Prevalence of Undiagnosed Inflammatory Bowel Disease in Spondyloarthritis Patients
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Selection Criteria
2.3. Study Procedures and Assessments
2.4. Statistical Analysis
3. Results
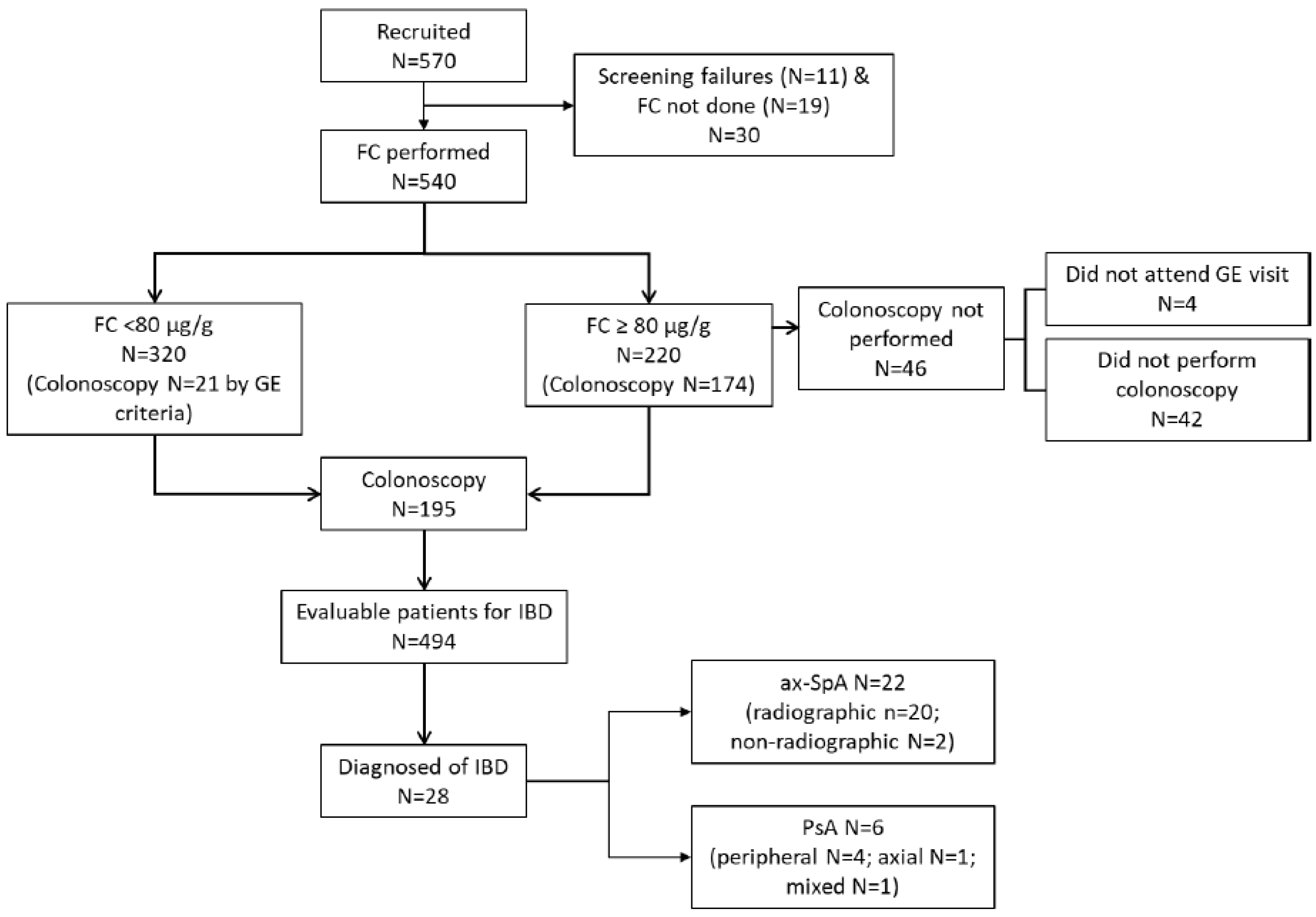
3.1. Patients’ Disposition and Characteristics
3.2. Fecal Calprotectin Levels
3.3. Prevalence of Inflammatory Bowel Disease
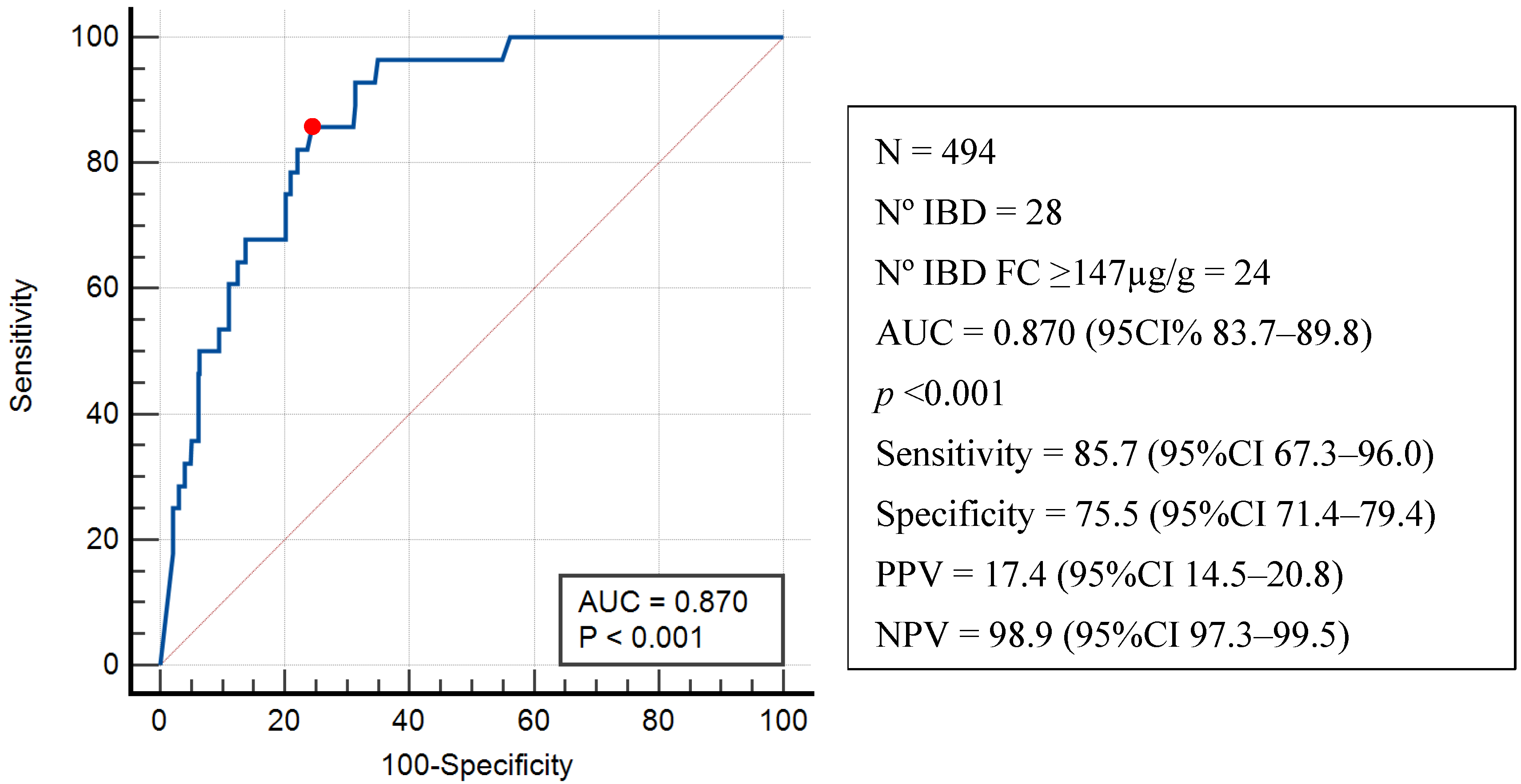
3.4. Diagnostic Performance of Fecal Calprotectin
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rudwaleit, M.; van der Heijde, D.; Landewé, R.; Akkoc, N.; Brandt, J.; Chou, C.T.; Dougados, M.; Huang, F.; Gu, J.; Kirazli, Y.; et al. The Assessment of SpondyloArthritis international Society classification criteria for peripheral spondyloarthritis and for spondyloarthritis in general. Ann. Rheum. Dis. 2011, 70, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Stolwijk, C.; van Tubergen, A.; Castillo-Ortiz, J.D.; Boonen, A. Prevalence of extra-articular manifestations in patients with ankylosing spondylitis: A systematic review and meta-analysis. Ann. Rheum. Dis. 2015, 74, 65–73. [Google Scholar] [CrossRef]
- Pittam, B.; Gupta, S.; Harrison, N.L.; Robertson, S.; Hughes, D.M.; Zhao, S.S. Prevalence of extra-articular manifestations in psoriatic arthritis: A systematic review and meta-analysis. Rheumatology 2020, 59, 2199–2206. [Google Scholar] [CrossRef]
- Essers, I.; Ramiro, S.; Stolwijk, C.; Blaauw, M.; Landewé, R.; van der Heijde, D.; Bosch, F.V.D.; Dougados, M.; van Tubergen, A. Characteristics associated with the presence and development of extra-articular manifestations in ankylosing spondylitis: 12-year results from OASIS. Rheumatology 2014, 54, 633–640. [Google Scholar] [CrossRef]
- Sanz, J.S.; Roura, X.J.; Seoane-Mato, D.; Montoro, M.; Gomollon, F.; Grupo de Trabajo del proyecto PIIASER. Screening of In-flammatory Bowel Disease and Spondyloarthritis for Referring Patients Between Rheumatology and Gastroenterology. Reumatol. Clin. 2018, 14, 68–74. [Google Scholar]
- Stebbings, S.; Jenks, K.; Treharne, G.J.; García, J.A.; Schultz, M.; Highton, J.; Dudley-Brown, S. Validation of the Dudley Inflammatory Bowel Symptom Questionnaire for the assessment of bowel symptoms in axial SpA: Prevalence of clinically relevant bowel symptoms and association with disease activity. Rheumatology 2011, 51, 858–865. [Google Scholar] [CrossRef][Green Version]
- Gutiérrez-Sánchez, J.; Parra-Izquierdo, V.; Flórez-Sarmiento, C.; Jaimes, D.A.; De Ávila, J.; Bello-Gualtero, J.M.; Ramos-Casallas, A.; Chila-Moreno, L.; Pacheco-Tena, C.; Beltrán-Ostos, A.; et al. Implementation of screening criteria for inflammatory bowel disease in patients with spondyloarthritis and its association with disease and endoscopic activity. Clin. Rheumatol. 2022, 42, 415–422. [Google Scholar] [CrossRef] [PubMed]
- D’haens, G.; Ferrante, M.; Vermeire, S.; Baert, F.; Noman, M.; Moortgat, L.; Geens, P.; Iwens, D.; Aerden, I.; Van Assche, G.; et al. Fecal calprotectin is a surrogate marker for endoscopic lesions in inflammatory bowel disease. Inflamm. Bowel Dis. 2012, 18, 2218–2224. [Google Scholar] [CrossRef]
- Rendek, Z.; Falk, M.; Grodzinsky, E.; Kechagias, S.; Hjortswang, H. Oral omeprazole and diclofenac intake is associated with increased faecal calprotectin levels: A randomised open-label clinical trial. Eur. J. Gastroenterol. Hepatol. 2022, 35, 52–58. [Google Scholar] [CrossRef]
- Fauny, M.; D’aMico, F.; Bonovas, S.; Netter, P.; Danese, S.; Loeuille, D.; Peyrin-Biroulet, L. Faecal Calprotectin for the Diagnosis of Bowel Inflammation in Patients With Rheumatological Diseases: A Systematic Review. J. Crohn’s Colitis 2019, 14, 688–693. [Google Scholar] [CrossRef]
- Taylor, W.; Gladman, D.; Helliwell, P.; Marchesoni, A.; Mease, P.; Mielants, H.; CASPAR Study Group. Classification criteria for psoriatic arthritis: Development of new criteria from a large international study. Arthritis Care Res. 2006, 54, 2665–2673. [Google Scholar] [CrossRef]
- Rudwaleit, M.; van der Heijde, D.; Landewé, R.; Listing, J.; Akkoc, N.; Brandt, J.; Braun, J.; Chou, C.T.; Collantes-Estevez, E.; Dougados, M.; et al. The development of Assessment of SpondyloArthritis international Society classification criteria for axial spondyloarthritis (part II): Validation and final selection. Ann. Rheum. Dis. 2009, 68, 777–783. [Google Scholar] [CrossRef] [PubMed]
- Lai, E.J.; Calderwood, A.H.; Doros, G.; Fix, O.K.; Jacobson, B.C. The Boston bowel preparation scale: A valid and reliable instrument for colonoscopy-oriented research. Gastrointest. Endosc. 2009, 69, 620–625. [Google Scholar] [CrossRef] [PubMed]
- Fauny, M.; Moulin, D.; D’AMico, F.; Netter, P.; Petitpain, N.; Arnone, D.; Jouzeau, J.-Y.; Loeuille, D.; Peyrin-Biroulet, L. Paradoxical gastrointestinal effects of interleukin-17 blockers. Ann. Rheum. Dis. 2020, 79, 1132–1138. [Google Scholar] [CrossRef]
- Garrett, S.; Jenkinson, T.; Kennedy, L.G.; Whitelock, H.; Gaisford, P.; Calin, A. A new approach to defining disease status in ankylosing spondylitis: The Bath Ankylosing Spondylitis Disease Activity Index. J. Rheumatol. 1994, 21, 2286–2291. [Google Scholar] [PubMed]
- Lukas, C.; Landewé, R.; Sieper, J.; Dougados, M.; Davis, J.; Braun, J.; van der Linden, S.; van der Heijde, D. Development of an ASAS-endorsed disease activity score (ASDAS) in patients with ankylosing spondylitis. Ann. Rheum. Dis. 2009, 68, 18–24. [Google Scholar] [CrossRef]
- Schoels, M.; Aletaha, D.; Funovits, J.; Kavanaugh, A.; Baker, D.; Smolen, J.S. Application of the DAREA/DAPSA score for assessment of disease activity in psoriatic arthritis. Ann. Rheum. Dis. 2010, 69, 1441–1447. [Google Scholar] [CrossRef]
- Daperno, M.; D’HAens, G.; Van Assche, G.; Baert, F.; Bulois, P.; Maunoury, V.; Sostegni, R.; Rocca, R.; Pera, A.; Gevers, A. Development and validation of a new, simplified endoscopic activity score for Crohn’s disease: The SES-CD. Gastrointest. Endosc. 2004, 60, 505–512. [Google Scholar] [CrossRef]
- Travis, S.P.L.; Schnell, D.; Krzeski, P.; Abreu, M.T.; Altman, D.G.; Colombel, J.-F.; Feagan, B.G.; Hanauer, S.B.; Lémann, M.; Lichtenstein, G.R.; et al. Developing an instrument to assess the endoscopic severity of ulcerative colitis: The Ulcerative Colitis Endoscopic Index of Severity (UCEIS). Gut 2011, 61, 535–542. [Google Scholar] [CrossRef]
- Magro, F.; Sabino, J.; Rosini, F.; Tripathi, M.; Borralho, P.; Baldin, P.; Danese, S.; Driessen, A.; Gordon, I.O.; Iacucci, M.; et al. ECCO Position on Harmonisation of Crohn’s Disease Mucosal Histopathology. J. Crohn’s Colitis 2022, 16, 876–883. [Google Scholar] [CrossRef]
- Gralnek, I.M.; Defranchis, R.; Seidman, E.; Leighton, J.A.; Legnani, P.; Lewis, B.S. Development of a capsule endoscopy scoring index for small bowel mucosal inflammatory change. Aliment. Pharmacol. Ther. 2007, 27, 146–154. [Google Scholar] [CrossRef] [PubMed]
- Tukey, M.; Pleskow, D.; Legnani, P.; Cheifetz, A.S.; Moss, A.C. The utility of capsule endoscopy in patients With suspected Crohn’s disease. Am. J. Gastroenterol. 2009, 104, 2734–2739. [Google Scholar] [CrossRef] [PubMed]
- Bergman, M.J.; Zueger, P.; Song, J.; Pivneva, I.; Betts, K.A.; Joshi, A.D. Inflammatory Bowel Disease Is Associated with a Substantial Economic Burden in Patients with Psoriatic Arthritis and in Patients with Ankylosing Spondylitis. In Arthritis & Rheumatol.; WILEY: Hoboken, NJ USA, 2018; Volume 70, (Suppl. S9). [Google Scholar]
- Przepiera-Będzak, H.; Fischer, K.; Brzosko, M. Axial spondyloarthritis and inflammatory bowel disease: Association between disease activity and endothelial dysfunction markers. Rheumatol. Int. 2021, 42, 273–277. [Google Scholar] [CrossRef]
- Sanchez-Bilbao, L.; Martinez-Lopez, D.; Palmou-Fontana, N.; Armesto, S.; González-Gay, M.A.; Blanco, R. Ab0829 Inflammatory Bowel Disease in Psoriatic Arthritis. Study of 306 Patients from a Single Universitary Center. Prevalence, Clinical Features and Relationship to Biologic Therapy. Ann. Rheum. Dis. 2020, 79, 1719. [Google Scholar] [CrossRef]
- Jadon, D.R.; Sengupta, R.; Nightingale, A.; Lindsay, M.; Korendowych, E.; Robinson, G.; Jobling, A.; Shaddick, G.; Bi, J.; Winchester, R.; et al. Axial Disease in Psoriatic Arthritis study: Defining the clinical and radiographic phenotype of psoriatic spondyloarthritis. Ann. Rheum. Dis. 2017, 76, 701–707. [Google Scholar] [CrossRef]
- Rudwaleit, M.; Haibel, H.; Baraliakos, X.; Listing, J.; Märker-Hermann, E.; Zeidler, H.; Braun, J.; Sieper, J. The early disease stage in axial spondylarthritis: Results from the German Spondyloarthritis Inception Cohort. Arthritis Rheum. 2009, 60, 717–727. [Google Scholar] [CrossRef]
- Baraliakos, X.; Braun, J. Non-radiographic axial spondyloarthritis and ankylosing spondylitis: What are the similarities and differences? RMD Open 2015, 1, e000053. [Google Scholar] [CrossRef] [PubMed]
- Kopylov, U.; Starr, M.; Watts, C.; Dionne, S.; Girardin, M.; Seidman, E.G. Detection of Crohn Disease in Patients with Spondyloar-thropathy: The SpACE Capsule Study. J. Rheumatol. 2018, 45, 498–505. [Google Scholar] [CrossRef]
- Leirisalo-Repo, M.; Turunen, U.; Stenman, S.; Helenius, P.; Seppälä, K. High frequency of silent inflammatory bowel disease in spondylarthropathy. Arthritis Rheum. 1994, 37, 23–31. [Google Scholar] [CrossRef]
- De Vos, M.; Mielants, H.; Cuvelier, C.; Elewaut, A.; Veys, E. Long-term evolution of gut inflammation in patients with spondy-loarthropathy. Gastroenterology 1996, 110, 1696–1703. [Google Scholar] [CrossRef]
- de Winter, J.J.; van Mens, L.J.; van der Heijde, D.; Landewé, R.; Baeten, D.L. Prevalence of peripheral and extra-articular disease in ankylosing spondylitis versus non-radiographic axial spondyloarthritis: A meta-analysis. Arthritis Res. Ther. 2016, 18, 196. [Google Scholar] [CrossRef] [PubMed]
- Stolwijk, C.; Essers, I.; van Tubergen, A.; Boonen, A.; Bazelier, M.T.; De Bruin, M.L.; de Vries, F. The epidemiology of extra-articular manifestations in ankylosing spondylitis: A population-based matched cohort study. Ann. Rheum. Dis. 2015, 74, 1373–1378. [Google Scholar] [CrossRef] [PubMed]
- Jayasooriya, N.; Baillie, S.; Blackwell, J.; Bottle, A.; Petersen, I.; Creese, H.; Saxena, S.; Pollok, R.C.; POP-IBD Study Group. Systematic review with meta-analysis: Time to diagnosis and the impact of delayed diagnosis on clinical outcomes in inflammatory bowel disease. Aliment. Pharmacol. Ther. 2023, 57, 635–652. [Google Scholar] [CrossRef] [PubMed]
- Mow, W.S.; Lo, S.K.; Targan, S.R.; Dubinsky, M.C.; Treyzon, L.; Abreu-Martin, M.T.; Papadakis, K.A.; Vasiliauskas, E.A. Initial experience with wireless capsule enteroscopy in the diagnosis and management of inflammatory bowel disease. Clin. Gastroenterol. Hepatol. 2004, 2, 31–40. [Google Scholar] [CrossRef]
- Østgård, R.; Deleuran, B.; Dam, M.; Hansen, I.; Jurik, A.; Glerup, H. Faecal calprotectin detects subclinical bowel inflammation and may predict treatment response in spondyloarthritis. Scand. J. Rheumatol. 2017, 47, 48–55. [Google Scholar] [CrossRef]
- Olofsson, T.; Lindqvist, E.; Mogard, E.; Andréasson, K.; Marsal, J.; Geijer, M.; Kristensen, L.E.; Wallman, J.K. Elevated faecal calprotectin is linked to worse disease status in axial spondyloarthritis: Results from the SPARTAKUS cohort. Rheumatology 2019, 58, 1176–1187. [Google Scholar] [CrossRef]
- Poullis, A.; Foster, R.; Mendall, M.A.; Shreeve, D.; Wiener, K. Proton pump inhibitors are associated with elevation of faecal cal-protectin and may affect specificity. Eur. J. Gastroenterol. Hepatol. 2003, 15, 573–574; author reply 574. [Google Scholar] [CrossRef]
| Characteristics | axSpA N = 273 | PsA N = 267 |
|---|---|---|
| Sex (women), n (%) | 123 (45.1) | 143 (53.6) |
| Age (years), mean ± SD | 50.1 ± 12.6 | 54.5 ± 12.7 |
| Age at diagnosis (years), mean ± SD | 35.6 ± 12.1 | 44.9 ± 12.7 |
| Disease duration (years), median (IQR) | 14.2 (3.7; 22.4) | 7.5 (2.3; 14.3) |
| Smoking, n (%) | ||
| Never smoker | 126 (46.2) | 135 (50.6) |
| Former smoker | 98 (35.9) | 84 (31.5) |
| Current smoker | 49 (17.9) | 48 (17.9) |
| Alcohol use (yes), n (%) | 128 (46.9) | 93 (34.8) |
| Characteristics | axSpA N = 273 | PsA N = 267 |
| HLA-B27, positive/evaluable (%) | 195/240 (81.3) | 32/171 (18.7) |
| Disease activity | ||
| BASDAI, mean ± SD | 3.6 ± 2.3 | 3.6 ± 2.4 |
| DAPSA, mean ± SD | - | 10.4 ± 8.3 |
| ASDAS_CRP, mean ± SD | 2.3 ± 1.0 | 2.2 ± 1.1 |
| ASDAS_ESR, mean ± SD | 2.4 ± 1.0 | 2.4 ± 1.1 |
| Patient Global Assessment, mean ± SD | 4.4 ± 2.7 | 3.9 ± 2.7 |
| Treatments | ||
| NSAID, n (%) | 189 (69.2) | 104 (39.0) |
| DMARD, n (%) | 31 (11.4) | 191 (71.5) |
| Methotrexate | 11 (4.0) | 147 (55.1) |
| Leflunomide | 2 (0.7) | 24 (9.0) |
| Sulfasalazine | 20 (7.3) | 13 (4.9) |
| Apremilast | - | 10 (3.7) |
| PPI, n (%) | 106 (38.8) | 92 (34.5) |
| All Patients | Excluding NSAID Users | Excluding PPI Users | Excluding NSAID + PPI Users | |||||
|---|---|---|---|---|---|---|---|---|
| FC N; FC µg/g (IQR) | FC ≥ 80 µg/g N (%) | FC N; FC µg/g (IQR); p-Value | FC ≥ 80 µg/g N (%); p-Value | FC N; FC µg/g (IQR); p-Value | FC ≥ 80 µg/g N (%); p-Value | FC N; FC µg/g (IQR); p-Value | FC ≥ 80 µg/g N (%); p-Value | |
| Overall | 540; 56.5 (30.0–192.0) | 220 (40.7) | 414; 53.5 (30.0–157.5); 0.502 | 166 (40.1); 0.894 | 342; 40.0 (30.0–94.3); <0.001 | 92 (26.9); <0.001 | 277; 39.0 (30.0–93.5); <0.001 | 75 (27.1); <0.001 |
| axSpA | 273; 66.0 (30.0–222.5) | 117 (42.9) | 187; 60.0 (30.0–171.0); 0.582 | 78 (41.7); 0.807 | 167; 46.0 (30.0–123.0); 0.029 | 53 (31.7); 0.021 | 122; 46.0 (30.0–117.3); 0.032 | 39 (32.0); 0.045 |
| PsA | 267; 47.0 (30.0–169.0) | 103 (38.6) | 227; 49.0 (30.0–155.0); 0.785 | 88 (38.8); 0.966 | 175; 35.0 (30.0–71.0); 0.001 | 39 (22.3); <0.001 | 155; 35.0 (30.0–75.0); 0.001 | 36 (23.2); 0.001 |
| Variables | Univariable Odds Ratio (IC95%); p-Value | Multivariable Odds Ratio (IC95%); p-Value |
|---|---|---|
| Diagnostic EspAax (ref. PsA) | 1.194 (0.847–1.684); 0.312 | |
| Radiographic | 1.439 (0.992–2.088); 0.055 | |
| Not radiographic | 0.704 (0.407–1.220); 0.211 | |
| Disease duration (years) | 1.023 (1.008–1.039); 0.002 | 1.026 (1.008–1.044); 0.004 |
| Patient global assessment | 1.054 (0.988–1.124); 0.109 | |
| EQ-5D-5L (VAS) visual analog scale | 0.984 (0.975–0.993); <0.001 | |
| Laboratory values | ||
| CRP | 0.998 (0.977–1.020); 0.873 | |
| ESR | 1.015 (1.001–1.030); 0.033 | |
| HLAB27 positive | 1.203 (0.810–1.787); 0.360 | |
| Disease activity | ||
| TJC68 | 0.978 (0.908–1.054); 0.559 | |
| SJC66 | 0.991 (0.891–1.102); 0.866 | |
| ASDAS-CRP | 1.100 (0.898–1.348); 0.356 | |
| BASDAI | 1.007 (0.920–1.102); 0.882 | |
| ASDAS-ESR | 1.212 (0.981–1.497); 0.075 | |
| Treatments | ||
| NSAID | 1.085 (0.768–1.531); 0.644 | |
| DMARD | 1.310 (0.925–1.856); 0.128 | 1.522 (1.016–2.281); 0.042 |
| PPI | 4.969 (3.409–7.243); <0.001 | 5.152 (3.454–7.683); <0.001 |
| Type of SpA | Sex | Age (Years) | FC (µg/g) | CRP (mg/L) | Family History of IBD | IBD Symptoms | Colonoscopy Findings | Histology Findings | VCE | VCE Findings | IBD DX |
|---|---|---|---|---|---|---|---|---|---|---|---|
| r-axSpA | F | 46 | 38 | 4.10 | N | Y | N | - | Y | Y | CD |
| r-axSpA | M | 54 | 88 | 0.80 | N | N | Y | Not done | Y | Y | CD |
| nr-axSpA | F | 20 | 90 | 3.70 | N | N | N | - | Y | Y | CD |
| r-axSpA | M | 42 | 148 | 6.00 | N | N | Y | CII | N | - | CD |
| r-axSpA | M | 54 | 177 | 3.15 | Y | N | Y | CII | Y | Y | CD |
| r-axSpA | M | 48 | 186 | 0.75 | N | N | Y | CII | N | - | CD |
| r-axSpA | F | 75 | 265 | 0.40 | Y | N | Y | CII | N | - | CD |
| r-axSpA | M | 53 | 285 | 3.86 | N | Y | N | - | Y | Y | CD |
| r-axSpA | M | 52 | 306 | 22.30 | N | N | Y | CII | Y | Y | CD |
| nr-axSpA | F | 27 | 306 | 4.40 | N | N | Y | Cryptitis | N | - | UC |
| r-axSpA | M | 43 | 360 | 5.70 | N | Y | N | - | Y | Y | CD |
| r-axSpA | F | 56 | 464 | 0.98 | N | N | Y | CII, Erosion/ulceration | N | - | Unclassified |
| r-axSpA | M | 52 | 500 | 6.70 | N | N | Y | Erosion/ulceration | Y | Y | CD |
| r-axSpA | M | 54 | 547 | 0.40 | Y | N | Y | Not done | Y | Y | CD |
| r-axSpA | M | 48 | 664 | 9.59 | N | Y | Y | CII and AD | N | - | CD |
| r-axSpA | M | 69 | 958 | 0.12 | N | N | Y | AD | N | - | CD |
| r-axSpA | F | 51 | 974 | 10.40 | N | N | Y | AD | Y | Y | CD |
| r-axSpA | M | 61 | 1000 | 7.90 | N | Y | Y | Mucin depletion, AD, Erosion/ulceration, basal Plasmacytosis and AII | N | - | CD |
| r-axSpA | M | 68 | 1000 | 12.90 | Y | N | Y | Not done | Y | Y | CD |
| r-axSpA | M | 73 | 1000 | 4.10 | N | N | Y | AII and CII | N | - | CD |
| r-axSpA | M | 44 | 1000 | 10.20 | N | N | Y | AD | N | - | CD |
| r-axSpA | F | 48 | 1000 | 31.80 | N | Y | Y | Erosion/ulceration, AII and CII | N | - | CD |
| peripheralPsA | F | 50 | 72 | 3.60 | N | Y | Y | Granulomas, Erosion/ulceration | N | - | CD |
| Mixed PsA | M | 61 | 166 | 2.00 | N | N | Y | Not done | N | - | Unclassified |
| peripheralPsA | M | 54 | 186 | 35.00 | N | N | Y | CII | N | - | CD |
| Axial PsA | F | 64 | 467 | 5.12 | N | N | Y | AD and AII | N | - | CD |
| peripheralPsA | M | 56 | 484 | 2.00 | N | N | Y | Erosion/ulceration, AII and CII | N | - | Unclassified |
| peripheralPsA | F | 30 | 732 | 1.10 | N | N | Y | Not done | Y | Y | CD |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sanz-Sanz, J.; Gutiérrez-Casbas, A.; Plaza, Z.; Gratacós, J.; Rodríguez-Lago, I.; Marín-Jiménez, I.; Trujillo-Martín, E.; Pérez-Pampín, E.; Barreiro-de Acosta, M.; Hernández-Hernández, M.V.; et al. Prevalence of Undiagnosed Inflammatory Bowel Disease in Spondyloarthritis Patients. J. Clin. Med. 2025, 14, 4569. https://doi.org/10.3390/jcm14134569
Sanz-Sanz J, Gutiérrez-Casbas A, Plaza Z, Gratacós J, Rodríguez-Lago I, Marín-Jiménez I, Trujillo-Martín E, Pérez-Pampín E, Barreiro-de Acosta M, Hernández-Hernández MV, et al. Prevalence of Undiagnosed Inflammatory Bowel Disease in Spondyloarthritis Patients. Journal of Clinical Medicine. 2025; 14(13):4569. https://doi.org/10.3390/jcm14134569
Chicago/Turabian StyleSanz-Sanz, Jesús, Ana Gutiérrez-Casbas, Zulema Plaza, Jordi Gratacós, Iago Rodríguez-Lago, Ignacio Marín-Jiménez, Elisa Trujillo-Martín, Eva Pérez-Pampín, Manuel Barreiro-de Acosta, María Vanesa Hernández-Hernández, and et al. 2025. "Prevalence of Undiagnosed Inflammatory Bowel Disease in Spondyloarthritis Patients" Journal of Clinical Medicine 14, no. 13: 4569. https://doi.org/10.3390/jcm14134569
APA StyleSanz-Sanz, J., Gutiérrez-Casbas, A., Plaza, Z., Gratacós, J., Rodríguez-Lago, I., Marín-Jiménez, I., Trujillo-Martín, E., Pérez-Pampín, E., Barreiro-de Acosta, M., Hernández-Hernández, M. V., Carrillo-Palau, M., García-Vivar, M. L., Muñoz-Villafranca, M. C., Ladehesa-Pineda, M. L., Iglesias-Flores, E., Merino-Argumánez, C., González-Lama, Y., Arévalo-Salaet, M., Calvet, X., & Díaz-Gonzalez, F., on behalf of the EISER Collaborative Group. (2025). Prevalence of Undiagnosed Inflammatory Bowel Disease in Spondyloarthritis Patients. Journal of Clinical Medicine, 14(13), 4569. https://doi.org/10.3390/jcm14134569






