Abstract
Background/Objectives: The surgical management of trigeminal schwannomas (TSs) has evolved considerably, with increasing interest in minimally invasive approaches. We performed a meta-regression analysis to characterise temporal trends in surgical strategies for TS and to explore factors influencing outcomes. Methods: This systematic review and meta-regression followed the PRISMA 2020 guidelines. Comparative studies published in English reporting surgical treatment of TS were included. Outcomes assessed were the extent of resection (EOR), improvement or worsening of trigeminal symptoms, and postoperative complications. Meta-analyses of pooled frequencies were performed, and meta-regression analyses evaluated associations between surgical approach, tumour localization, year of publication, and outcomes. Surgical approaches were categorized as microsurgical antero-lateral (M-AL-Apr), retrosigmoid (RSA), endoscopic endonasal (EEA), and endoscopic transorbital (ETOA). Tumour localization was stratified using the Samii classification. Results: Fifteen studies (583 surgeries) were included. Endoscopic approaches accounted for 20.1% of cases, with increasing use over time (β = 0.12—p < 0.001), largely driven by transorbital access for Samii type A and C tumours. The use of M-AL-Apr declined. The pooled gross-total resection (GTR) rate was 73% (I2 = 78.8%). The stratified meta-regression identified a temporal decrease in GTR for Samii type C tumours alone, while resection rates for types A, B, and D remained stable, likely reflecting the increasing proportion of anatomically complex cases in recent series Trigeminal impairment improved postoperatively in 17% (I2 = 84.5%), while worsening of trigeminal symptoms was rare (β = 0.07%—I2 = 0%). Complication rates were 11.6% (I2 = 32.7%) but with a temporal increase (β = 0.041, p = 0.047). Tumour type was the dominant predictor of EOR, functional outcomes, and complications. Conclusions: Surgical management of TS has evolved towards minimally invasive techniques, particularly endoscopic routes, reflecting advances in technology and a focus on functional preservation. Tumour anatomy remains the key determinant of surgical outcomes, highlighting the importance of tailored, anatomy-driven surgical planning.
1. Introduction
Trigeminal schwannomas (TS) are rare, typically benign tumours originating from the Schwann cells of the fifth cranial nerve. Accounting for approximately 0.8% to 8% of all intracranial schwannomas, these lesions can arise along any portion of the trigeminal pathway, from the root entry zone at the brainstem to the extracranial peripheral branches [1]. (Figure 1) Due to their anatomical variability and frequent proximity to critical neurovascular structures of the skull base, surgical resection remains complex. Although stereotactic radiosurgery may be an option for selected small or asymptomatic tumours, surgical resection is often the treatment of choice for symptomatic or enlarging lesions [2].
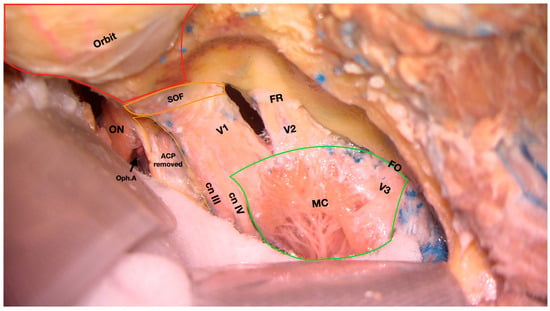
Figure 1.
Cadaveric dissection showing the middle cranial fossa following removal of the superior and lateral walls of the right orbit. Meckel’s cave is exposed following the removal of the periosteal layer of the dura mater. ON, optic nerve; Oph.A, ophthalmic artery; ACP, anterior clinoid process; cn III, cranial nerve III; cn IV, cranial nerve IV; V1, first branch of the trigeminal nerve; V2, second branch of the trigeminal nerve; V3, third branch of the trigeminal nerve; FR, foramen rotundum; FO, foramen ovale. SOF, superior orbital fissure; MC, Meckel’s cave. (First author’s dissection).
Historically, TSs have been managed through traditional craniotomy-based approaches, including posterior routes (retrosigmoid), and lateral approaches such as subtemporal, frontotemporal (pterional), and fronto-orbitozygomatic (FOZ) variants [3]. These techniques offer wide exposure and the ability to manage tumours involving the middle and posterior cranial fossae. However, they often require significant brain retraction and manipulation of surrounding structures, with associated risks of postoperative morbidity. Despite refinements in microsurgical technique, these approaches are inherently invasive and may not be optimal for all tumour locations or patient profiles [4].
In recent years, minimally invasive strategies have gained increasing attention, particularly the endoscopic endonasal (EEA) and endoscopic transorbital (ETOA) approaches. While the EEA route offers a direct midline trajectory to lesions involving Meckel’s cave and the central skull base, the ETOA has emerged as a versatile and promising option for lesions involving the antero-lateral skull base, cavernous sinus, and middle fossa [5]. The ETOA route, in particular, allows for lateral access with minimal brain retraction and improved cosmetic outcomes, making it an increasingly attractive alternative or complement to conventional approaches [6,7,8]. Nonetheless, both endoscopic techniques come with their own set of challenges, including restricted working corridors, demanding reconstruction, and a steep learning curve [9]. Comparative data on indications, the extent of resection, functional outcomes, and complication rates are limited.
This systematic review and meta-analysis primarily aimed to investigate whether a shift in the surgical management strategy of TSs has occurred over time. Specifically, it assessed temporal trends in the adoption of various surgical approaches, including both traditional open craniotomies and more recent endoscopic techniques. Secondarily, the study evaluated whether different treatment strategies over time have resulted in variations in the extent of resection (EOR), complication rates, and functional outcomes. By synthesizing current evidence, this analysis seeks to clarify the evolving role of each surgical corridor and guide decision-making based on anatomical, pathological, and temporal considerations (Figure 1).
2. Materials and Methods
2.1. Study Design and Eligibility Criteria
This systematic review and meta-analysis were conducted in accordance with the PRISMA 2020 guidelines [10].
Eligible studies were observational in design and reported on surgical series of patients with TSs undergoing resection via either traditional open craniotomy approaches (e.g., subtemporal, frontotemporal, fronto-orbitozygomatic [FOZ], or retrosigmoid [RSA]) or endoscopic techniques (EEA or ETOA). Studies were required to provide information on at least one of the outcomes of interest and to report the surgical approach used.
To ensure consistency in anatomical comparison, tumour localization was classified according to the Samii classification, which stratifies TSs into four types based on their anatomical extension: Type A (middle fossa), Type B (posterior fossa), Type C (dumbbell-shaped, involving both compartments) and Type D (extracranial extension) [11] (Figure 2).
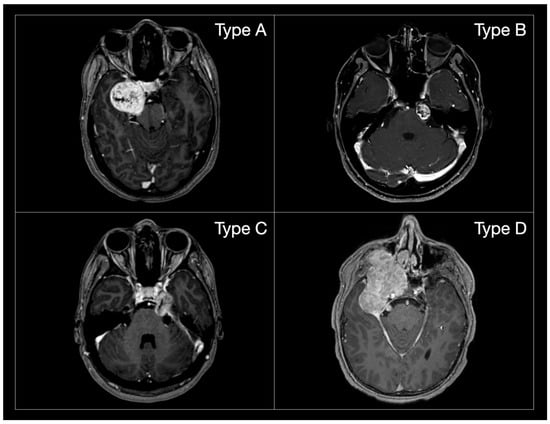
Figure 2.
MR post-gadolinium T1 axial images of the different Samii TS subtypes. (Type A): tumours mainly extending in the middle cranial fossa; (Type B): tumours mainly extending in the posterior cranial fossa; (Type C): dumbbell tumours; (Type D): tumours extending in the extra-cranial compartment.
No restrictions were placed on the study period or language, provided that an English version was available. Case reports and small case series (less than 10 cases), reviews, editorials, and conference abstracts were excluded.
2.2. Literature Search and Study Selection
A comprehensive literature search was conducted across MEDLINE (PubMed), Embase, Scopus, and Web of Science, using combinations of the following MeSH terms and free-text keywords: “trigeminal schwannoma”, “trigeminal nerve tumour”, “skull base surgery”, “endoscopic endonasal”, “transorbital”, “craniotomy”, “retrosigmoid”, “subtemporal”, “fronto-orbitozygomatic”, and “surgical outcomes”. References of included studies were also screened to identify additional eligible articles. The final search update was performed in May 2025.
2.3. Data Extraction
Two authors (EP and GF) independently screened titles, abstracts, and full texts for inclusion. Any discrepancies were resolved by consensus. Data extraction was also performed independently by the same reviewers, using a predefined template. Extracted variables included study characteristics (author, year of publication, number of patients), tumour distribution according to Samii classification, the surgical approach used (EEA, ETOA, RSA, and microsurgical antero-lateral approaches [M-AL-Apr] [such as pre- and subtemporal, FOZ, and Kawase approaches]), and surgical outcomes.
2.4. Outcomes
The primary outcomes were EOR (gross-total [GTR] and subtotal [STR]), the presence of trigeminal impairment (hypoesthesia, dysesthesia, or facial pain) before and after surgery, changes in trigeminal symptoms (either improvement or worsening), and the rate of postoperative complications (cranial nerve deficits, CSF leak, infection, vascular injury, brain-retraction-related complications).
In order to explore whether surgical practice and outcomes have changed over time and whether they are influenced by tumour type or surgical approach, we constructed a comprehensive dataset that included the proportion of patients treated with each surgical approach, as well as the distribution of Samii tumour types within each study. We also quantified the percentage of each Samii tumour type over time and visualized their temporal evolution using an alluvial diagram to assess whether more challenging tumour subtypes have become more commonly treated in recent years.
2.5. Statistical Analysis
For each outcome, pooled incidence rates were estimated using random-effects meta-analysis of proportions (GLMM method). Statistical heterogeneity was quantified using the I2 and τ2 statistics. Forest plots were used to display the pooled effects, the confidence intervals (CI), and the estimated measures of inter-study heterogeneity.
To investigate whether outcomes varied according to surgical approach, tumour type, or publication year, meta-regression analyses were conducted using binomial generalized linear models (GLMs). Separate univariable models were first fitted to assess the influence of each predictor individually. Subsequently, multivariable models were constructed incorporating all key variables, namely surgical approach, Samii tumour type, and year of publication. The significance of each factor within the multivariable framework was assessed using likelihood ratio tests.
To explore if any variation in GTR rates was driven by specific tumour subtypes, we performed subgroup analyses stratified by dominant Samii type. For each group, GTR rates were modelled over time using GLMs with year quartile as a categorical variable, and the effect of time was evaluated using likelihood ratio testing.
No imputation was performed. Studies reporting zero events were included in the analyses as such. All statistical analyses were conducted in R software 4.2.2 (R Foundation for Statistical Computing, Vienna, Austria; https://www.r-project.org/ [accessed on 31 May 2025]).
2.6. Risk of Bias
Risk of bias was assessed independently by two reviewers (EP and GF). The Joanna Briggs Institute (JBI) checklist for case series and cohort studies was used [12]. Studies scoring ≥ 7 were considered methodologically acceptable. Discrepancies were resolved through consensus.
2.7. Assessment of Reporting Bias and Sensitivity Analysis
Influence analyses were conducted to assess whether individual studies disproportionately affected pooled results. Sensitivity analyses excluded studies at high risk of bias or those lacking stratified outcomes.
3. Results
3.1. Study Selection and Characteristics
A total of 15 studies was included in this meta-analysis [3,4,6,11,13,14,15,16,17,18,19,20,21,22,23], encompassing 583 patients who underwent surgeries for TS. The studies spanned publication years 1988 to 2025, with cohort sizes ranging from 12 to 96 patients. The PRISMA flow diagram is provided in Figure 3.
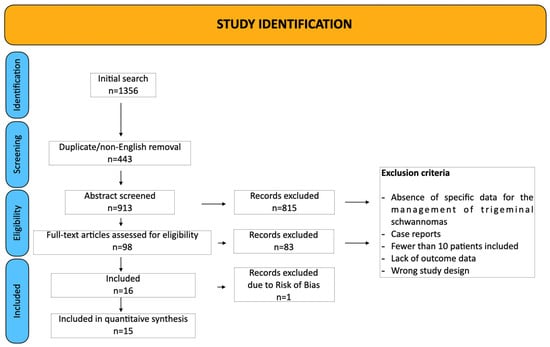
Figure 3.
PRISMA chart.
The distribution of Samii tumour types was as follows: type A (22%), type B (11%), type C (42%), and type D (25%). Surgical approaches included M-AL Apr (64.2%, n = 374), RSA (11.3%, n = 66), ETOA (10.5%, n = 61), EEA (9.6%, n = 56), combined/two-stage approaches (Comb/2sps; 3.9%, n = 23), and the trans-maxillary approach (TMA; 0.5%, n = 3) (Table 1). Follow-up time was variable across the studies included (48.8 ± 52.6 months).

Table 1.
General data of the studies included.
3.2. Choice of Surgical Approaches and Evolution over Time
Meta-regression confirmed that Samii tumour type significantly determined the choice of approach (p < 0.001). EEA or ETOA was adopted in 40.7% of Samii type A tumours, 2.6% of type B, 21.1% of type C, and 37.9% of type D. M-AL approaches predominated for type C tumours (73.4%), and RSA was the preferred approach for type B tumours (68.4%). This distribution is illustrated in Figure 4 and Table 2.
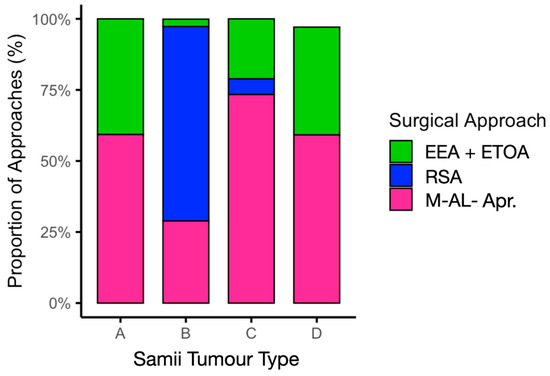
Figure 4.
Surgical approach distribution by Samii tumour type. M-AL-Apr, microsurgical antero-lateral approaches.

Table 2.
Surgical approach distribution by Samii tumour type.
The use of endoscopic approaches increased significantly over time (β = +0.12, 95% CI 0.07–0.17, p < 0.001), corresponding to an estimated +12.7% relative increase per year. In contrast, the use of M-AL-Apr declined significantly (β = −0.13, 95% CI −0.21 to −0.06, p < 0.001), corresponding to a −12.2% relative decrease per year. The use of RSA remained stable (β = +0.01, 95% CI −0.02 to +0.04, p = 0.47) (Figure 5).
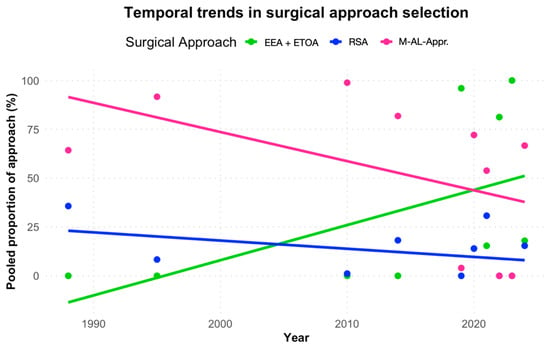
Figure 5.
Surgical approach distribution over time. M-AL-Apr, microsurgical antero-lateral approaches.
To contextualize the shifting surgical strategies, we also examined how the proportion of Samii tumour types evolved across the included studies. The alluvial plot (Figure 6) illustrates a gradual increase in the relative frequency of Samii type C and D tumours over the past three decades, whereas type A tumours declined in more recent years.
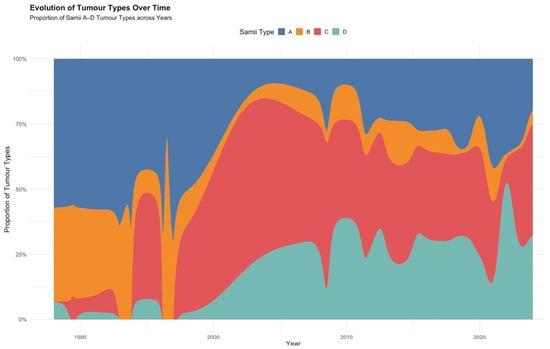
Figure 6.
Alluvial diagram showing the evolution of Samii tumour types over time.
3.3. Extent of Resection Outcomes
The pooled rate of GTR was 73.2% (95% CI 63.4–81.2%; I2 = 78.8%; Figure 6). Meta-regression showed a decrease in GTR rates over time (β = −0.054, 95% CI −0.087 to −0.022, p < 0.001), corresponding to a 5.3% relative reduction per year.
In multivariable analysis, RSA use (β = −13.66, p < 0.001) and higher proportions of Samii type B (β = −42.33, p = 0.010), type C (β = −59.75, p = 0.002) and type D tumours (β = −60.30, p = 0.002) were independently associated with lower likelihood of achieving GTR (Supplementary Table S1).
To further investigate whether the observed reduction in GTR rates was uniformly distributed across tumour types, we analysed GTR trends stratified by Samii classification. While no significant change was detected for Samii types B and D, and only marginal changes for type A, a marked and statistically significant decline in GTR was observed in Samii type C tumours (p < 0.001; Supplementary Table S2).
The pooled rate of subtotal resection (STR) was 6.5% (95% CI 2.7–14.9%; I2 = 76.0%). STR rates increased significantly over time (β = +0.067, 95% CI +0.024 to +0.116, p = 0.004), corresponding to a 6.9% relative increase per year (Figure 7A,B).
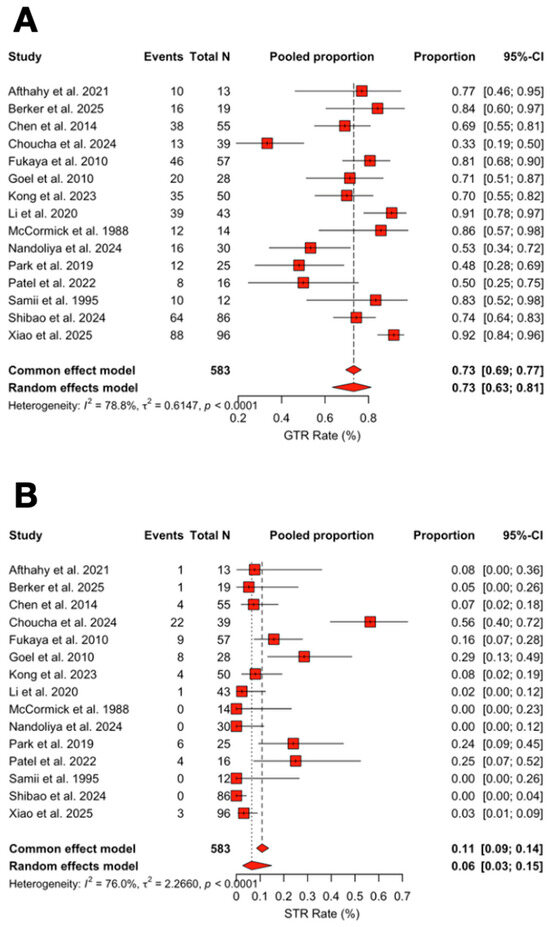
Figure 7.
(A) Forest plot showing GTR rate across studies included. (B) Forest plot showing STR rate across studies included [3,4,6,11,13,14,15,16,17,18,19,20,21,22,23]. Red refers to Extent of Resection rates. The overall meta-analysed measure of effect is represented on the plot as a dashed vertical line. Squares represent individual study effect sizes (with relative solid lines representing confidence intervals). Diamonds summarize the overall pooled effect and its confidence interval.
3.4. Functional Outcomes
The pooled rate of preoperative trigeminal symptoms was 72.1% (95% CI 61.6–80.6%; I2 = 56.4%). The pooled rate of postoperative trigeminal impairment was 46.5% (95% CI 22.9–71.7%; I2 = 75.3%).
Improvement rates of trigeminal symptoms (delta improved) were 17.0% (95% CI 7.1–35.6%; I2 = 84.5%), while their worsening accounted for 0.07% of cases (95% CI 0–6.5%; I2 = 0%) (Figure 8A–C).
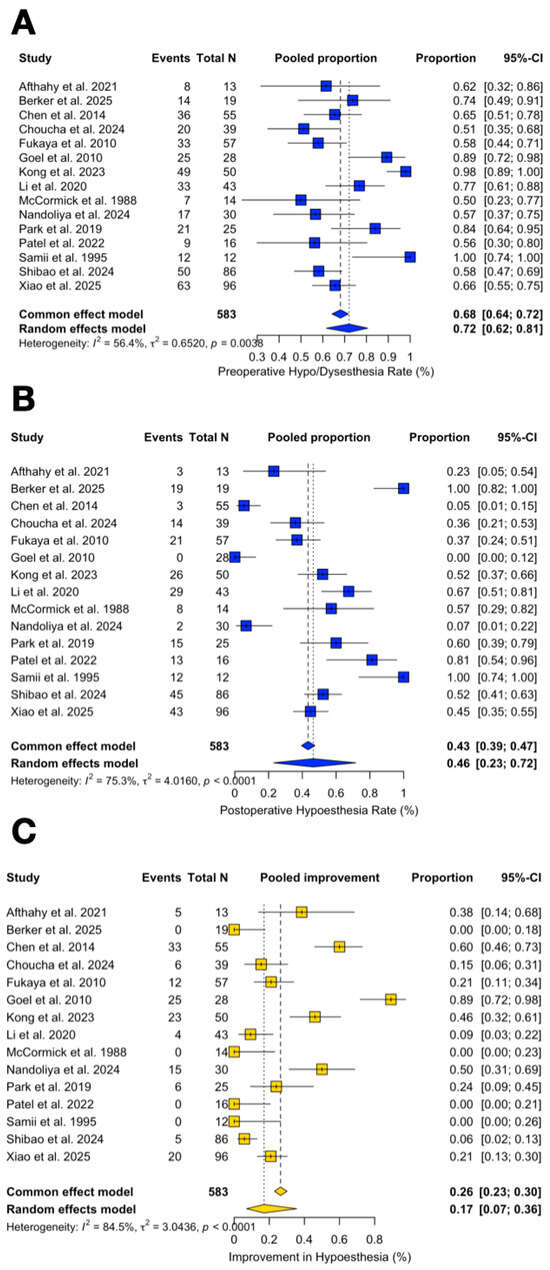
Figure 8.
Forest plot showing the preoperative (A) and postoperative (B) trigeminal impairment rate across studies included and its improvement rate (C) [3,4,6,11,13,14,15,16,17,18,19,20,21,22,23]. Blue refers to trigeminal impairment. Yellow refers to improvement rate. The overall meta-analysed measure of effect is represented on the plot as a dashed vertical line. Squares represent individual study effect sizes (with relative solid lines representing confidence intervals). Diamonds summarize the overall pooled effect and its confidence interval.
Meta-regression revealed that, compared to Samii type A, improvement was significantly more likely in tumours with higher proportions of Samii type B (β = +6.36, p = 0.007) and type D (β = +4.70, p < 0.001), while no significant difference was observed for type C tumours (p = 0.37) (Supplementary Tables S3 and S4).
No significant relationship was observed between year of surgery and improvement rates (β = +0.003, p = 0.83).
Although a full model including endoscopic, RSA, and M-AL-Apr was fitted, multicollinearity precluded reliable interpretation of individual surgical approaches (Supplementary Table S5).
3.5. Complications
The pooled rate of complications was 11.6% (95% CI 6.7–19.4%; I2 = 32.7%). Meta-regression showed an increasing trend in complications over time (β = +0.041, 95% CI +0.001 to +0.089, p = 0.066), corresponding to an estimated +4.2% relative increase per year.
In multivariable analysis, higher proportions of Samii type A (β = +103.04, p = 0.004), type C (β = +112.69, p = 0.002), and type D (β = +96.04, p = 0.007) tumours were independently associated with increased complication rates. No surgical approach remained independently associated with complication rates after adjustment (Figure 9).
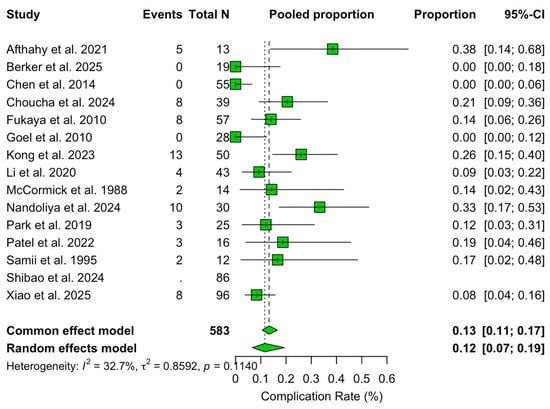
Figure 9.
Forest plot showing the complication rate across studies included [3,4,6,11,13,14,15,16,17,18,19,20,21,22,23]. Green refers to complication rate. The overall meta-analysed measure of effect is represented on the plot as a dashed vertical line. Squares represent individual study effect sizes (with relative solid lines representing confidence intervals). Diamonds summarize the overall pooled effect and its confidence interval.
The studies included did not report any cases of mortality.
A breakdown of major postoperative complications is reported in Table 3.

Table 3.
Major postoperative complications.
3.6. Quality Assessment
Using the JBI checklist for case series, 15 studies were assessed as having a low risk of bias and were therefore included. One study was excluded due to a high risk of bias [24] (Table 4).

Table 4.
Risk of bias—JBI checklist for case series.
4. Discussion
This meta-analysis provides a comprehensive synthesis of surgical outcomes in trigeminal schwannoma resection, drawing on over 580 cases reported in contemporary series. It elucidates evolving operative strategies, predictors of oncological and functional outcomes, and the delicate balance between efficacy and safety in this rare disease [11,16].
A clear temporal trend toward endoscopic techniques was observed, mirroring the integration of ETOAs into modern skull base surgery. Most frequently employed for Samii type A tumours, typically those involving the middle cranial fossa and lateral skull base, ETOAs provide direct access to regions such as the lateral cavernous sinus and Meckel’s cave with minimal brain retraction. Their use in type B tumours remains limited due to posterior fossa confinement; RCA remains the mainstay. For dumbbell-shaped type C tumours and extracranial-extending type D tumours, ETOAs may be selectively combined with other routes to enhance access while minimizing morbidity. These nuanced applications underscore the importance of tailoring surgical strategy to tumour anatomy. More broadly, this evolution reflects not only a refined surgical philosophy centred on anatomical preservation and functional protection, but also the growing adoption of less invasive approaches made possible by advances in endoscopic technology without compromising oncological control [11,25,26].
The stratified meta-regression identified a temporal decrease in GTR for Samii type C tumours alone, while resection rates for types A, B, and D remained stable. While no significant change occurred for Samii types B and D, and only marginal changes were seen in type A, a marked and statistically significant decline in GTR was observed in type C tumours. This trend likely reflects the increasing proportion of anatomically complex cases in recent series, rather than a general reduction in surgical ambition (see Figure 6). Concurrently, advancements in adjuvant therapies have encouraged a paradigm shift: preserving function may at times take precedence over radical resection, particularly in high-risk cases [6,17,27,28]. Within this framework, STR has gained broader acceptance. The availability of stereotactic radiosurgery (SRS) as an effective adjuvant tool has enabled deliberate conservative resections in select cases, allowing for excellent disease control while mitigating neurological risk. This strategy exemplifies an individualized, multidisciplinary approach to TS management [2].
Surgical treatment remains highly effective in alleviating trigeminal dysfunction. Improvement in pre-existing symptoms was frequently achieved, underscoring the potential for surgery to offer both oncological and symptomatic benefits. Yet, tumour type continues to shape these outcomes. Specifically, higher proportions of Samii type B and D tumours were significantly associated with improved outcomes, whereas type A and especially type C tumours, often more invasive or anatomically complex, were less likely to demonstrate postoperative recovery. These results reinforce the primacy of tumour-specific planning in predicting neurological improvement, independent of surgical era or technique [6,17,27,29,30].
Interestingly, we observed a temporal rise in reported complications, despite the increasing adoption of minimally invasive approaches. This paradox is best explained by the concurrent increase in anatomically complex tumours, again underscoring tumour type as the key driver of morbidity. Importantly, the surgical approach per se was not independently associated with complication risk. This reinforces that approach selection should be dictated by anatomical suitability rather than a priori assumptions about safety profiles [6,11,17,19]. Notably, when specific attention was given to complications associated with individual surgical approaches, an increased incidence of oculomotor impairment was observed with the use of the ETOA, and a higher rate of CSF leakage was noted with the EEA. Conversely, endoscopic techniques were associated with a reduced prevalence of brain retraction-related complications compared to traditional open approaches [6,17,20].
Limitations
This study has several limitations. First, the included literature consisted predominantly of retrospective observational series, carrying inherent risks of selection bias and heterogeneity in reporting. Although we applied rigorous eligibility criteria and conducted meta-regression analyses to explore sources of heterogeneity, residual confounding cannot be excluded. Second, the lack of direct comparative studies between surgical approaches precluded the ability to perform pairwise comparisons with relative risks; as such, our inferences rely on meta-regression, which, while informative, does not replace direct comparative evidence. Third, the classification of surgical approaches and tumour types, although standardised for analysis, may vary across centres and publications. Fourth, the progressive incorporation of adjunctive therapies over time could influence surgical decision-making and outcomes but was not consistently reported across studies. Fifth, the extreme variation in follow-up durations across studies and the lack of standardised timing for postoperative trigeminal impairment assessments limit the ability to differentiate between early transient deficits and long-term functional outcomes. Sixth, due to the highly specialized nature of this pathology, additional sources such as select book chapters are available; however, these were excluded from the analysis owing to the absence of data specifically pertinent to the objectives of this meta-regression [31,32]. Finally, the low event rate for certain outcomes, particularly complication subtypes, limited the possibility to analyse them systematically.
5. Conclusions
This meta-analysis delineates evolving trends and key predictors in the surgical management of trigeminal schwannomas. While the adoption of minimally invasive approaches has expanded, the apparent decline in resection rates and rise in complications reflect an increasing anatomical complexity in modern surgical series. Tumour type remains a pivotal determinant of resection extent, symptomatic recovery, and complication risk, underscoring the need for personalized, anatomy-guided surgical planning. These findings may inform future refinements in surgical decision-making and support the ongoing evolution of patient-centred care in skull-base surgery.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/jcm14134488/s1, Table S1: Multivariable Meta-Regression Results for GTR; Table S2: Meta-regression by association of GTR and Samii Type over Year Quartiles; Table S3: Multivariable Model for Trigeminal Symptom Improvement; Table S4: Predictors of Trigeminal Function Improvement; Table S5: Meta-regression: Functional Improvement by Surgical Approach.
Author Contributions
Conceptualization, E.P. and G.F.; methodology, G.F. and E.P.; validation, E.P., G.F. and C.C.; formal analysis, G.F.; investigation, E.P.; writing—original draft preparation, E.P. and G.F.; writing—review and editing, G.A.B., H.J.M., M.S. and M.B.; visualization, G.F.; supervision, F.D. and M.L. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Italian Ministry of Health (RRC). H. J. Marcus is part-funded by the UCLH/UCL NIHR Biomedical Research Centre.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
H. J. Marcus is employed by Panda Surgical and holds shares in the company. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Abbreviations
| CI | Confidence interval |
| Comb./2sps | Combined approach or two-step surgical strategy |
| EEA | Endoscopic endonasal approach |
| ETOA | Endoscopic transorbital approach |
| FOZ | Fronto-orbitozygomatic |
| GLM | General linear model |
| GTR | Gross-total resection |
| M-AL-Appr | Microsurgical antero-lateral approaches |
| NTR | Near-total resection |
| RSA | Retrosigmoid approach |
| SRS | Stereotactic radiosurgery |
| STR | Subtotal resection |
| TMA | Trans-maxillary approach |
| TS | Trigeminal schwannoma |
References
- Srinivas, D.; Somanna, S.; Ashwathnarayana, C.B.; Bhagavatula, I.D. Multicompartmental trigeminal schwannomas: Management strategies and outcome. Skull Base 2011, 21, 351–358. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Zhang, J.; Yu, X.; Qi, S.; Du, Y.; Ni, W.; Hu, Y.; Tian, Z. Stereotactic radiosurgery for trigeminal schwannoma: A clinical retrospective study in 52 cases. Stereotact. Funct. Neurosurg. 2013, 91, 236–242. [Google Scholar] [CrossRef]
- Fukaya, R.; Yoshida, K.; Ohira, T.; Kawase, T. Trigeminal schwannomas: Experience with 57 cases and a review of the literature. Neurosurg. Rev. 2010, 34, 159–171. [Google Scholar] [CrossRef] [PubMed]
- Aftahy, A.K.; Groll, M.; Barz, M.; Wagner, A.; Lange, N.; Butenschön, V.M.; Delbridge, C.; Bernhardt, D.; Meyer, B.; Negwer, C.; et al. Surgical Outcome of Trigeminal Schwannomas. Cancers 2021, 13, 1310. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, F.V.; Sousa, M.P.; Palavani, L.B.; Andreão, F.F.; Neto, A.R.; Pereira, M.A.O.M.; Fukunaga, C.K.; Paleare, L.F.F.; Montecino, L.M.; Cabral, S.G.; et al. Endonasal endoscopic surgical approach for treating trigeminal schwannomas: A systematic review and meta-analysis. Neurochirurgie 2025, 71, 101676. [Google Scholar] [CrossRef]
- Park, H.H.; Hong, S.D.; Kim, Y.H.; Hong, C.-K.; Woo, K.I.; Yun, I.-S.; Kong, D.-S. Endoscopic transorbital and endonasal approach for trigeminal schwannomas: A retrospective multicenter analysis (KOSEN-005). J. Neurosurg. 2020, 133, 467–476. [Google Scholar] [CrossRef]
- Corrivetti, F.; de Notaris, M.; Seneca, V.; Di Nuzzo, G.; Catapano, G. Is It Time for a Paradigm Shift in the Surgical Management of Trigeminal Schwannomas? Evaluating the Role of the Transorbital Approach: An Anatomo-Clinical Study and Systematic Literature Review. World Neurosurg. 2024, 190, e1025–e1037. [Google Scholar] [CrossRef]
- de Notaris, M.; Kong, D.-S.; Di Somma, A.; Enseñat, J.; Hong, C.-K.; Moe, K.; Schwartz, T.H. Superior eyelid transorbital approaches: A modular classification system. J. Neurosurg. 2024, 141, 278–283. [Google Scholar] [CrossRef]
- Niranjan, A.; Barnett, S.; Anand, V.; Agazzi, S. Multimodality Management of Trigeminal Schwannomas. J. Neurol. Surg. B Skull Base 2016, 77, 371–378. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Samii, M.; Migliori, M.M.; Tatagiba, M.; Babu, R. Surgical treatment of trigeminal schwannomas. J. Neurosurg. 1995, 82, 711–718. [Google Scholar] [CrossRef] [PubMed]
- Munn, Z.; Barker, T.H.; Moola, S.; Tufanaru, C.; Stern, C.; McArthur, A.; Stephenson, M.; Aromataris, E. Methodological quality of case series studies: An introduction to the JBI critical appraisal tool. JBI Evid. Synth. 2020, 18, 2127–2133. [Google Scholar] [CrossRef] [PubMed]
- Berker, B.B.; Güngör, A.; Doğruel, Y.; Rahmanov, S.; Türe, H.; Türe, U. Endoscope Assisted Microsurgical Removal of Trigeminal Schwannomas. World Neurosurg. 2025, 199, 124098. [Google Scholar] [CrossRef]
- Chen, L.-F.; Yang, Y.; Yu, X.-G.; Gui, Q.-P.; Bu, B.; Xu, B.-N.; Zhou, D.-B. Operative management of trigeminal neuromas: An analysis of a surgical experience with 55 cases. Acta Neurochir. 2014, 156, 1105–1114. [Google Scholar] [CrossRef]
- Choucha, A.; Troude, L.; Morin, L.; Fernandes, S.; Baucher, G.; De Simone, M.; Lihi, A.; Mazen, K.; Alseirihi, M.; Passeri, T.; et al. Management of large Trigeminal Schwannoma: Long-term oncologic and functional outcome from a multicentric retrospective cohort. Acta Neurochir. 2024, 166, 440. [Google Scholar] [CrossRef]
- Goel, A.; Shah, A.; Muzumdar, D.; Nadkarni, T.; Chagla, A. Trigeminal neurinomas with extracranial extension: Analysis of 28 surgically treated cases. J. Neurosurg. 2010, 113, 1079–1084. [Google Scholar] [CrossRef]
- Kong, D.-S.; Kim, Y.H.; Lee, W.-J.; Kim, Y.-H.; Hong, C.-K. Indications and outcomes of endoscopic transorbital surgery for trigeminal schwannoma based on tumor classification: A multicenter study with 50 cases. J. Neurosurg. 2023, 138, 1653–1661. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Wang, X.; Chen, G.; Liang, J.; Guo, H.; Song, G.; Bao, Y. Trigeminal schwannoma: A single-center experience with 43 cases and review of literature. Br. J. Neurosurg. 2021, 35, 49–56. [Google Scholar] [CrossRef]
- McCormick, P.C.; Bello, J.A.; Post, K.D. Trigeminal schwannoma. Surgical series of 14 cases with review of the literature. J. Neurosurg. 1988, 69, 850–860. [Google Scholar] [CrossRef]
- Nandoliya, K.R.; Vignolles-Jeong, J.; Karras, C.L.; Govind, S.; Finger, G.; Thirunavu, V.; Sonabend, A.M.; Magill, S.T.; Prevedello, D.M.; Chandler, J.P. Clinical characteristics and outcomes after trigeminal schwannoma resection: A multi-institutional experience. Neurosurg. Rev. 2024, 47, 340. [Google Scholar] [CrossRef]
- Shibao, S.; Yoshida, K.; Ueda, R.; Toda, M. Risk factors of postoperative trigeminal neuropathy in surgical treatment of trigeminal schwannomas. Acta Neurochir. 2024, 166, 387. [Google Scholar] [CrossRef]
- Xiao, Q.; Peng, H.; Tang, G.; Yuan, J.; Peng, G.; Zhang, C.; Wang, X.; Huang, W.; Qin, C.; Liu, Q. Individual surgical management of trigeminal schwannomas guided by an extended classification: A consecutive series of 96 clinical cases at a single institution. Neurosurg. Rev. 2025, 48, 289. [Google Scholar] [CrossRef]
- Patel, V.A.; Polster, S.P.; Abou-Al-Shaar, H.; Kalmar, C.L.; Zenonos, G.A.; Wang, E.W.; Gardner, P.A.; Snyderman, C.H. Trigeminal Schwannoma: A Retrospective Analysis of Endoscopic Endonasal Management, Treatment Outcomes, and Neuropathic Sequelae. J. Neurol. Surg. B Skull Base 2023, 84, 444–451. [Google Scholar] [CrossRef] [PubMed]
- Bordi, L.; Compton, J.; Symon, L. Trigeminal neuroma. A report of eleven cases. Surg. Neurol. 1989, 31, 272–276. [Google Scholar] [CrossRef]
- Raza, S.M.; Donaldson, A.M.; Mehta, A.; Tsiouris, A.J.; Anand, V.K.; Schwartz, T.H. Surgical management of trigeminal schwannomas: Defining the role for endoscopic endonasal approaches. Neurosurg. Focus 2014, 37, E17. [Google Scholar] [CrossRef] [PubMed]
- Mariniello, G.; Cappabianca, P.; Buonamassa, S.; de Divitiis, E. Surgical treatment of intracavernous trigeminal schwannomas via a fronto-temporal epidural approach. Clin. Neurol. Neurosurg. 2004, 106, 104–109. [Google Scholar] [CrossRef]
- Di Somma, A.; Andaluz, N.; Cavallo, L.M.; Topczewski, T.E.; Frio, F.; Gerardi, R.M.; Pineda, J.; Solari, D.; Enseñat, J.; Prats-Galino, A.; et al. Endoscopic transorbital route to the petrous apex: A feasibility anatomic study. Acta Neurochir. 2018, 160, 707–720. [Google Scholar] [CrossRef]
- Shin, S.S.; Gardner, P.A.; Stefko, S.T.; Madhok, R.; Fernandez-Miranda, J.C.; Snyderman, C.H. Endoscopic endonasal approach for nonvestibular schwannomas. Neurosurgery 2011, 69, 1046–1057; discussion 1057. [Google Scholar] [CrossRef] [PubMed]
- Yoo, J.; Park, H.H.; Yun, I.S.; Hong, C.K. Clinical applications of the endoscopic transorbital approach for various lesions. Acta Neurochir. 2021, 163, 2269–2277. [Google Scholar] [CrossRef]
- Kassam, A.B.; Prevedello, D.M.; Carrau, R.L.; Snyderman, C.H.; Gardner, P.; Osawa, S.; Seker, A.; Rhoton, A.L. The front door to meckel’s cave: An anteromedial corridor via expanded endoscopic endonasal approach- technical considerations and clinical series. Neurosurgery 2009, 64 (Suppl. S3), ons71–ons82; discussion ons82–ons83. [Google Scholar] [CrossRef]
- Dolenc, V.V.; Rogers, L. Microsurgical Anatomy and Surgery of the Central Skull Base; Springer: Wien, Austria, 2003. [Google Scholar]
- Dolenc, V.; Rogers, L. Cavernous Sinus: Developments and Future Perspectives; Springer: New York, NY, USA, 2009; pp. 1–227. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).