | - | 100 | 100 |
| Creatinine (mg) | 218 | 218 | 218 | 218 | 218 | 218 | 218 | 218 | 218 | 218 | 218 | 218 |
| Mannitol (g) | 5 | 5 | 5 | 2.5 | 4 | 4 | 4 | 2.5 | 2.5 | 2.5 | 0.1 | 25 |
| Sodium Nitroprusside (mg) | 5 | 17.5 | 10 | 20 | 22 | - | - | - | - | - | - | - |
| Velitri (ug) | 83.3 | - | - | - | - | 164.93 | - | 83.3 | 83.3 | 49.98 | - | 16.66 |
| 8.4% NaHCO₃ (mL) | 30 | 30 | 40 | 60 | 85 | 70 | 30 | - | 20 | 15 | 15 | 35 |
| Fluid loss replacement (5% albumin) (mL) | - | 320 | 100 | 700 | - | 450 | - | 250 | 2750 | 1900 | 250 | 2510 |
References
- Torabi, J.; Todd, R.; van Leeuwen, L.L.; Bekki, Y.; Holzner, M.; Moon, J.; Schiano, T.; Florman, S.S.; Akhtar, M.Z.B. A Decade of Liver Transplantation in the United States: Drivers of Discard and Underutilization. Transplant. Direct 2024, 10, E1605. [Google Scholar] [CrossRef] [PubMed]
- McKenney, C.; Torabi, J.; Todd, R.; Akhtar, M.Z.; Tedla, F.M.; Shapiro, R.; Florman, S.S.; Holzner, M.L.; van Leeuwen, L.L. Wasted Potential: Decoding the Trifecta of Donor Kidney Shortage, Underutilization, and Rising Discard Rates. Transplantology 2024, 5, 51–64. [Google Scholar] [CrossRef]
- van Leeuwen, L.L.; Ruigrok, M.J.R.; Kessler, B.M.; Leuvenink, H.G.D.; Olinga, P. Targeted delivery of galunisertib using machine perfusion reduces fibrogenesis in an integrated ex vivo renal transplant and fibrogenesis model. Br. J. Pharmacol. 2024, 181, 464–479. [Google Scholar] [CrossRef] [PubMed]
- Hosgood, S.A.; Callaghan, C.J.; Wilson, C.H.; Smith, L.; Mullings, J.; Mehew, J.; Oniscu, G.C.; Phillips, B.L.; Bates, L.; Nicholson, M.L. Normothermic machine perfusion versus static cold storage in donation after circulatory death kidney transplantation: A randomized controlled trial. Nat. Med. 2023, 29, 1511–1519. [Google Scholar] [CrossRef]
- Nasralla, D.; Coussios, C.C.; Mergental, H.; Akhtar, M.Z.; Butler, A.J.; Ceresa, C.D.L.; Chiocchia, V.; Dutton, S.J.; García-Valdecasas, J.C.; Heaton, N.; et al. A randomized trial of normothermic preservation in liver transplantation. Nature 2018, 557, 50–56. [Google Scholar] [CrossRef]
- van Leeuwen, L.L.; Irizar, H.; Kim-Schluger, L.; Florman, S.; Akhtar, M.Z. The Potential of Machine Learning to Predict Early Allograft Dysfunction after Normothermic Machine Perfusion in Liver transplantation. J. Hepatol. 2024, 81, e298–e300. [Google Scholar] [CrossRef]
- Eshmuminov, D.; Becker, D.; Bautista Borrego, L.; Hefti, M.; Schuler, M.J.; Hagedorn, C.; Muller, X.; Mueller, M.; Onder, C.; Graf, R.; et al. An integrated perfusion machine preserves injured human livers for 1 week. Nat. Biotechnol. 2020, 38, 189–198. [Google Scholar] [CrossRef]
- Hamelink, T.L.; Ogurlu, B.; Pamplona, C.C.; Castelein, J.; Bennedsgaard, S.S.; Qi, H.; Weiss, T.; Lantinga, V.A.; Pool, M.B.; Laustsen, C.; et al. Magnetic resonance imaging as a noninvasive adjunct to conventional assessment of functional differences between kidneys in vivo and during ex vivo normothermic machine perfusion. Am. J. Transplant. 2024, 24, 1761–1771. [Google Scholar] [CrossRef]
- Lantinga, V.A.; Arykbaeva, A.S.; Spraakman, N.A.; Blom, E.W.P.; Huijink, T.M.; de Vries, D.K.; Ploeg, R.J.; Alwayn, I.P.J.; Leuvenink, H.G.D.; Moers, C.; et al. Impact of device variability and protocol differences on kidney function during normothermic machine perfusion: A comparative study using porcine and human kidneys. Artif. Organs 2025, 49, 93–107. [Google Scholar] [CrossRef]
- Barlow, A.D.; Hamed, M.O.; Mallon, D.H.; Brais, R.J.; Gribble, F.M.; Scott, M.A.; Howat, W.J.; Bradley, J.A.; Bolton, E.M.; Pettigrew, G.J.; et al. Use of Ex Vivo Normothermic Perfusion for Quality Assessment of Discarded Human Donor Pancreases. Am. J. Transplant. 2015, 15, 2475–2482. [Google Scholar] [CrossRef]
- Abraham, N.; Ludwig, E.K.; Schaaf, C.R.; Veerasammy, B.; Stewart, A.S.; McKinney, C.; Freund, J.B.; Brassil, J.; Samy, K.P.; Gao, Q.M.; et al. Orthotopic Transplantation of the Full-length Porcine Intestine After Normothermic Machine Perfusion. Transplant. Direct 2022, 8, e1390. [Google Scholar] [CrossRef] [PubMed]
- Ludwig, E.K.; Abraham, N.; Schaaf, C.R.; McKinney, C.A.; Freund, J.; Stewart, A.S.; Veerasammy, B.A.; Thomas, M.; Cardona, D.M.; Garman, K.; et al. Comparison of the effects of normothermic machine perfusion and cold storage preservation on porcine intestinal allograft regenerative potential and viability. Am. J. Transplant. 2024, 24, 564–576. [Google Scholar] [CrossRef] [PubMed]
- Anthony Celi, L.; Mark, R.G.; Stone, D.J.; Montgomery, R.A. “Big Data” in the Intensive Care Unit. Closing the Data Loop. Am. J. Respir. Crit. Care Med. 2013, 187, 1157–1160. [Google Scholar] [CrossRef]
- Abdulmalek, S.; Nasir, A.; Jabbar, W.A.; Almuhaya, M.A.M.; Bairagi, A.K.; Khan, M.A.-M.; Kee, S.-H. IoT-Based Healthcare-Monitoring System towards Improving Quality of Life: A Review. Healthcare 2022, 10, 1993. [Google Scholar] [CrossRef]
- Prieto, M.; Baron, P.; Andreone, P.A.; Runge, W.J.; Edwards, B.; Jamieson, S.W.; Kaye, M.P. Multiple ex vivo organ preservation with warm whole blood. J. Heart Transplant. 1988, 7, 227–237. [Google Scholar]
- Chien, S.; Diana, J.N.; Oeltgen, P.R.; Todd, E.P.; O’Connor, W.N.; Chitwood, W.R. Eighteen to 37 hours’ preservation of major organs using a new autoperfusion multiorgan preparation. Ann. Thorac. Surg. 1989, 47, 860–867. [Google Scholar] [CrossRef]
- Chen, C.; Chen, M.; Lin, X.; Guo, Y.; Ma, Y.; Chen, Z.; Ju, W.; He, X. En bloc procurement of porcine abdominal multiple organ block for ex situ normothermic machine perfusion: A technique for avoiding initial cold preservation. Ann. Transl. Med. 2021, 9, 1116. [Google Scholar] [CrossRef]
- Stevens, L.J.; van de Steeg, E.; Doppenberg, J.B.; Alwayn, I.P.J.; Knibbe, C.A.J.; Dubbeld, J. Ex vivo gut-hepato-biliary organ perfusion model to characterize oral absorption, gut-wall metabolism, pre-systemic hepatic metabolism and biliary excretion; application to midazolam. Eur. J. Pharm. Sci. 2024, 196, 106760. [Google Scholar] [CrossRef]
- Horné, F.; Drefs, M.; Schirren, M.J.; Koch, D.T.; Cepele, G.; Jacobi, S.J.; Payani, E.; Börner, N.; Werner, J.; Guba, M.O.; et al. Hypothermic Oxygenated Machine Perfusion (HOPE) Prior to Liver Transplantation Mitigates Post-Reperfusion Syndrome and Perioperative Electrolyte Shifts. J. Clin. Med. 2022, 11, 7381. [Google Scholar] [CrossRef]
- Hansen, T.; Hollemann, D.; Pitton, M.B.; Heise, M.; Hoppe-Lotichius, M.; Schuchmann, M.; Kirkpatrick, C.J.; Otto, G. Histological examination and evaluation of donor bile ducts received during orthotopic liver transplantation—A morphological clue to ischemic-type biliary lesion? Virchows Arch. 2012, 461, 41–48. [Google Scholar] [CrossRef]
- op den Dries, S.; Westerkamp, A.C.; Karimian, N.; Gouw, A.S.H.; Bruinsma, B.G.; Markmann, J.F.; Lisman, T.; Yeh, H.; Uygun, K.; Martins, P.N.; et al. Injury to peribiliary glands and vascular plexus before liver transplantation predicts formation of non-anastomotic biliary strictures. J. Hepatol. 2014, 60, 1172–1179. [Google Scholar] [CrossRef] [PubMed]
- Parmentier, C.; Ray, S.; Mazilescu, L.I.; Kawamura, M.; Noguchi, Y.; Nogueira, E.; Ganesh, S.; Arulratnam, B.; Kalimuthu, S.N.; Selzner, M.; et al. Normothermic Ex Vivo Machine Perfusion of Discarded Human Pancreas Allografts: A Feasibility Study. Transpl. Int. 2023, 36, 10936. [Google Scholar] [CrossRef] [PubMed]
- Khalid, U.; Pino-Chavez, G.; Nesargikar, P.; Jenkins, R.H.; Bowen, T.; Fraser, D.J.; Chavez, R. Kidney ischaemia reperfusion injury in the rat: The EGTI scoring system as a valid and reliable tool for histological assessment. J. Histol. Histopathol. 2016, 3, 1. [Google Scholar] [CrossRef]
- Karakaş, B.R.; Sırcan-Küçüksayan, A.; G Elpek, Ö.; Canpolat, M. Investigating viability of intestine using spectroscopy: A pilot study. J. Surg. Res. 2014, 191, 91–98. [Google Scholar] [CrossRef]
- Mazilescu, L.I.; Parmentier, C.; Kalimuthu, S.N.; Ganesh, S.; Kawamura, M.; Goto, T.; Noguchi, Y.; Selzner, M.; Reichman, T.W. Normothermic ex situ pancreas perfusion for the preservation of porcine pancreas grafts. Am. J. Transplant. 2022, 22, 1339–1349. [Google Scholar] [CrossRef]
- Ogbemudia, A.E.; Hakim, G.; Dengu, F.; El-Gilani, F.; Dumbill, R.; Mulvey, J.; Sayal, K.; Prudhomme, T.; Mesnard, B.; Rozenberg, K.; et al. Development of ex situ normothermic reperfusion as an innovative method to assess pancreases after preservation. Transpl. Int. 2021, 34, 1630–1642. [Google Scholar] [CrossRef]
- Kumar, R.; Chung, W.Y.; Runau, F.; Isherwood, J.D.; Kuan, K.G.; West, K.; Garcea, G.; Dennison, A.R. Ex vivo normothermic porcine pancreas: A physiological model for preservation and transplant study. Int. J. Surg. 2018, 54, 206–215. [Google Scholar] [CrossRef]
- Sanders, K.M. Spontaneous Electrical Activity and Rhythmicity in Gastrointestinal Smooth Muscles. In Smooth Muscle Spontaneous Activity; Springer: Berlin/Heidelberg, Germany, 2019; pp. 3–46. [Google Scholar]
- Hou, W.; Yang, S.; Lu, J.; Shi, Y.; Chen, J.; Chen, D.; Wang, F.; Liu, L. Hypothermic machine perfusion alleviates ischemia-reperfusion injury of intestinal transplantation in pigs. Front. Immunol. 2023, 14, 1117292. [Google Scholar] [CrossRef]
- Lenaerts, K.; Ceulemans, L.J.; Hundscheid, I.H.R.; Grootjans, J.; Dejong, C.H.C.; Olde Damink, S.W.M. New insights in intestinal ischemia–reperfusion injury. Curr. Opin. Organ Transplant. 2013, 18, 298–303. [Google Scholar] [CrossRef]
- Meyerholz, D.K.; Burrough, E.R.; Kirchhof, N.; Anderson, D.J.; Helke, K.L. Swine models in translational research and medicine. Vet. Pathol. 2024, 61, 512–523. [Google Scholar] [CrossRef]
- Raza, S.S.; Hara, H.; Eyestone, W.; Ayares, D.; Cleveland, D.C.; Cooper, D.K.C. Pigs in Transplantation Research and Their Potential as Sources of Organs in Clinical Xenotransplantation. Comp. Med. 2024, 74, 33–48. [Google Scholar] [CrossRef] [PubMed]
- Jochmans, I.; Brat, A.; Davies, L.; Hofker, H.S.; van de Leemkolk, F.E.M.; Leuvenink, H.G.D.; Knight, S.R.; Pirenne, J.; Ploeg, R.J.; Abramowicz, D.; et al. Oxygenated versus standard cold perfusion preservation in kidney transplantation (COMPARE): A randomised, double-blind, paired, phase 3 trial. Lancet 2020, 396, 1653–1662. [Google Scholar] [CrossRef] [PubMed]
- Hessheimer, A.; Coll, E.; Valdivieso, A.; Gómez, M.; Santoyo, J.; Ramírez, P.; Gómez-Bravo, M.Á.; López-Andujar, R.; Villar, J.; Jiménez, C.; et al. Superior Outcomes Using Normothermic Regional Perfusion in CDCD Liver Transplantation. Transplantation 2018, 102 (Suppl. 7), S380. [Google Scholar] [CrossRef]


| Demographics | Experimental Groups | p-Value | ||||
|---|---|---|---|---|---|---|
| Gen 1 | Gen 2 | Gen 3 | G1 vs. G2 | G1 vs. G3 | G2 vs. G3 | |
| WIT (min) | 9.4 ± 3.1 | 8.3 ± 1.2 | 3.3 ± 1.7 | 0.8086 | 0.0085 | 0.0447 |
| Cold flush (min) | 11.8 ± 0.8 | 18.7 ± 13.9 | 17.3 ± 2.5 | 0.3812 | 0.4774 | 0.9590 |
| CIT (min) | 368.6 ± 55.8 | 265.3 ± 61.9 | 184.8 ± 32.3 | 0.0506 | 0.0011 | 0.1497 |
| Duration of HMP (min) | 110.8 ± 21.78 | - | - | - | - | - |
| Duration of NMP (min) | 438.0 ± 47.6 | 440.0 ± 69.3 | 480.0 ± 176.6 | 0.9997 | 0.8438 | 0.8873 |
| Mean MAP during NMP (mmHg) | 110.3 ± 22.31 | 73.15 ± 13.32 | 63.60 ± 7.565 | <0.0001 | <0.0001 | 0.0956 |
| NMP Parameters | Experimental Groups | p-Value | ||||
|---|---|---|---|---|---|---|
| Gen 1 | Gen 2 | Gen 3 | G1 vs. G2 | G1 vs. G3 | G2 vs. G3 | |
| Aortic flow (L/min) | 1.018 ± 0.506 | 0.617 ± 0.201 | 2.089 ± 0.633 | 0.0498 | <0.0001 | <0.0001 |
| Aortic resistance | 0.224 ± 0.127 | 0.195 ± 0.148 | 0.038 ± 0.027 | 0.6916 | <0.0001 | <0.0001 |
| Total urine output (mL) | 51.90 ± 99.97 | 2 ± 3.46 | 271.3 ± 324.8 | 0.9375 | 0.278 | 0.233 |
| Oxygen consumption (mL O2/min) | 43.56 ± 16.57 | 19.42 ± 5.21 | 49.52 ± 15.79 | 0.0082 | 0.6583 | 0.0009 |
| Lactate clearance (mmol/L) | 10.44 ± 3.72 | 3.26 ± 2.37 | 3.10 ± 2.21 | <0.0001 | <0.0001 | 0.993 |
| Perfusate pH | 7.27 ± 0.34 | 7.29 ± 0.14 | 7.30 ± 0.13 | 0.9843 | 0.961 | 0.9961 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
van Leeuwen, L.L.; Holzner, M.L.; McKenney, C.; Todd, R.; Frost, J.K.; Gudibendi, S.; Kim-Schluger, L.; Schiano, T.; Florman, S.; Akhtar, M.Z. An Iterative Design Approach to Development of an Ex Situ Normothermic Multivisceral Perfusion Platform. J. Clin. Med. 2025, 14, 4620. https://doi.org/10.3390/jcm14134620
van Leeuwen LL, Holzner ML, McKenney C, Todd R, Frost JK, Gudibendi S, Kim-Schluger L, Schiano T, Florman S, Akhtar MZ. An Iterative Design Approach to Development of an Ex Situ Normothermic Multivisceral Perfusion Platform. Journal of Clinical Medicine. 2025; 14(13):4620. https://doi.org/10.3390/jcm14134620
Chicago/Turabian Stylevan Leeuwen, L. Leonie, Matthew L. Holzner, Ceilidh McKenney, Rachel Todd, Jamie K. Frost, Sneha Gudibendi, Leona Kim-Schluger, Thomas Schiano, Sander Florman, and M. Zeeshan Akhtar. 2025. "An Iterative Design Approach to Development of an Ex Situ Normothermic Multivisceral Perfusion Platform" Journal of Clinical Medicine 14, no. 13: 4620. https://doi.org/10.3390/jcm14134620
APA Stylevan Leeuwen, L. L., Holzner, M. L., McKenney, C., Todd, R., Frost, J. K., Gudibendi, S., Kim-Schluger, L., Schiano, T., Florman, S., & Akhtar, M. Z. (2025). An Iterative Design Approach to Development of an Ex Situ Normothermic Multivisceral Perfusion Platform. Journal of Clinical Medicine, 14(13), 4620. https://doi.org/10.3390/jcm14134620






