Keratoconus: The Local Manifestation of a Systemic Disease?
Abstract
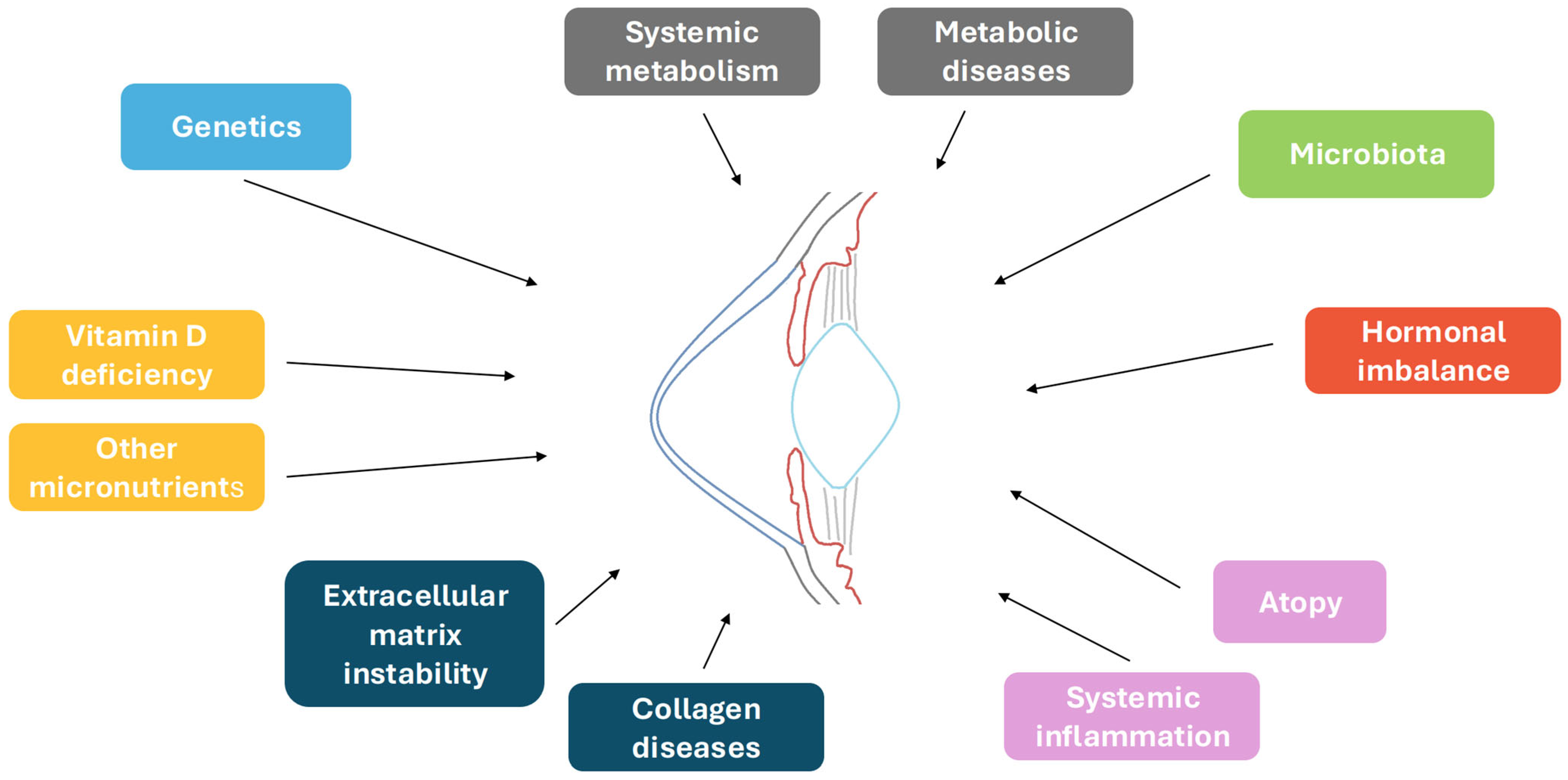
1. Introduction
2. Genetics
3. Systemic Inflammatory Pathways and Innate Immunity
4. Atopy and Eye Rubbing
5. Vitamin D: A Modulator of Systemic Inflammation
6. Other Micronutrients with a Possible Role in KC
7. The Ocular Surface Microbiota
8. Does Systemic Metabolism Impact KC?
9. Association with Metabolic Disorders
10. Hormonal Balance and KC Risk
11. Association with Collagen Diseases
12. Possible Association with Psychiatric Conditions
13. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Alió, J.L. (Ed.) Keratoconus; Springer International Publishing: Cham, Switzerland, 2017. [Google Scholar] [CrossRef]
- Gomes, J.A.P.; Tan, D.; Rapuano, C.J.; Belin, M.W.; Ambrósio, R.; Guell, J.L.; Malecaze, F.; Nishida, K.; Sangwan, V.; the Group of Panelists for the Global Delphi Panel of Keratoconus and Ectatic Diseases. Global consensus on keratoconus and ectatic diseases. Cornea 2015, 34, 359–369. [Google Scholar] [CrossRef] [PubMed]
- Santodomingo-Rubido, J.; Carracedo, G.; Suzaki, A.; Villa-Collar, C.; Vincent, S.J.; Wolffsohn, J.S. Keratoconus: An updated review. Cont. Lens Anterior Eye 2022, 45, 101559. [Google Scholar] [CrossRef]
- Smolin, G.; Thoft, R.; Foster, C.; Azar, D.; Dohlman, C. Smolin and Thoft’s the Cornea: Scientific Foundations and Clinical Practice; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2005. [Google Scholar]
- Krachmer, J.H.; Feder, R.S.; Belin, M.W. Keratoconus and related noninflammatory corneal thinning disorders. Surv. Ophthalmol. 1984, 28, 293–322. [Google Scholar] [CrossRef] [PubMed]
- Mannis, M.J.; Holland, E.J. Cornea: Fundamentals, diagnosis, and management. Am. J. Ophthalmol. 2022, 140, 779. [Google Scholar]
- Loh, I.P.; Sherwin, T. Is Keratoconus an Inflammatory Disease? The Implication of Inflammatory Pathways. Ocul. Immunol. Inflamm. 2022, 30, 246–255. [Google Scholar] [CrossRef]
- Bykhovskaya, Y.; Rabinowitz, Y.S. Update on the genetics of keratoconus. Exp. Eye Res. 2021, 202, 108398. [Google Scholar] [CrossRef]
- Rabinowitz, Y.S.; Galvis, V.; Tello, A.; Rueda, D.; García, J.D. Genetics vs chronic corneal mechanical trauma in the etiology of keratoconus. Exp. Eye Res. 2021, 202, 108328. [Google Scholar] [CrossRef]
- Ferrari, G.; Rama, P. The keratoconus enigma: A review with emphasis on pathogenesis. Ocul. Surf. 2020, 18, 363–373. [Google Scholar] [CrossRef]
- Kennedy, R.H.; Bourne, W.M.; Dyer, J.A. A 48-year clinical and epidemiologic study of keratoconus. Am. J. Ophthalmol. 1986, 101, 267–273. [Google Scholar] [CrossRef]
- Maher, E.R.; Yates, J.R.W.; Ferguson-Smith, M.A. Statistical analysis of the two stage mutation model in von Hippel-Lindau disease, and in sporadic cerebellar haemangioblastoma and renal cell carcinoma. J. Med. Genet. 1990, 27, 311–314. [Google Scholar] [CrossRef]
- Gordon-Shaag, A.; Millodot, M.; Essa, M.; Garth, J.; Ghara, M.; Shneor, E. Is consanguinity a risk factor for keratoconus? Optom. Vis. Sci. 2013, 90, 448–454. [Google Scholar] [CrossRef] [PubMed]
- Tuft, S.J.; Hassan, H.; George, S.; Frazer, D.G.; Willoughby, C.E.; Liskova, P. Keratoconus in 18 pairs of twins. Acta Ophthalmol. 2012, 90, e482–e486. [Google Scholar] [CrossRef] [PubMed]
- Valgaeren, H.; Koppen, C.; Van Camp, G. A new perspective on the genetics of keratoconus: Why have we not been more successful? Ophthalmic Genet. 2018, 39, 158–174. [Google Scholar] [CrossRef]
- Brancati, F.; Valente, E.M.; Sarkozy, A.; Fehèr, J.; Castori, M.; Del Duca, P.; Mingarelli, R.; Pizzuti, A.; Dallapiccola, B. A locus for autosomal dominant keratoconus maps to human chromosome 3p14-q13. J. Med. Genet. 2004, 41, 188–192. [Google Scholar] [CrossRef]
- Burdon, K.P.; Coster, D.J.; Charlesworth, J.C.; Mills, R.A.; Laurie, K.J.; Giunta, C.; Hewitt, A.W.; Latimer, P.; Craig, J.E. Apparent autosomal dominant keratoconus in a large Australian pedigree accounted for by digenic inheritance of two novel loci. Hum. Genet. 2008, 124, 379–386. [Google Scholar] [CrossRef]
- Jacobs, D.S.; Dohlman, C.H. Is keratoconus genetic? Int. Ophthalmol. Clin. 1993, 33, 249–260. [Google Scholar] [CrossRef]
- De Bonis, P.; Laborante, A.; Pizzicoli, C.; Stallone, R.; Barbano, R.; Longo, C.; Mazzilli, E.; Zelante, L.; Bisceglia, L. Mutational screening of VSX1, SPARC, SOD1, LOX, and TIMP3 in keratoconus. Mol. Vis. 2011, 17, 2482–2494. [Google Scholar]
- Saee-Rad, S.; Hashemi, H.; Miraftab, M.; Noori-Daloii, M.R.; Chaleshtori, M.H.; Raoofian, R.; Jafari, F.; Greene, W.; Fakhraie, G.; Rezvan, F.; et al. Mutation analysis of VSX1 and SOD1 in Iranian patients with keratoconus. Mol. Vis. 2011, 17, 3128–3136. [Google Scholar]
- Vincent, A.L.; Jordan, C.A.; Cadzow, M.J.; Merriman, T.R.; McGhee, C.N. Mutations in the zinc finger protein gene, ZNF469, contribute to the pathogenesis of keratoconus. Investig. Ophthalmol. Vis. Sci. 2014, 55, 5629–5635. [Google Scholar] [CrossRef]
- Lechner, J.; Porter, L.F.; Rice, A.; Vitart, V.; Armstrong, D.J.; Schorderet, D.F.; Munier, F.L.; Wright, A.F.; Inglehearn, C.F.; Black, G.C.; et al. Enrichment of pathogenic alleles in the brittle cornea gene, ZNF469, in keratoconus. Hum. Mol. Genet. 2014, 23, 5527–5535. [Google Scholar] [CrossRef]
- Karolak, J.A.; Gambin, T.; Rydzanicz, M.; Szaflik, J.P.; Polakowski, P.; Frajdenberg, A.; Mrugacz, M.; Podfigurna-Musielak, M.; Stankiewicz, P.; Gajecka, M. Evidence against ZNF469 being causative for keratoconus in Polish patients. Acta Ophthalmol. 2016, 94, 289–294. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.G.; Picornell, Y.; Su, X.; Li, X.; Yang, H.; Rabinowitz, Y.S. Three VSX1 gene mutations, L159M, R166W, and H244R, are not associated with keratoconus. Cornea 2008, 27, 189–192. [Google Scholar] [CrossRef] [PubMed]
- Barbaro, V.; Di Iorio, E.; Ferrari, S.; Bisceglia, L.; Ruzza, A.; De Luca, M.; Pellegrini, G. Expression of VSX1 in human corneal keratocytes during differentiation into myofibroblasts in response to wound healing. Investig. Ophthalmol. Vis. Sci. 2006, 47, 5243–5250. [Google Scholar] [CrossRef]
- Aldave, A.J.; Yellore, V.S.; Salem, A.K.; Yoo, G.L.; Rayner, S.A.; Yang, H.; Tang, G.Y.; Piconell, Y.; Rabinowitz, Y.S. No VSX1 gene mutations associated with keratoconus. Investig. Ophthalmol. Vis. Sci. 2006, 47, 2820–2822. [Google Scholar] [CrossRef]
- Štabuc-Šilih, M.; Stražišar, M.; Hawlina, M.; Glavač, D. Absence of pathogenic mutations in VSX1 and SOD1 genes in patients with keratoconus. Cornea 2010, 29, 172–176. [Google Scholar] [CrossRef]
- Liskova, P.; Ebenezer, N.D.; Hysi, P.G.; Gwilliam, R.; El-Ashry, M.F.; Moodaley, L.C.; . Hau, S.; Twa, M.; Tuft, S.J.; Bhatacharya, S.S. Molecular analysis of the VSX1 gene in familial keratoconus. Mol. Vis. 2007, 13, 1887–1891. [Google Scholar]
- Stabuc-Silih, M.; Ravnik-Glavac, M.; Glavac, D.; Hawlina, M.; Strazisar, M. Polymorphisms in COL4A3 and COL4A4 genes associated with keratoconus. Mol. Vis. 2009, 15, 2848–2860. [Google Scholar]
- Kokolakis, N.S.; Gazouli, M.; Chatziralli, I.P.; Koutsandrea, C.; Gatzioufas, Z.; Peponis, V.G.; Droutsas, K.D.; Kalogeropoulos, C.; Anagnou, N.; Miltsakakis, D.; et al. Polymorphism analysis of COL4A3 and COL4A4 genes in Greek patients with keratoconus. Ophthalmic Genet. 2014, 35, 226–228. [Google Scholar] [CrossRef]
- Lu, Y.; Vitart, V.; Burdon, K.P.; Khor, C.C.; Bykhovskaya, Y.; Mirshahi, A.; Hewitt, A.W.; Koehn, D.; Hysi, P.G.; Ramdas, W.D.; et al. Genome-wide association analyses identify multiple loci associated with central corneal thickness and keratoconus. Nat. Genet. 2013, 45, 155–163. [Google Scholar] [CrossRef]
- Hughes, A.E.; Bradley, D.T.; Campbell, M.; Lechner, J.; Dash, D.P.; Simpson, D.A.; Willoughby, C.E. Mutation altering the miR-184 seed region causes familial keratoconus with cataract. Am. J. Hum. Genet. 2011, 89, 628–633. [Google Scholar] [CrossRef]
- Iliff, B.W.; Amer Riazuddin, S.; Gottsch, J.D. A single-base substitution in the seed region of miR-184 causes EDICT syndrome. Investig. Ophthalmol. Vis. Sci. 2012, 53, 348–353. [Google Scholar] [CrossRef] [PubMed]
- Yanovitch, T.; Li, Y.J.; Metlapally, R.; Abbott, D.; Viet, K.N.T.; Young, T.L. Hepatocyte growth factor and myopia: Genetic association analyses in a Caucasian population. Mol. Vis. 2009, 15, 1028–1035. [Google Scholar] [PubMed]
- Li, X.; Bykhovskaya, Y.; Haritunians, T.; Siscovick, D.; Aldave, A.; Szczotka-flynn, L.; Iyengar, S.K.; Rotter, J.I.; Taylor, K.D.; Rabinowitz, Y.S. A genome-wide association study identifies a potential novel gene locus for keratoconus, one of the commonest causes for corneal transplantation in developed countries. Hum. Mol. Genet. 2012, 21, 421–429. [Google Scholar] [CrossRef]
- Wang, Y.; Wei, W.; Zhang, C.; Zhang, X.H.; Liu, M.; Zhu, X.; Xu, K. Association of Interleukin-1 Gene Single Nucleotide Polymorphisms with Keratoconus in Chinese Han Population. Curr. Eye Res. 2016, 41, 630–635. [Google Scholar] [CrossRef]
- Mikami, T.; Meguro, A.; Teshigawara, T.; Takeuchi, M.; Uemoto, R.; Kawagoe, T.; Nomura, E.; Asukata, Y.; Ishioka, M.; Iwasaki, M.; et al. Interleukin 1 beta promoter polymorphism is associated with keratoconus in a Japanese population. Mol. Vis. 2013, 19, 845–851. [Google Scholar]
- Kim, S.H.; Mok, J.W.; Kim, H.S.; Joo, C.K. Association of -31T>C and -511 C>T polymorphisms in the interleukin 1 beta (IL1B) promoter in Korean keratoconus patients. Mol. Vis. 2008, 14, 2109–2116. [Google Scholar]
- Palamar, M.; Onay, H.; Ozdemir, T.R.; Arslan, E.; Egrilmez, S.; Ozkinay, F.; Yagci, A. Relationship between IL1β-511C>T and ILRN VNTR polymorphisms and keratoconus. Cornea 2014, 33, 145–147. [Google Scholar] [CrossRef]
- Kannabiran, C. Genetics of corneal endothelial dystrophies. J. Genet. 2009, 88, 487–494. [Google Scholar] [CrossRef]
- Guan, T.; Liu, C.; Ma, Z.; Ding, S. The point mutation and polymorphism in keratoconus candidate gene TGFBI in Chinese population. Gene 2012, 503, 137–139. [Google Scholar] [CrossRef]
- Karolak, J.A.; Polakowski, P.; Szaflik, J.; Szaflik, J.P.; Gajecka, M. Molecular screening of keratoconus susceptibility sequence variants in VSX1, TGFBI, DOCK9, STK24, and IPO5 genes in Polish patients and novel TGFBI variant identification. Ophthalmic Genet. 2016, 37, 37–43. [Google Scholar] [CrossRef]
- Udar, N.; Kenney, M.C.; Chalukya, M.; Anderson, T.; Morales, L.; Brown, D.; Nesburn, A.; Small, K. Keratoconus—No association with the transforming growth factor beta-induced gene in a cohort of American patients. Cornea 2004, 23, 13–17. [Google Scholar] [CrossRef] [PubMed]
- Gajecka, M.; Radhakrishna, U.; Winters, D.; Nath, S.K.; Rydzanicz, M.; Ratnamala, U.; Ewing, K.; Molinari, A.; Pitarque, J.A.; Kwanghyuk, L.; et al. Localization of a gene for keratoconus to a 5.6-Mb interval on 13q32. Investig. Ophthalmol. Vis. Sci. 2009, 50, 1531–1539. [Google Scholar] [CrossRef] [PubMed]
- Adzhubei, I.A.; Schmidt, S.; Peshkin, L.; Ramensky, V.E.; Gerasimova, A.; Bork, P.; Kondrashov, A.S.; Sunyaev, S.R. A method and server for predicting damaging missense mutations. Nat. Methods 2010, 7, 248–249. [Google Scholar] [CrossRef] [PubMed]
- Hasanian-Langroudi, F.; Saravani, R.; Validad, M.H.; Bahari, G.; Yari, D. Association of Lysyl oxidase (LOX) polymorphisms with the risk of keratoconus in an Iranian population. Ophthalmic Genet. 2015, 36, 309–314. [Google Scholar] [CrossRef]
- Hao, X.D.; Chen, P.; Chen, Z.L.; Li, S.X.; Wang, Y. Evaluating the association between keratoconus and reported genetic loci in a Han Chinese population. Ophthalmic Genet. 2015, 36, 132–136. [Google Scholar] [CrossRef]
- Dudakova, L.; Palos, M.; Jirsova, K.; Stranecky, V.; Krepelova, A.; Hysi, P.G.; Liskova, P. Validation of rs2956540:G>C and rs3735520:G>A association with keratoconus in a population of European descent. Eur. J. Hum. Genet. 2015, 23, 1581–1583. [Google Scholar] [CrossRef][Green Version]
- Bykhovskaya, Y.; Li, X.; Taylor, K.D.; Haritunians, T.; Rotter, J.I.; Rabinowitz, Y.S. Linkage analysis of high-density SNPs confirms keratoconus locus at 5q chromosomal region. Ophthalmic Genet. 2016, 37, 109–110. [Google Scholar] [CrossRef][Green Version]
- Li, X.; Bykhovskaya, Y.; Tang, Y.G.; Picornell, Y.; Haritunians, T.; Aldave, A.J.; Szczotka-Flynn, L.; Iyengar, S.K.; Rotter, J.I.; Taylor, K.D.; et al. An association between the calpastatin (CAST) gene and keratoconus. Cornea 2013, 32, 696–701. [Google Scholar] [CrossRef]
- Burdon, K.P.; Macgregor, S.; Bykhovskaya, Y.; Javadiyan, S.; Li, X.; Laurie, K.J.; Muszynska, D.; Lindsay, R.; Lechner, J.; Haritunians, T.; et al. Association of polymorphisms in the hepatocyte growth factor gene promoter with keratoconus. Investig. Ophthalmol. Vis. Sci. 2011, 52, 8514–8519. [Google Scholar] [CrossRef]
- Shetty, R.; Sathyanarayanamoorthy, A.; Ramachandra, R.A.; Arora, V.; Ghosh, A.; Srivatsa, P.R.; Pahuja, N.; Nuijts, R.M.M.A.; Sinha-Roy, A.; Mohan, R.R.; et al. Attenuation of lysyl oxidase and collagen gene expression in keratoconus patient corneal epithelium corresponds to disease severity. Mol. Vis. 2015, 21, 12–25. [Google Scholar]
- Kristianslund, O.; Drolsum, L. Prevalence of keratoconus in persons with Down syndrome: A review. BMJ Open Ophthalmol. 2021, 6, e000754. [Google Scholar] [CrossRef] [PubMed]
- Alio, J.L.; Vega-Estrada, A.; Sanz, P.; Osman, A.A.; Kamal, A.M.; Mamoon, A.; Soliman, H. Corneal morphologic characteristics in patients with Down syndrome. JAMA Ophthalmol. 2018, 136, 971–978. [Google Scholar] [CrossRef] [PubMed]
- Mathan, J.J.; Gokul, A.; Simkin, S.K.; Meyer, J.J.; McGhee, C.N.J. Keratoconus in Down syndrome: Prevalence, risk factors, severity and corneal tomographic characteristics. Clin. Exp. Ophthalmol. 2024, 52, 22–30. [Google Scholar] [CrossRef]
- Woodward, M.A.; Blachley, T.S.; Stein, J.D. The association between sociodemographic factors, common systemic diseases, and keratoconus: An analysis of a nationwide heath care claims database. Ophthalmology. 2016, 123, 457–465.e2. [Google Scholar] [CrossRef]
- Mathan, J.J.; Gokul, A.; Simkin, S.K.; Meyer, J.J.; Patel, D.V.; McGhee, C.N.J. Topographic screening reveals keratoconus to be extremely common in Down syndrome. Clin. Exp. Ophthalmol. 2020, 48, 1160–1167. [Google Scholar] [CrossRef]
- Hashemi, H.; Miraftab, M.; Amanzadeh, K.; Seyedian, M.A.; Vinciguerra, R.; Ambrósio, R.; Roberts, C.; Makateb, A.; Vinciguerra, P.; Asgari, S. Keratoconus detection by novel indices in patients with Down syndrome: A cohort population-based study. Jpn. J. Ophthalmol. 2020, 64, 285–291. [Google Scholar] [CrossRef]
- Vincent, A.L.; Weiser, B.A.; Cupryn, M.; Stein, R.M.; Abdolell, M.; Levin, A.V. Computerized corneal topography in a paediatric population with Down syndrome. Clin. Exp. Ophthalmol. 2005, 33, 47–52. [Google Scholar] [CrossRef]
- Gittenberger-de Groot, A.C.; Bartram, U.; Oosthoek, P.W.; Bartelings, M.M.; Hogers, B.; Poelmann, R.E.; Jongewaard, I.N.; Klewer, S.E. Collagen type VI expression during cardiac development and in human fetuses with trisomy 21. Anat. Rec. A Discov. Mol. Cell Evol. Biol. 2003, 275, 1109–1116. [Google Scholar] [CrossRef]
- Davies, G.E.; Howard, C.M.; Farrer, M.J.; Coleman, M.M.; Bennett, L.B.; Cullen, L.M.; Wyse, R.K.; Burn, J.; Williamson, R.; Kessling, A.M. Genetic variation in the COL6A1 region is associated with congenital heart defects in trisomy 21 (Down’s syndrome). Ann. Hum. Genet. 1995, 59, 253–269. [Google Scholar] [CrossRef]
- von Kaisenberg, C.S.; Brand-Saberi, B.; Christ, B.; Vallian, S.; Farzaneh, F.; Nicolaides, K.H. Collagen type VI gene expression in the skin of trisomy 21 fetuses. Obstet. Gynecol. 1998, 91, 319–323. [Google Scholar] [CrossRef]
- Quarello, E.; Guimiot, F.; Moalic, J.M.; Simoneau, M.; Ville, Y.; Delezoide, A.L. Quantitative evaluation of collagen type VI and SOD gene expression in the nuchal skin of human fetuses with trisomy 21. Prenat. Diagn. 2007, 27, 926–931. [Google Scholar] [CrossRef] [PubMed]
- Gulesserian, T.; Seidl, R.; Hardmeier, R.; Cairns, N.; Lubec, G. Superoxide dismutase SOD1, encoded on chromosome 21, but not SOD2 is overexpressed in brains of patients with Down syndrome. J. Investig. Med. 2001, 49, 41–46. [Google Scholar] [CrossRef] [PubMed]
- Nemet, A.Y.; Vinker, S.; Bahar, I.; Kaiserman, I. The association of keratoconus with immune disorders. Cornea 2010, 29, 1261–1264. [Google Scholar] [CrossRef]
- Chen, X.; Chen, L. Causal Links Between Systemic Disorders and Keratoconus in European Population. Am. J. Ophthalmol. 2024, 265, 189–199. [Google Scholar] [CrossRef]
- Lee, H.K.; Jung, E.H.; Cho, B.J. Epidemiological Association Between Systemic Diseases and Keratoconus in a Korean Population: A 10-Year Nationwide Cohort Study. Cornea 2020, 39, 348–353. [Google Scholar] [CrossRef]
- Cejka, C.; Cejkova, J. Oxidative stress to the cornea, changes in corneal optical properties, and advances in treatment of corneal oxidative injuries. Oxid. Med. Cell Longev. 2015, 2015, 591530. [Google Scholar] [CrossRef]
- Buddi, R.; Lin, B.; Atilano, S.R.; Zorapapel, N.C.; Kenney, M.C.; Brown, D.J. Evidence of oxidative stress in human corneal diseases. J. Histochem. Cytochem. 2002, 50, 341–351. [Google Scholar] [CrossRef]
- Chwa, M.; Atilano, S.R.; Reddy, V.; Jordan, N.; Kim, D.W.; Kenney, M.C. Increased stress-induced generation of reactive oxygen species and apoptosis in human keratoconus fibroblasts. Investig. Ophthalmol. Vis. Sci. 2006, 47, 1902–1910. [Google Scholar] [CrossRef]
- Arnal, E.; Peris-Martínez, C.; Menezo, J.L.; Johnsen-Soriano, S.; Romero, F.J. Oxidative stress in keratoconus? Investig. Ophthalmol. Vis. Sci. 2011, 52, 8592–8597. [Google Scholar] [CrossRef]
- Atilano, S.; Lee, D.; Fukuhara, P.; Chwa, M.; Nesburn, A.; Udar, N.; Kenney, M.C. Corneal Oxidative Damage in Keratoconus Cells due to Decreased Oxidant Elimination from Modified Expression Levels of SOD Enzymes, PRDX6, SCARA3, CPSF3, and FOXM1. J. Ophthalmic Vis. Res. 2019, 14, 62–70. [Google Scholar]
- Saijyothi, A.V.; Fowjana, J.; Madhumathi, S.; Rajeshwari, M.; Thennarasu, M.; Prema, P.; Angayarkanni, N. Tear fluid small molecular antioxidants profiling shows lowered glutathione in keratoconus. Exp. Eye Res. 2012, 103, 41–46. [Google Scholar] [CrossRef] [PubMed]
- Karamichos, D.; Zieske, J.D.; Sejersen, H.; Sarker-Nag, A.; Asara, J.M.; Hjortdal, J. Tear metabolite changes in keratoconus. Exp. Eye Res. 2015, 132, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Gondhowiardjo, T.D.; Van Haeringen, N.J.; Volker-Dieben, H.J.; Beekhuis, H.W.; Kok, J.H.C.; Van Rij, G.; Pels, L.; Kijlstra, A. Analysis of corneal aldehyde dehydrogenase patterns in pathologic corneas. Cornea 1993, 12, 146–154. [Google Scholar] [CrossRef] [PubMed]
- Navel, V.; Malecaze, J.; Pereira, B.; Baker, J.S.; Malecaze, F.; Sapin, V.; Chiambaretta, F.; Dutheil, F. Oxidative and antioxidative stress markers in keratoconus: A systematic review and meta-analysis. Acta Ophthalmol. 2021, 99, e777–e794. [Google Scholar] [CrossRef]
- Behndig, A.; Karlsson, K.; Johansson, B.O.; Brännström, T.; Marklund, S.L. Superoxide dismutase isoenzymes in the normal and diseased human cornea. Investig. Ophthalmol. Vis. Sci. 2001, 42, 2293–2296. [Google Scholar]
- Kenney, M.C.; Brown, D.J. The cascade hypothesis of keratoconus. Cont. Lens Anterior Eye. 2003, 26, 139–146. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, X.; Li, Q.; Wei, C.; Guo, X.; Zhao, L.; Liu, T.; Bao, Q.; Dou, S.; Seitz, B.; et al. CYR61/CCN1: A novel mediator of redox response in corneal stromal cells of keratoconus. Exp. Eye Res. 2024, 248, 110093. [Google Scholar] [CrossRef]
- Cantemir, A.; Alexa, A.; Ciobica, A.; Balmus, I.; Antioch, I.; Stoica, B.; Chiselita, D.; Costin, D. Evaluation of antioxidant enzymes in keratoconus. Revista de Chimie 2016, 67, 1725–1727. [Google Scholar]
- Tekin, S.; Seven, E. Assessment of serum catalase, reduced glutathione, and superoxide dismutase activities and malondialdehyde levels in keratoconus patients. Eye 2022, 36, 2062–2066. [Google Scholar] [CrossRef]
- Toprak, I.; Kucukatay, V.; Yildirim, C.; Kilic-Toprak, E.; Kilic-Erkek, O. Increased systemic oxidative stress in patients with keratoconus. Eye 2014, 28, 285–289. [Google Scholar] [CrossRef]
- Teh, A.L.; Jayapalan, J.J.; Loke, M.F.; Wan Abdul Kadir, A.J.; Subrayan, V. Identification of potential serum metabolic biomarkers for patient with keratoconus using untargeted metabolomics approach. Exp. Eye Res. 2021, 211, 108734. [Google Scholar] [CrossRef]
- Galvis, V.; Sherwin, T.; Tello, A.; Merayo, J.; Barrera, R.; Acera, A. Keratoconus: An inflammatory disorder? Eye 2015, 29, 843–859. [Google Scholar] [CrossRef] [PubMed]
- Lema, I.; Durán, J.A. Inflammatory molecules in the tears of patients with keratoconus. Ophthalmology 2005, 112, 654–659. [Google Scholar] [CrossRef] [PubMed]
- Lema, I.; Sobrino, T.; Durán, J.A.; Brea, D.; Díez-Feijoo, E. Subclinical keratoconus and inflammatory molecules from tears. Br. J. Ophthalmol. 2009, 93, 820–824. [Google Scholar] [CrossRef]
- Pásztor, D.; Kolozsvári, B.L.; Csutak, A.; Berta, A.; Hassan, Z.; Ujhelyi, B.; Gogolák, P.; Fodor, M. Tear mediators in corneal ectatic disorders. PLoS ONE 2016, 11, e0153186. [Google Scholar] [CrossRef]
- Sorkhabi, R.; Ghorbanihaghjo, A.; Taheri, N.; Ahoor, M.H. Tear film inflammatory mediators in patients with keratoconus. Int. Ophthalmol. 2015, 35, 467–472. [Google Scholar] [CrossRef]
- Sobrino, T.; Regueiro, U.; Malfeito, M.; Vieites-Prado, A.; Pérez-Mato, M.; Campos, F.; Lema, I. Higher expression of Toll-like receptors 2 and 4 in blood cells of keratoconus patients. Sci. Rep. 2017, 7, 12975. [Google Scholar] [CrossRef]
- Marques, J.C.; Ladislau de Carvalho, K.I.; Xavier, R.; Nosé, W.; Rizzo, L.V. Inflammatory profile of keratoconic corneal epithelium. BMC Ophthalmol. 2023, 23, 326. [Google Scholar] [CrossRef]
- Karaca, E.E.; Özmen, M.C.; Ekici, F.; Yüksel, E.; Türkoğlu, Z. Neutrophil-to-lymphocyte ratio may predict progression in patients with keratoconus. Cornea 2014, 33, 1168–1173. [Google Scholar] [CrossRef]
- Regueiro, U.; López-López, M.; Hervella, P.; Sobrino, T.; Lema, I. Corneal and conjunctival alteration of innate immune expression in first-degree relatives of keratoconus patients. Graefes Arch. Clin. Exp. Ophthalmol. 2021, 259, 459–467. [Google Scholar] [CrossRef]
- Regueiro, U.; López-López, M.; Varela-Fernández, R.; Sobrino, T.; Diez-Feijoo, E.; Lema, I. Immunomodulatory effect of human lactoferrin on Toll-like receptors 2 expression as therapeutic approach for keratoconus. Int. J. Mol. Sci. 2022, 23, 12350. [Google Scholar] [CrossRef] [PubMed]
- Ben-Shaul, O.; Segal, A.; Schwartz, S.; Stein, N.; Hyams, M.; Saliba, W.; Mimouni, M. Factors associated with keratoconus in Israel—A cross-sectional population-based study. Acta Ophthalmol. 2024, 102, e1011–e1017. [Google Scholar] [CrossRef] [PubMed]
- Alqasimi, N.A.; Aljohani, L.H.; Ambrósio, R.; AlQahtani, B.S.; Al Haydar, N.S.; Alanazi, B.R.; Alfurayhan, D.T.; Saber, L.S.H.; Alsalem, F.S.; Alqahtani, N.A.; et al. Assessment of awareness of keratoconus and its relation to eye rubbing among Saudi Arabia population. Front. Ophthalmol. 2025, 5, 1545030. [Google Scholar] [CrossRef]
- Shah, Z.; Purohit, D.; Danayak, P. Keratoconus characteristics and associations: A cross-sectional keratoconus study in western India (CKSWI). Indian J. Ophthalmol. 2024, 72, 704–711. [Google Scholar] [CrossRef]
- Lo, J.E.; Huang, Y.H.; Bhattacharyya, N.; Moulton, E.A.; Ma, K.S.K. Allergic rhinitis and keratoconus: A systematic review and meta-analysis. J. Allergy Clin. Immunol. Pract. 2024, 12, 3096–3104. [Google Scholar] [CrossRef]
- Chang, Y.; Huang, T.; Yang, S.; Li, Y.; Chen, D. Causal association between atopic dermatitis and keratoconus: A Mendelian randomization study. Transl. Vis. Sci. Technol. 2024, 13, 13. [Google Scholar] [CrossRef]
- Balogun, M.M.; Fashola, M.B. Association between keratoconus and allergic conjunctivitis in children attending a tertiary hospital in Nigeria. Rom. J. Ophthalmol. 2023, 67, 134–139. [Google Scholar] [CrossRef]
- Xu, H.; Wen, Y.; Zheng, H.; Jiang, D.; Chen, W. Allergic disease and keratoconus: A two-sample univariable and multivariable Mendelian randomization study. World Allergy Organ. J. 2024, 17, 100993. [Google Scholar] [CrossRef]
- Wang, Q.; Deng, Y.; Li, S.; Du, X.; Zhao, X.; Zhang, T.; Yuan, J. Corneal biomechanical changes in allergic conjunctivitis. Eye Vis. 2021, 8, 17. [Google Scholar] [CrossRef]
- Naderan, M.; Rajabi, M.T.; Zarrinbakhsh, P.; Bakhshi, A. Effect of allergic diseases on keratoconus severity. Ocul. Immunol. Inflamm. 2017, 25, 418–423. [Google Scholar] [CrossRef]
- Gijs, M.; Adelaar, T.I.; Vergouwen, D.P.C.; Visser, N.; Dickman, M.M.; Ollivier, R.C.I.; Berendschot, T.T.J.M.; Nuijts, R.M.M.A. Tear fluid inflammatory proteome analysis highlights similarities between keratoconus and allergic conjunctivitis. Investig. Ophthalmol. Vis. Sci. 2023, 64, 9. [Google Scholar] [CrossRef] [PubMed]
- Merdler, I.; Hassidim, A.; Sorkin, N.; Shapira, S.; Gronovich, Y.; Korach, Z. Keratoconus and allergic diseases among Israeli adolescents between 2005 and 2013. Cornea 2005, 34, 525–529. [Google Scholar] [CrossRef] [PubMed]
- Wajnsztajn, D.; Solomon, A. Vernal keratoconjunctivitis and keratoconus. Curr. Opin. Allergy Clin. Immunol. 2021, 21, 507–514. [Google Scholar] [CrossRef] [PubMed]
- Marx-Gross, S.; Fieß, A.; Münzel, T.; Wild, P.S.; Beutel, M.E.; Schmidtmann, I.; Lackner, K.J.; Pfeiffer, N.; Schuster, A.K.G. Much higher prevalence of keratoconus than announced results of the Gutenberg Health Study (GHS). Graefes Arch. Clin. Exp. Ophthalmol. 2023, 261, 3241–3247. [Google Scholar] [CrossRef]
- Guo, X.H.; Bian, J.; Yang, K.; Liu, X.; Sun, Y.; Liu, M.; Qi, X.; Ren, S.; Dong, Y.; Gao, H. Eye Rubbing in Chinese Patients With Keratoconus: A Multicenter Analysis. J. Refract. Surg. 2023, 39, 712–718. [Google Scholar] [CrossRef]
- AlSomali, A.; Almithn, D.; Alamer, A.; Al-Omair, A.; Almuhaysin, F.; Almulhim, N. Awareness of Keratoconus and Its Relationship With Eye Rubbing Among the Population of the Eastern Province of Saudi Arabia. Cureus 2024, 16, e51627. [Google Scholar] [CrossRef]
- Duarte-Bueno, M.L.; Tello, A.; Diaz-Martínez, A.L.; Escobar, S.; Galvis, V. Evaluating the Influence of Eye Rubbing and Genetic Predisposition on Keratoconus in Bucaramanga (Colombia): A Case-control Study. Cesk Slov. Oftalmol. 2025, 81, 1–10. [Google Scholar] [CrossRef]
- Mazharian, A.; Flamant, R.; Elahi, S.; Panthier, C.; Rampat, R.; Gatinel, D. Medium to long term follow up study of the efficacy of cessation of eye-rubbing to halt progression of keratoconus. Front. Med. 2023, 10, 1152266. [Google Scholar] [CrossRef]
- Hage, A.; Knoeri, J.; Leveziel, L.; Majoulet, A.; Blanc, J.V.; Buffault, J.; Labbé, A.; Baudouin, C. EYERUBBICS: The Eye Rubbing Cycle Study. J. Clin. Med. 2023, 12, 1529. [Google Scholar] [CrossRef]
- Yin, S.; Xu, L.; Yang, K.; Fan, Q.; Gu, Y.; Yin, C.; Zang, Y.; Wang, Y.; Yuan, Y.; Chang, A.; et al. Gene–Environment Interaction Between CAST Gene and Eye-Rubbing in the Chinese Keratoconus Cohort Study: A Case-Only Study. Investig. Ophthalmol. Vis. Sci. 2024, 65, 36. [Google Scholar] [CrossRef]
- McMonnies, C.W. Mechanisms of rubbing-related corneal trauma in keratoconus. Cornea 2009, 28, 607–615. [Google Scholar] [CrossRef] [PubMed]
- Ferrini, E.; Posarelli, C.; Figus, M.; Lisi, D.; Gabbriellini, G. A pilot study on Langerhans cells in keratoconus patients by in vivo confocal microscopy before and after corneal cross-linking and correlation with eye rubbing. Cont. Lens Anterior Eye 2024, 47, 102170. [Google Scholar] [CrossRef] [PubMed]
- Alamri, A.; Alrizqi, A.A.; Aljohani, A.A.; Alzahrani, D.A.; Alassaf, O.M.; Hamzi, Y.A.; Alharbi, N.M.; Alharbi, B.A.; Taha, M., Sr. Awareness of Keratoconus and Its Association With Eye Rubbing Among the Population in Aseer Province. Cureus 2023, 15, e41271. [Google Scholar] [CrossRef]
- Bikle, D.D. Vitamin D regulation of immune function. Vitam. Horm. 2011, 86, 1–21. [Google Scholar] [CrossRef]
- McMillan, J. Spectrum of Darkness, Agent of Light: Myopia, Keratoconus, Ocular Surface Disease, and Evidence for a Profoundly Vitamin D-dependent Eye. Cureus 2018, 10, e2744. [Google Scholar] [CrossRef]
- Lu, X.; Watsky, M.A. Influence of Vitamin D on Corneal Epithelial Cell Desmosomes and Hemidesmosomes. Investig. Ophthalmol. Vis. Sci. 2019, 60, 4074–4083. [Google Scholar] [CrossRef]
- Yin, Z.; Pintea, V.; Lin, Y.; Hammock, B.D.; Watsky, M.A. Vitamin D enhances corneal epithelial barrier function. Investig. Ophthalmol. Vis. Sci. 2011, 52, 7359–7364. [Google Scholar] [CrossRef]
- Cankaya, C.; Cumurcu, T.; Gunduz, A. Corneal endothelial changes in patients with vitamin D deficiency. Indian J. Ophthalmol. 2018, 66, 1256–1261. [Google Scholar] [CrossRef]
- Reins, R.Y.; Baidouri, H.; McDermott, A.M. Vitamin D Activation and Function in Human Corneal Epithelial Cells During TLR-Induced Inflammation. Investig. Ophthalmol. Vis. Sci. 2015, 56, 7715–7727. [Google Scholar] [CrossRef]
- Wimalawansa, S.J. Vitamin D Deficiency: Effects on Oxidative Stress, Epigenetics, Gene Regulation, and Aging. Biology 2019, 8, 30. [Google Scholar] [CrossRef]
- Liu, W.; Zhang, L.; Xu, H.J.; Li, Y.; Hu, C.M.; Yang, J.Y.; Sun, M.Y. The Anti-Inflammatory Effects of Vitamin D in Tumorigenesis. Int. J. Mol. Sci. 2018, 19, 2736. [Google Scholar] [CrossRef] [PubMed]
- Lai, Y.T.; Cerquinho, R.G.; Perez, M.M.; Alves Bda, C.A.; Pereira, E.C.; Azzalis, L.A.; Campos Junqueira, V.B.; Soares, L.R.; Fonseca, F.L.A. Determination of vitamin D in tears of healthy individuals by the electrochemiluminescence method. J. Clin. Lab. Anal. 2019, 33, e22830. [Google Scholar] [CrossRef] [PubMed]
- Alsalem, J.A.; Patel, D.; Susarla, R.; Coca-Prados, M.; Bland, R.; Walker, E.A.; Rauz, S.; Wallace, G.R. Characterization of vitamin D production by human ocular barrier cells. Investig. Ophthalmol. Vis. Sci. 2014, 55, 2140–2147. [Google Scholar] [CrossRef] [PubMed]
- Knapp, A.A. Results of vitamin-D-complex treatment of keratoconus. Preliminary study. Am. J. Ophthalmol. 1939, 22, 289–292. [Google Scholar] [CrossRef]
- Akkaya, S.; Ulusoy, D.M. Serum Vitamin D Levels in Patients with Keratoconus. Ocul. Immunol. Inflamm. 2020, 28, 348–353. [Google Scholar] [CrossRef]
- Aslan, M.G.; Findik, H.; Okutucu, M.; Aydin, E.; Oruç, Y.; Arpa, M.; Uzun, F. Serum 25-Hydroxy Vitamin D, Vitamin B12, and Folic Acid Levels in Progressive and Nonprogressive Keratoconus. Cornea 2021, 40, 334–341. [Google Scholar] [CrossRef]
- Zarei-Ghanavati, S.; Yahaghi, B.; Hassanzadeh, S.; Mobarhan, M.G.; Hakimi, H.R.; Eghbali, P. Serum 25-Hydroxyvitamin D, Selenium, Zinc and Copper in Patients with Keratoconus. J. Curr. Ophthalmol. 2020, 32, 26–31. [Google Scholar] [CrossRef]
- Mackawy, A.M.H.; Al-Ayed, B.M.; Al-Rashidi, B.M. Vitamin D deficiency and its association with thyroid disease. Int J Health Sci (Qassim). 2013, 7, 267–275. [Google Scholar] [CrossRef]
- Stagi, S.; Lapi, E.; Romano, S.; Bargiacchi, S.; Brambilla, A.; Giglio, S.; Seminara, s.; De Martino, M. Determinants of vitamin D levels in children and adolescents with Down syndrome. Int. J. Endocrinol. 2015, 2015, 896758. [Google Scholar] [CrossRef]
- Eren, E.; Ellidag, H.Y.; Cekin, Y.; Ayoglu, R.U.; Sekercioglu, A.O.; Yilmaz, N. Heart valve disease: The role of calcidiol deficiency, elevated parathyroid hormone levels and oxidative stress in mitral and aortic valve insufficiency. Redox Rep. 2014, 19, 34–39. [Google Scholar] [CrossRef]
- Archontogeorgis, K.; Nena, E.; Papanas, N.; Steiropoulos, P. The role of vitamin D in obstructive sleep apnoea syndrome. Breathe (Sheff) 2018, 14, 206–215. [Google Scholar] [CrossRef] [PubMed]
- Hollams, E.M.; Hart, P.H.; Holt, B.J.; Serralha, M.; Parsons, F.; De Klerk, N.H.; Zhang, G.; Sly, P.D.; Holt, P.G. Vitamin D and atopy and asthma phenotypes in children: A longitudinal cohort study. Eur. Respir. J. 2011, 38, 1320–1327. [Google Scholar] [CrossRef] [PubMed]
- Stock, R.A.; Thumé, T.; Bonamigo, E.L. Acute corneal hydrops during pregnancy with spontaneous resolution after corneal cross-linking for keratoconus: A case report. J. Med. Case Rep. 2017, 11, 53. [Google Scholar] [CrossRef] [PubMed]
- Bilgihan, K.; Hondur, A.; Sul, S.; Ozturk, S. Pregnancy-induced progression of keratoconus. Cornea 2011, 30, 991–994. [Google Scholar] [CrossRef]
- Manios, Y.; Moschonis, G.; Lambrinou, C.P.; Tsoutsoulopoulou, K.; Binou, P.; Karachaliou, A.; Breidenassel, C.; Gonzalez-Gross, M.; Kiely, M.; Cashman, K.D. A systematic review of vitamin D status in southern European countries. Eur. J. Nutr. 2018, 57, 2001–2036. [Google Scholar] [CrossRef]
- Huh, S.Y.; Gordon, C.M. Vitamin D deficiency in children and adolescents: Epidemiology, impact and treatment. Rev. Endocr. Metab. Disord. 2008, 9, 161–170. [Google Scholar] [CrossRef]
- Naderan, M.; Rajabi, M.T.; Zarrinbakhsh, P.; Farjadnia, M. Is keratoconus more severe in pediatric population? Int. Ophthalmol. 2017, 37, 1169–1173. [Google Scholar] [CrossRef]
- Naderan, M.; Jahanrad, A. Topographic, tomographic and biomechanical corneal changes during pregnancy in patients with keratoconus: A cohort study. Acta Ophthalmol. 2017, 95, e291–e296. [Google Scholar] [CrossRef]
- López-López, M.; Regueiro, U.; Bravo, S.B.; Chantada-Vázquez Mdel, P.; Varela-Fernández, R.; Ávila-Gómez, P.; Hervella, P.; Lema, I. Tear Proteomics in Keratoconus: A Quantitative SWATH-MS Analysis. Investig. Ophthalmol. Vis. Sci. 2021, 62, 30. [Google Scholar] [CrossRef]
- Gupta, P.; Pathak, M.; Thakur, B.; Fogla, R.; Agarwal, A.; Ram, J. Association of keratoconus with serum levels of 25-hydroxyvitamin D and antioxidant trace elements: A systematic review and meta-analysis. Indian J. Ophthalmol. 2022, 70, 2818–2824. [Google Scholar] [CrossRef]
- Knapp, A.A. Vitamin D Complex in Keratoconus: Etiology, Pathology and Treatment of Conical Cornea: Preliminary Report. J. Am. Med. Assoc. 1938, 110, 1993–1994. [Google Scholar] [CrossRef]
- Mutti, D.O.; Marks, A.R. Blood levels of vitamin D in teens and young adults with myopia. Optom. Vis. Sci. 2011, 88, 377–382. [Google Scholar] [CrossRef] [PubMed]
- Vitar, R.M.L.; Fonteyne, P.; Knutsson, K.A.; Bertuzzi, F.; Galli, L.; Rama, P.; Ferrari, G. Vitamin D Supplementation Impacts Systemic Biomarkers of Collagen Degradation and Copper Metabolism in Patients With Keratoconus. Transl. Vis. Sci. Technol. 2022, 11, 16. [Google Scholar] [CrossRef] [PubMed]
- Bartolomeo, N.; Pederzolli, M.; Palombella, S.; Fonteyne, P.; Suanno, G.; Tilaro, G.; de Pretis, S.; Borgo, F.; Bertuzzi, F.; Senni, C.; et al. The Effects of Vitamin D on Keratoconus Progression. Am. J. Ophthalmol. 2025, 276, 235–251. [Google Scholar] [CrossRef]
- Mutch, J.R.; Richards, M.B. Keratoconus Experimentally Produced in the Rat by Vitamin A Deficiency. Br. J. Ophthalmol. 1939, 23, 381–387. [Google Scholar] [CrossRef]
- Wu, J.; Wang, J.; Wang, L.; Huang, Y. Topical retinoic acid induces corneal strengthening by upregulating transglutaminase 2 in murine cornea. Exp. Eye Res. 2022, 214, 108850. [Google Scholar] [CrossRef]
- Yu, H.; Wu, J.; Li, K.; Huang, Y.; Wu, T.; Wang, L.; Huang, Y. Integrated analysis of murine cornea identifies JAK/STAT signaling pathway upregulated specifically in female Vitamin A Deficient mice. Exp. Eye Res. 2023, 237, 109714. [Google Scholar] [CrossRef]
- Sozer, O.; Ozalp, O.; Atalay, E.; Demir, S.S.; Alatas, İ.O.; Yildirim, N. Comparison of blood levels of vitamin B12, folic acid, riboflavin, and homocysteine in keratoconus and healthy subjects. J. Cataract. Refract. Surg. 2023, 49, 589–594. [Google Scholar] [CrossRef]
- Bamdad, S.; Owji, N.; Bolkheir, A. Association Between Advanced Keratoconus and Serum Levels of Zinc, Calcium, Magnesium, Iron, Copper, and Selenium. Cornea 2018, 37, 1306–1310. [Google Scholar] [CrossRef]
- Kiliç, R.; Bayraktar, A.C.; Bayraktar, S.; Kurt, A.; Kavutçu, M. Evaluation of Serum Superoxide Dismutase Activity, Malondialdehyde, and Zinc and Copper Levels in Patients With Keratoconus. Cornea 2016, 35, 1512–1515. [Google Scholar] [CrossRef]
- Ortak, H.; Söǧüt, E.; Taş, U.; Mesci, C.; Mendil, D. The relation between keratoconus and plasma levels of MMP-2, zinc, and SOD. Cornea 2012, 31, 1048–1051. [Google Scholar] [CrossRef] [PubMed]
- Dudakova, L.; Liskova, P.; Jirsova, K. Is copper imbalance an environmental factor influencing keratoconus development? Med. Hypotheses 2015, 84, 518–524. [Google Scholar] [CrossRef]
- Avetisov, S.E.; Mamikonian, V.R.; Novikov, I.A. The role of tear acidity and Cu-cofactor of lysyl oxidase activity in the pathogenesis of keratoconus. Vestn. Oftalmol. 2011, 127, 3–8. [Google Scholar]
- Avetisov, S.E.; Mamikonyan, V.R.; Novikov, I.A.; Pateyuk, L.S.; Osipyan, G.A.; Kiryushchenkova, N.P. Abnormal distribution of trace elements in keratoconic corneas. Vestn. Oftalmol. 2015, 131, 34–42. [Google Scholar] [CrossRef]
- Balasubramanian, S.A.; Pye, D.C.; Willcox, M.D.P. Levels of lactoferrin, secretory IgA and serum albumin in the tear film of people with keratoconus. Exp. Eye Res. 2012, 96, 132–137. [Google Scholar] [CrossRef]
- Chaerkady, R.; Shao, H.; Scott, S.G.; Pandey, A.; Jun, A.S.; Chakravarti, S. The keratoconus corneal proteome: Loss of epithelial integrity and stromal degeneration. J. Proteom. 2013, 87, 122–131. [Google Scholar] [CrossRef]
- Wójcik, K.A.; Synowiec, E.; Jiménez-García, M.P.; Kaminska, A.; Polakowski, P.; Blasiak, J.; Szaflik, J.; Szaflik, J.P. Polymorphism of the transferrin gene in eye diseases: Keratoconus and Fuchs endothelial corneal dystrophy. BioMed Res. Int. 2013, 2013, 247438. [Google Scholar] [CrossRef]
- Rouault, T.A. The role of iron regulatory proteins in mammalian iron homeostasis and disease. Nat. Chem. Biol. 2006, 2, 406–414. [Google Scholar] [CrossRef]
- Gao, J.F.; Dong, Y.Y.; Jin, X.; Dai, L.J.; Wang, J.R.; Zhang, H. Identification and Verification of Ferroptosis-Related Genes in Keratoconus Using Bioinformatics Analysis. J. Inflamm. Res. 2024, 17, 2383–2397. [Google Scholar] [CrossRef]
- Kumar, N.R.; Khamar, P.; Kannan, R.; Padmanabhan, A.; Shetty, R.; D’Souza, S.; Vaidya, T.; Sethu, S.; Ghosh, A. Distinct Ocular Surface Microbiome in Keratoconus Patients Correlate With Local Immune Dysregulation. Investig. Ophthalmol. Vis. Sci. 2025, 66, 60. [Google Scholar] [CrossRef]
- Rocha-de-Lossada, C.; Mazzotta, C.; Gabrielli, F.; Papa, F.T.; Gómez-Huertas, C.; García-López, C.; Urbinati, F.; Rachwani-Anil, R.; García-Lorente, M.; Sánchez-González, J.M.; et al. Ocular Surface Microbiota in Naïve Keratoconus: A Multicenter Validation Study. J. Clin. Med. 2023, 12, 6354. [Google Scholar] [CrossRef] [PubMed]
- Tunç, U.; Çelebi, A.C.; Ekren, B.Y.; Yıldırım, Y.; Kepez Yıldız, B.; Okullu, S.Ö.; Sezerman, O.U. Corneal bacterial microbiome in patients with keratoconus using next-generation sequencing-based 16S rRNA gene analysis. Exp. Eye Res. 2023, 228, 109402. [Google Scholar] [CrossRef] [PubMed]
- Kaur, P.; Moon, L.; Srikumaran, D.; Salzberg, S.L.; Lu, J.; Simner, P.J.; Soiberman, U.S. No Evidence of Chronic Infection in a Metagenomic Sequencing Study of the Keratoconus Corneal Epithelium. J. Clin. Med. 2024, 13, 3399. [Google Scholar] [CrossRef] [PubMed]
- Sharif, R.; Sejersen, H.; Frank, G.; Hjortdal, J.; Karamichos, D. Effects of collagen cross-linking on the keratoconus metabolic network. Eye 2018, 32, 1271–1281. [Google Scholar] [CrossRef]
- McKay, T.B.; Hjortdal, J.; Sejersen, H.; Asara, J.M.; Wu, J.; Karamichos, D. Endocrine and Metabolic Pathways Linked to Keratoconus: Implications for the Role of Hormones in the Stromal Microenvironment. Sci. Rep. 2016, 6, 25534. [Google Scholar] [CrossRef]
- Karamichos, D.; Hutcheon, A.E.K.; Rich, C.B.; Trinkaus-Randall, V.; Asara, J.M.; Zieske, J.D. In vitro model suggests oxidative stress involved in keratoconus disease. Sci. Rep. 2014, 4, 4608. [Google Scholar] [CrossRef]
- Blokhina, O.; Virolainen, E.; Fagerstedt, K.V. Antioxidants, oxidative damage and oxygen deprivation stress: A review. Ann. Bot. 2003, 91, 179–194. [Google Scholar] [CrossRef]
- McKay, T.B.; Lyon, D.; Sarker-Nag, A.; Priyadarsini, S.; Asara, J.M.; Karamichos, D. Quercetin attenuates lactate production and extracellular matrix secretion in keratoconus. Sci. Rep. 2015, 5, 9003. [Google Scholar] [CrossRef]
- Whelchel, A.E.; McKay, T.B.; Priyadarsini, S.; Rowsey, T.; Karamichos, D. Association between Diabetes and Keratoconus: A Retrospective Analysis. Sci. Rep. 2019, 9, 18521. [Google Scholar] [CrossRef]
- Gu, S.; Liu, Z.; Pan, S.; Jiang, Z.; Lu, H.; Amit, O.; Bradbury, E.M.; Hu, C.A.A.; Chen, X. Global investigation of p53-induced apoptosis through quantitative proteomic profiling using comparative amino acid-coded tagging. Mol. Cell. Proteom. 2004, 3, 998–1008. [Google Scholar] [CrossRef]
- McKay, T.B.; Hjortdal, J.; Sejersen, H.; Karamichos, D. Differential Effects of Hormones on Cellular Metabolism in Keratoconus In Vitro. Sci. Rep. 2017, 7, 42896. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, S.; Häberle, J.; Kido, J.; Mitsubuchi, H.; Endo, F.; Nakamura, K. Urea cycle disorders–update. J. Hum. Genet. 2019, 64, 833–847. [Google Scholar] [CrossRef] [PubMed]
- Wojakowska, A.; Pietrowska, A.; Widlak, P.; Dobrowolski, D.; Wylegała, E.; Tarnawska, D. Metabolomic Signature Discriminates Normal Human Cornea from Keratoconus—A Pilot GC/MS Study. Molecules 2020, 25, 2933. [Google Scholar] [CrossRef]
- McKay, T.B.; Hjortdal, J.; Priyadarsini, S.; Karamichos, D. Acute hypoxia influences collagen and matrix metalloproteinase expression by human keratoconus cells in vitro. PLoS ONE 2017, 12, e0176017. [Google Scholar] [CrossRef] [PubMed]
- Peris-Martínez, C.; Piá-Ludeña, J.V.; Rog-Revert, M.J.; Fernández-López, E.; Domingo, J.C. Antioxidant and Anti-Inflammatory Effects of Oral Supplementation with a Highly-Concentrated Docosahexaenoic Acid (DHA) Triglyceride in Patients with Keratoconus: A Randomized Controlled Preliminary Study. Nutrients 2023, 15, 1300. [Google Scholar] [CrossRef]
- Pihlblad, M.S.; Schaefer, D.P. Eyelid laxity, obesity, and obstructive sleep apnea in keratoconus. Cornea 2013, 32, 1232–1236. [Google Scholar] [CrossRef]
- Eliasi, E.; Bez, M.; Megreli, J.; Avramovich, E.; Fischer, N.; Barak, A.; Levine, H. The Association Between Keratoconus and Body Mass Index: A Population-Based Cross-Sectional Study Among Half a Million Adolescents. Am. J. Ophthalmol. 2021, 224, 200–206. [Google Scholar] [CrossRef]
- Wang, J.; Liu, F.; Mo, J.; Gong, D.; Zheng, F.; Su, J.; Ding, S.; Yang, W.; Guo, P. Exploring the causal relationship between body mass index and keratoconus: A Mendelian randomization study. Front. Med. 2024, 11, 1402108. [Google Scholar] [CrossRef]
- Ren, S.; Tu, R.; Xu, L.; Gu, Y.; Fan, Q.; Wang, Q.; Zhu, M.; Yin, S.; Pang, C.; Zhao, D.; et al. A high body mass index strengthens the association between the time of eye rubbing and keratoconus in a Chinese population: A case control study. BMC Public Health 2023, 23, 2032. [Google Scholar] [CrossRef]
- Skorin, L.; Knutson, R. Ophthalmic Diseases in Patients With Obstructive Sleep Apnea. J. Am. Osteopath. Assoc. 2016, 116, 522–529. [Google Scholar] [CrossRef][Green Version]
- Salinas, R.; Puig, M.; Fry, C.L.; Johnson, D.A.; Kheirkhah, A. Floppy eyelid syndrome: A comprehensive review. Ocul. Surf. 2020, 18, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Bulloch, G.; Seth, I.; Zhu, Z.; Sukumar, S.; McNab, A. Ocular manifestations of obstructive sleep apnea: A systematic review and meta-analysis. Graefes Arch. Clin. Exp. Ophthalmol. 2024, 262, 19–32. [Google Scholar] [CrossRef] [PubMed]
- Karaca, U.; Akıncıoğlu, D.; Ayyildiz, O.; Dogan, D.; Ozge, G.; Usta, G.; Mutlu, F.M. Comparison of obstructive sleep apnea syndrome and keratoconus patients on elevation maps. Int. Ophthalmol. 2022, 42, 933–938. [Google Scholar] [CrossRef]
- Seiler, T.; Huhle, S.; Spoerl, E.; Kunath, H. Manifest diabetes and keratoconus: A retrospective case-control study. Graefes Arch. Clin. Exp. Ophthalmol. 2000, 238, 822–825. [Google Scholar] [CrossRef]
- Naderan, M.; Naderan, M.; Rezagholizadeh, F.; Zolfaghari, M.; Pahlevani, R.; Rajabi, M.T. Association between diabetes and keratoconus: A case-control study. Cornea 2014, 33, 1271–1273. [Google Scholar] [CrossRef]
- Naderan, M.; Shoar, S.; Rezagholizadeh, F.; Zolfaghari, M.; Naderan, M. Characteristics and associations of keratoconus patients. Cont. Lens Anterior Eye 2015, 38, 199–205. [Google Scholar] [CrossRef]
- Akowuah, P.K.; Arthur, C.; Otabil, F.A.; Ofori, C.A.; Osei-Poku, K.; Fummey, J.; Boadi, P.; Dadzie, E.E. Association between diabetes and keratoconus–a systematic review and meta-analysis. Eur. J. Ophthalmol. 2022, 32, 23–30. [Google Scholar] [CrossRef]
- Dong, X.X.; Liu, K.F.; Zhou, M.; Liang, G.; Pan, C.W. Diabetes Mellitus and Keratoconus: A Systematic Review and Meta-Analysis. Cornea 2022, 41, 1398–1404. [Google Scholar] [CrossRef]
- Kuo, I.C.; Broman, A.; Pirouzmanesh, A.; Melia, M. Is there an association between diabetes and keratoconus? Ophthalmology 2006, 113, 184–190. [Google Scholar] [CrossRef]
- El-Massry, A.; Doheim, M.F.; Iqbal, M.; Fawzy, O.; Said, O.M.; Yousif, M.O.; Badawi, A.E.; Tawfik, A.; Abousamra, A. Association Between Keratoconus and Thyroid Gland Dysfunction: A Cross-Sectional Case-Control Study. J. Refract. Surg. 2020, 36, 253–257. [Google Scholar] [CrossRef]
- Said, O.M.; Iqbal, M.; El-Massry, A.; Elgharieb, M.E.; Mady, M.; Sharawy, A.M.; Abdelaziz, K. Thyroid gland dysfunction and keratoconus. Med. Hypothesis Discov. Innov. Ophthalmol. 2024, 13, 104–111. [Google Scholar] [CrossRef] [PubMed]
- Chung, I.K.; Kim, B.S.; Han, K.D.; Yoo, Y.S.; Kim, H.; Jeong, C. Ten-year incidence of keratoconus in relation to sex, age, and thyroid gland dysfunction: A nationwide population-based cohort study (2009–2018). Ann. Transl. Med. 2024, 12, 45. [Google Scholar] [CrossRef] [PubMed]
- Roszkowska, A.M.; Alessandrello, F.; Waśniewska, M.; Tropeano, A.; Gargano, R.; Aragona, P. Is keratoconus associated to thyroid diseases? Assessment of the corneal parameters in patients with congenital hypothyroidism. Eur. J. Ophthalmol. 2022, 32, 31–35. [Google Scholar] [CrossRef]
- Razafimino, S.; Flockerzi, E.; Zemova, E.; Munteanu, C.; Seitz, B. Impact of Hypothyroidism on Tomography and Biomechanics in Keratoconus—Cross-Sectional and Longitudinal Assessment within the Homburg Keratoconus Center at the Time of Inclusion and after 1 Year. Klin. Monbl Augenheilkd 2023, 240, 1185–1191. [Google Scholar] [CrossRef]
- Kahán, I.L.; Varsányi-Nagy, M.; Tóth, M.; Nádrai, A. The possible role of tear fluid thyroxine in keratoconus development. Exp. Eye Res. 1990, 50, 339–343. [Google Scholar] [CrossRef]
- Thanos, S.; Oellers, P.; Meyer Zu Hörste, M.; Prokosch, V.; Schlatt, S.; Seitz, B.; Gatzioufas, Z. Role of Thyroxine in the Development of Keratoconus. Cornea 2016, 35, 1338–1346. [Google Scholar] [CrossRef]
- Stachon, T.; Stachon, A.; Hartmann, U.; Seitz, B.; Langenbucher, A.; Szentmáry, N. Urea, Uric Acid, Prolactin and fT4 Concentrations in Aqueous Humor of Keratoconus Patients. Curr. Eye Res. 2017, 42, 842–846. [Google Scholar] [CrossRef]
- Stachon, T.; Omar Ali, M.; Latta, L.; Huessein, G.H.; Mohamed, T.A.; Soliman, W.; Seitz, B.; Szentmary, N. Effect of Thyroxine on Transforming Growth Factor β1, Collagen I, and V Expression in Keratoconus Corneal Fibroblasts and Keratocytes, in Vitro. Curr. Eye Res. 2022, 47, 206–213. [Google Scholar] [CrossRef]
- Claessens, J.L.J.; Godefrooij, D.A.; Vink, G.; Frank, L.E.; Wisse, R.P.L. Nationwide epidemiological approach to identify associations between keratoconus and immune-mediated diseases. Br. J. Ophthalmol. 2022, 106, 1350–1354. [Google Scholar] [CrossRef]
- Karamichos, D.; Escandon, P.; Vasini, B.; Nicholas, S.E.; Van, L.; Dang, D.H.; Cunningham, R.L.; Riaz, K. Anterior pituitary, sex hormones, and keratoconus: Beyond traditional targets. Prog. Retin. Eye Res. 2022, 88, 101016. [Google Scholar] [CrossRef]
- Wagner, H.; Barr, J.T.; Zadnik, K. Collaborative Longitudinal Evaluation of Keratoconus (CLEK) Study: Methods and findings to date. Cont. Lens Anterior Eye. 2007, 30, 223–232. [Google Scholar] [CrossRef] [PubMed]
- Hashemi, H.; Beiranvand, A.; Khabazkhoob, M.; Asgari, S.; Emamian, M.H.; Shariati, M.; Fotouhi, A. Prevalence of keratoconus in a population-based study in Shahroud. Cornea 2013, 32, 1441–1445. [Google Scholar] [CrossRef] [PubMed]
- Hashemi, H.; Khabazkhoob, M.; Yazdani, N.; Ostadimoghaddam, H.; Norouzirad, R.; Amanzadeh, K.; Miraftab, M.; Derakhshan, A.; Yekta, A.A. The prevalence of keratoconus in a young population in Mashhad, Iran. Ophthalmic Physiol. Opt. 2014, 34, 519–527. [Google Scholar] [CrossRef]
- Rabinowitz, Y.S. Keratoconus. Surv. Ophthalmol. 1998, 42, 297–319. [Google Scholar] [CrossRef]
- Yin, H.; Luo, C.; Tian, Y.; Deng, Y. Altered expression of sex hormone receptors in keratoconus corneas. Biomed. Res.-India 2017, 28, 5089–5092. [Google Scholar]
- Ayan, B.; Yuksel, N.; Carhan, A.; Gumuşkaya Ocal, B.; Akcay, E.; Cagil, N.; Asik, M.D. Evaluation estrogen, progesteron and androgen receptor expressions in corneal epithelium in keratoconus. Cont. Lens Anterior Eye. 2019, 42, 492–496. [Google Scholar] [CrossRef]
- Sharif, R.; Bak-Nielsen, S.; Sejersen, H.; Ding, K.; Hjortdal, J.; Karamichos, D. Prolactin-Induced Protein is a novel biomarker for Keratoconus. Exp. Eye Res. 2019, 179, 55–63. [Google Scholar] [CrossRef]
- Zhao, X.; Yuan, Y.; Sun, T.; Zhang, Y.; Chen, Y. Associations Between Keratoconus and the Level of Sex Hormones: A Cross-Sectional Study. Front. Med. 2022, 9, 828233. [Google Scholar] [CrossRef]
- Karamichos, D.; Barrientez, B.; Nicholas, S.; Ma, S.; Van, L.; Bak-Nielsen, S.; Hjortdal, J. Gonadotropins in Keratoconus: The Unexpected Suspects. Cells 2019, 8, 1494. [Google Scholar] [CrossRef]
- Jamali, H.; Heydari, M.; Masihpour, N.; Khosravi, A.; Zare, M.; Shams, M.; Omrani, G.R. Serum androgens and prolactin levels in patients with keratoconus. Clin. Exp. Optom. 2023, 106, 484–488. [Google Scholar] [CrossRef]
- Deitel, C.M.; Chen, K.H.; Uber, I.C. Possible association of keratoconus progression with gender-affirming hormone therapy: A case report. Am. J. Ophthalmol. Case Rep. 2023, 30, 101850. [Google Scholar] [CrossRef]
- Beardsley, T.L.; Foulks, G.N. An association of keratoconus and mitral valve prolapse. Ophthalmology 1982, 89, 35–37. [Google Scholar] [CrossRef] [PubMed]
- Sharif, K.W.; Casey, T.A.; Coltart, J. Prevalence of mitral valve prolapse in keratoconus patients. J. R. Soc. Med. 1992, 85, 446–448. [Google Scholar] [CrossRef] [PubMed]
- Lichter, H.; Loya, N.; Sagie, A.; Cohen, N.; Muzmacher, L.; Yassur, Y.; Weinberger, D. Keratoconus and mitral valve prolapse. Am. J. Ophthalmol. 2000, 129, 667–668. [Google Scholar] [CrossRef]
- Kalkan Akcay, E.; Akcay, M.; Uysal, B.S.; Kosekahya, P.; Aslan, A.N.; Caglayan, M.; Koseoglu, C.; Yulek, F.; Cagil, N. Impaired corneal biomechanical properties and the prevalence of keratoconus in mitral valve prolapse. J. Ophthalmol. 2014, 2014, 402193. [Google Scholar] [CrossRef]
- Rabbanikhah, Z.; Javadi, M.A.; Rostami, P.; Aghdaie, A.; Yaseri, M.; Yahyapour, F.; Katibeh, M. Association between acute corneal hydrops in patients with keratoconus and mitral valve prolapse. Cornea 2011, 30, 154–157. [Google Scholar] [CrossRef]
- Chang, Y.S.; Tai, M.C.; Weng, S.F.; Wang, J.J.; Tseng, S.H.; Jan, R.L. Risk of Mitral Valve Prolapse in Patients with Keratoconus in Taiwan: A Population-Based Cohort Study. Int. J. Environ. Res. Public Health 2020, 17, 6049. [Google Scholar] [CrossRef]
- Ritelli, M.; Colombi, M. Molecular Genetics and Pathogenesis of Ehlers-Danlos Syndrome and Related Connective Tissue Disorders. Genes 2020, 11, 547. [Google Scholar] [CrossRef]
- Woodward, E.G.; Morris, M.T. Joint hypermobility in keratoconus. Ophthalmic Physiol. Opt. 1990, 10, 360–362. [Google Scholar] [CrossRef]
- Fransen, E.; Valgaeren, H.; Janssens, K.; Sommen, M.; De Ridder, R.; Vandeweyer, G.; Bisceglia, L.; Soler, V.; Hoischen, A.; Mortier, G.; et al. Resequencing of candidate genes for Keratoconus reveals a role for Ehlers-Danlos Syndrome genes. Eur. J. Hum. Genet. 2021, 29, 1745–1755. [Google Scholar] [CrossRef]
- Maumenee, I.H. The eye in the Marfan syndrome. Trans. Am. Ophthalmol. Soc. 1981, 79, 684–733. [Google Scholar] [PubMed]
- Mashor, R.S.; Kumar, N.L.; Ritenour, R.J.; Rootman, D.S. Keratoconus caused by eye rubbing in patients with Tourette Syndrome. Can. J. Ophthalmol. 2011, 46, 83–86. [Google Scholar] [CrossRef]
- Knutsson, K.A.; Paganoni, G.; Ambrosio, O.; Ferrari, G.; Rama, P. Corneal collagen cross-linking for management of keratoconus in patients affected by Tourette syndrome. Eur. J. Ophthalmol. 2021, 31, 2233–2236. [Google Scholar] [CrossRef] [PubMed]
- Safir, M.; Hecht, I.; Heller, D.; Pras, E.; Lifshitz, M.; Einan-Lifshitz, A. Psychiatric Comorbidities Associated with Keratoconus. JAMA Ophthalmol. 2023, 141, 1145–1150. [Google Scholar] [CrossRef] [PubMed]
- Alfardan, F.; Alsanad, M.H.; Altoub, H.A. Prevalence of Psychiatric Illness Among Keratoconus Patients. Cureus 2023, 15, e42141. [Google Scholar] [CrossRef]
- Moschos, M.M.; Gouliopoulos, N.S.; Kalogeropoulos, C.; Androudi, S.; Kitsos, G.; Ladas, D.; Tsatsos, M.; Chatziralli, I. Psychological Aspects and Depression in Patients with Symptomatic Keratoconus. J. Ophthalmol. 2018, 2018, 7314308. [Google Scholar] [CrossRef]
- Moshfeghinia, R.; Arman, A.; Sobhi, N.; Mahmoudinezhad, G.; Molavi Vardanjani, H. Depression among keratoconus patients: A systematic review and meta-analysis. Front. Public Health. 2024, 12, 1477411. [Google Scholar] [CrossRef]
- Schlötzer Schrehardt, U.M.; Koca, M.R.; Naumann, G.O.H.; Volkholz, H. Pseudoexfoliation syndrome. Ocular manifestation of a systemic disorder? Arch. Ophthalmol. 1992, 110, 1752–1756. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pederzolli, M.; Procopio, F.; Tombolini, B.; Marra, S.; De Micheli, M.; Bandello, F.; Ferrari, G. Keratoconus: The Local Manifestation of a Systemic Disease? J. Clin. Med. 2025, 14, 4587. https://doi.org/10.3390/jcm14134587
Pederzolli M, Procopio F, Tombolini B, Marra S, De Micheli M, Bandello F, Ferrari G. Keratoconus: The Local Manifestation of a Systemic Disease? Journal of Clinical Medicine. 2025; 14(13):4587. https://doi.org/10.3390/jcm14134587
Chicago/Turabian StylePederzolli, Matteo, Federico Procopio, Beatrice Tombolini, Simone Marra, Massimo De Micheli, Francesco Bandello, and Giulio Ferrari. 2025. "Keratoconus: The Local Manifestation of a Systemic Disease?" Journal of Clinical Medicine 14, no. 13: 4587. https://doi.org/10.3390/jcm14134587
APA StylePederzolli, M., Procopio, F., Tombolini, B., Marra, S., De Micheli, M., Bandello, F., & Ferrari, G. (2025). Keratoconus: The Local Manifestation of a Systemic Disease? Journal of Clinical Medicine, 14(13), 4587. https://doi.org/10.3390/jcm14134587







