Effects of Cardiac Contractility Modulation on Right Ventricular and Left Atrial Strain in Patients with Chronic Heart Failure
Abstract
1. Background
2. Methods
3. Statistics
4. Results
Effects of CCM Therapy
5. Discussion
Limitations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chan, J.; Shiino, K.; Obonyo, N.G.; Hanna, J.; Chamberlain, R.; Small, A.; Scalia, I.G.; Scalia, W.; Yamada, A.; Hamilton-Craig, C.R.; et al. Left Ventricular Global Strain Analysis by Two-Dimensional Speckle-Tracking Echocardiography: The Learning Curve. J. Am. Soc. Echocardiogr. 2017, 30, 1081–1090. [Google Scholar] [CrossRef] [PubMed]
- Ayach, B.; Fine, N.M.; Rudski, L.G. Right ventricular strain: Measurement and clinical application. Curr. Opin. Cardiol. 2018, 33, 486–492. [Google Scholar] [CrossRef]
- Muraru, D.; Haugaa, K.; Donal, E.; Stankovic, I.; Voigt, J.U.; Petersen, S.E.; Popescu, B.A.; Marwick, T. Right ventricular longitudinal strain in the clinical routine: A state-of-the-art review. Eur. Heart J. Cardiovasc. Imaging 2022, 23, 898–912. [Google Scholar] [CrossRef]
- Galderisi, M.; Cosyns, B.; Edvardsen, T.; Cardim, N.; Delgado, V.; Di Salvo, G.; Donal, E.; Sade, L.E.; Ernande, L.; Garbi, M.; et al. Standardization of adult transthoracic echocardiography reporting in agreement with recent chamber quantification, diastolic function, and heart valve disease recommendations: An expert consensus document of the European Association of Cardiovascular Imaging. Eur. Heart J. Cardiovasc. Imaging 2017, 18, 1301–1310. [Google Scholar] [CrossRef]
- Muraru, D.; Onciul, S.; Peluso, D.; Soriani, N.; Cucchini, U.; Aruta, P.; Romeo, G.; Cavalli, G.; Iliceto, S.; Badano, L.P. Sex- and Method-Specific Reference Values for Right Ventricular Strain by 2-Dimensional Speckle-Tracking Echocardiography. Circ. Cardiovasc. Imaging 2016, 9, e003866. [Google Scholar] [CrossRef] [PubMed]
- Lang, R.M.; Badano, L.P.; Mor-Avi, V.; Afilalo, J.; Armstrong, A.; Ernande, L.; Flachskampf, F.A.; Foster, E.; Goldstein, S.A.; Kuznetsova, T.; et al. Recommendations for cardiac chamber quantification by echocardiography in adults: An update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J. Am. Soc. Echocardiogr. 2015, 28, 1–39.e14. [Google Scholar] [CrossRef] [PubMed]
- Houard, L.; Benaets, M.B.; de Meester de Ravenstein, C.; Rousseau, M.F.; Ahn, S.A.; Amzulescu, M.S.; Roy, C.; Slimani, A.; Vancraeynest, D.; Pasquet, A.; et al. Additional Prognostic Value of 2D Right Ventricular Speckle-Tracking Strain for Prediction of Survival in Heart Failure and Reduced Ejection Fraction: A Comparative Study with Cardiac Magnetic Resonance. JACC Cardiovasc. Imaging 2019, 12, 2373–2385. [Google Scholar] [CrossRef]
- Badano, L.P.; Kolias, T.J.; Muraru, D.; Abraham, T.P.; Aurigemma, G.; Edvardsen, T.; D’Hooge, J.; Donal, E.; Fraser, A.G.; Marwick, T.; et al. Standardization of left atrial, right ventricular, and right atrial deformation imaging using two-dimensional speckle tracking echocardiography: A consensus document of the EACVI/ASE/Industry Task Force to standardize deformation imaging. Eur. Heart J. Cardiovasc. Imaging 2018, 19, 591–600. [Google Scholar] [CrossRef]
- Frydas, A.; Morris, D.A.; Belyavskiy, E.; Radhakrishnan, A.K.; Kropf, M.; Tadic, M.; Roessig, L.; Lam, C.S.P.; Shah, S.J.; Solomon, S.D.; et al. Left atrial strain as sensitive marker of left ventricular diastolic dysfunction in heart failure. ESC Heart Fail. 2020, 7, 1956–1965. [Google Scholar] [CrossRef]
- Cameli, M.; Incampo, E.; Mondillo, S. Left atrial deformation: Useful index for early detection of cardiac damage in chronic mitral regurgitation. Int. J. Cardiol. Heart Vasc. 2017, 17, 17–22. [Google Scholar] [CrossRef]
- Carpenito, M.; Fanti, D.; Mega, S.; Benfari, G.; Bono, M.C.; Rossi, A.; Ribichini, F.L.; Grigioni, F. The Central Role of Left Atrium in Heart Failure. Front. Cardiovasc. Med. 2021, 8, 704762. [Google Scholar] [CrossRef] [PubMed]
- Pathan, F.; D’Elia, N.; Nolan, M.T.; Marwick, T.H.; Negishi, K. Normal Ranges of Left Atrial Strain by Speckle-Tracking Echocardiography: A Systematic Review and Meta-Analysis. J. Am. Soc. Echocardiogr. 2017, 30, 59–70.e8. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Hwang, I.C.; Park, J.J.; Park, J.B.; Cho, G.Y. Prognostic power of left atrial strain in patients with acute heart failure. Eur. Heart J. Cardiovasc. Imaging 2021, 22, 210–219. [Google Scholar] [CrossRef]
- Maffeis, C.; Rossi, A.; Cannata, L.; Zocco, C.; Belyavskiy, E.; Radhakrishnan, A.K.; Feuerstein, A.; Morris, D.A.; Pieske-Kraigher, E.; Pieske, B.; et al. Left atrial strain predicts exercise capacity in heart failure independently of left ventricular ejection fraction. ESC Heart Fail. 2022, 9, 842–852. [Google Scholar] [CrossRef]
- Park, J.J.; Park, J.B.; Park, J.H.; Cho, G.Y. Global Longitudinal Strain to Predict Mortality in Patients with Acute Heart Failure. J. Am. Coll. Cardiol. 2018, 71, 1947–1957. [Google Scholar] [CrossRef]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Bohm, M.; Burri, H.; Butler, J.; Celutkiene, J.; Chioncel, O.; et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. Heart J. 2021, 42, 3599–3726. [Google Scholar] [CrossRef] [PubMed]
- Kuschyk, J.; Falk, P.; Demming, T.; Marx, O.; Morley, D.; Rao, I.; Burkhoff, D. Long-term clinical experience with cardiac contractility modulation therapy delivered by the Optimizer Smart system. Eur. J. Heart Fail. 2021, 23, 1160–1169. [Google Scholar] [CrossRef]
- Campbell, C.M.; Kahwash, R.; Abraham, W.T. Optimizer Smart in the treatment of moderate-to-severe chronic heart failure. Future Cardiol. 2020, 16, 13–25. [Google Scholar] [CrossRef]
- Giallauria, F.; Vigorito, C.; Piepoli, M.F.; Stewart Coats, A.J. Effects of cardiac contractility modulation by non-excitatory electrical stimulation on exercise capacity and quality of life: An individual patient’s data meta-analysis of randomized controlled trials. Int. J. Cardiol. 2014, 175, 352–357. [Google Scholar] [CrossRef]
- Borggrefe, M.; Burkhoff, D. Clinical effects of cardiac contractility modulation (CCM) as a treatment for chronic heart failure. Eur. J. Heart Fail. 2012, 14, 703–712. [Google Scholar] [CrossRef]
- Gupta, R.C.; Mishra, S.; Rastogi, S.; Wang, M.; Rousso, B.; Mika, Y.; Remppis, A.; Sabbah, H.N. Ca2+-binding proteins in dogs with heart failure: Effects of cardiac contractility modulation electrical signals. Clin. Transl. Sci. 2009, 2, 211–215. [Google Scholar] [CrossRef] [PubMed]
- Stix, G.; Borggrefe, M.; Wolpert, C.; Hindricks, G.; Kottkamp, H.; Böcker, D.; Wichter, T.; Mika, Y.; Ben-Haim, S.; Burkhoff, D.; et al. Chronic electrical stimulation during the absolute refractory period of the myocardium improves severe heart failure. Eur. Heart J. 2004, 25, 650–655. [Google Scholar] [CrossRef]
- Abraham, W.T.; Kuck, K.H.; Goldsmith, R.L.; Lindenfeld, J.; Reddy, V.Y.; Carson, P.E.; Mann, D.L.; Saville, B.; Parise, H.; Chan, R.; et al. A Randomized Controlled Trial to Evaluate the Safety and Efficacy of Cardiac Contractility Modulation. JACC Heart Fail. 2018, 6, 874–883. [Google Scholar] [CrossRef] [PubMed]
- Abi-Samra, F.; Gutterman, D. Cardiac contractility modulation: A novel approach for the treatment of heart failure. Heart Fail. Rev. 2016, 21, 645–660. [Google Scholar] [CrossRef]
- Abraham, W.T.; Nademanee, K.; Volosin, K.; Krueger, S.; Neelagaru, S.; Raval, N.; Obel, O.; Weiner, S.; Wish, M.; Carson, P.; et al. Subgroup analysis of a randomized controlled trial evaluating the safety and efficacy of cardiac contractility modulation in advanced heart failure. J. Card. Fail. 2011, 17, 710–717. [Google Scholar] [CrossRef] [PubMed]
- Kloppe, A.; Lawo, T.; Mijic, D.; Schiedat, F.; Muegge, A.; Lemke, B. Long-term survival with Cardiac Contractility Modulation in patients with NYHA II or III symptoms and normal QRS duration. Int. J. Cardiol. 2016, 209, 291–295. [Google Scholar] [CrossRef]
- Kadish, A.; Nademanee, K.; Volosin, K.; Krueger, S.; Neelagaru, S.; Raval, N.; Obel, O.; Weiner, S.; Wish, M.; Carson, P.; et al. A randomized controlled trial evaluating the safety and efficacy of cardiac contractility modulation in advanced heart failure. Am. Heart J. 2011, 161, 329–337.e2. [Google Scholar] [CrossRef]
- Borggrefe, M.M.; Lawo, T.; Butter, C.; Schmidinger, H.; Lunati, M.; Pieske, B.; Misier, A.R.; Curnis, A.; Bocker, D.; Remppis, A.; et al. Randomized, double blind study of non-excitatory, cardiac contractility modulation electrical impulses for symptomatic heart failure. Eur. Heart J. 2008, 29, 1019–1028. [Google Scholar] [CrossRef]
- Raab, C.; Roehl, P.; Wiora, M.; Ebelt, H. Cardiac Contractility Modulation Improves Left Ventricular Function, Including Global Longitudinal Strain, in Patients with Chronic Heart Failure. J. Clin. Med. 2025, 14, 2251. [Google Scholar] [CrossRef]
- Sidiropoulos, G.; Karakasis, P.; Antoniadis, A.; Saplaouras, A.; Karamitsos, T.; Fragakis, N. The Effect of Cardiac Resynchronization Therapy on Right Ventricular Function: A Systematic Review and Meta-Analysis. J. Clin. Med. 2024, 13, 4173. [Google Scholar] [CrossRef]
- Bannehr, M.; Kahn, U.; Liebchen, J.; Okamoto, M.; Hähnel, V.; Georgi, C.; Dworok, V.; Edlinger, C.; Lichtenauer, M.; Kücken, T.; et al. Right Ventricular Longitudinal Strain Predicts Survival in Patients with Functional Tricuspid Regurgitation. Can. J. Cardiol. 2021, 37, 1086–1093. [Google Scholar] [CrossRef] [PubMed]
- Ji, M.; Wu, W.; He, L.; Gao, L.; Zhang, Y.; Lin, Y.; Qian, M.; Wang, J.; Zhang, L.; Xie, M.; et al. Right Ventricular Longitudinal Strain in Patients with Heart Failure. Diagnostics 2022, 12, 445. [Google Scholar] [CrossRef] [PubMed]
- Jani, V.P.; Strom, J.B.; Gami, A.; Beussink-Nelson, L.; Patel, R.; Michos, E.D.; Shah, S.J.; Freed, B.H.; Mukherjee, M. Optimal Method for Assessing Right Ventricular to Pulmonary Arterial Coupling in Older Healthy Adults: The Multi-Ethnic Study of Atherosclerosis. Am. J. Cardiol. 2024, 222, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Ro, R.; Prandi, F.R.; Zaid, S.; Anastasius, M.O.; Tang, G.H.L.; Seetharam, K.; Argulian, E.; Massaro, G.; Sharma, S.; Kini, A.; et al. Acute effect of edge-to-edge repair of mitral regurgitation on left heart mechanics and health status. J. Cardiovasc. Med. 2022, 23, 787–797. [Google Scholar] [CrossRef]
- Inoue, K.; Khan, F.H.; Remme, E.W.; Ohte, N.; García-Izquierdo, E.; Chetrit, M.; Moñivas-Palomero, V.; Mingo-Santos, S.; Andersen, Ø.S.; Gude, E.; et al. Determinants of left atrial reservoir and pump strain and use of atrial strain for evaluation of left ventricular filling pressure. Eur. Heart J. Cardiovasc. Imaging 2021, 23, 61–70. [Google Scholar] [CrossRef]
- Zhang, F.; Dang, Y.; Li, Y.; Hao, Q.; Li, R.; Qi, X. Cardiac Contractility Modulation Attenuate Myocardial Fibrosis by Inhibiting TGF-beta1/Smad3 Signaling Pathway in a Rabbit Model of Chronic Heart Failure. Cell. Physiol. Biochem. 2016, 39, 294–302. [Google Scholar] [CrossRef]
- Tschope, C.; Van Linthout, S.; Spillmann, F.; Klein, O.; Biewener, S.; Remppis, A.; Gutterman, D.; Linke, W.A.; Pieske, B.; Hamdani, N.; et al. Cardiac contractility modulation signals improve exercise intolerance and maladaptive regulation of cardiac key proteins for systolic and diastolic function in HFpEF. Int. J. Cardiol. 2016, 203, 1061–1066. [Google Scholar] [CrossRef]
- Tschope, C.; Kherad, B.; Klein, O.; Lipp, A.; Blaschke, F.; Gutterman, D.; Burkhoff, D.; Hamdani, N.; Spillmann, F.; Van Linthout, S. Cardiac contractility modulation: Mechanisms of action in heart failure with reduced ejection fraction and beyond. Eur. J. Heart Fail. 2019, 21, 14–22. [Google Scholar] [CrossRef]
- Mandoli, G.E.; Pastore, M.C.; Benfari, G.; Setti, M.; Nistor, D.; D’Ascenzi, F.; Focardi, M.; Baccani, B.; Patti, G.; Valente, S.; et al. New echocardiographic indices of shift to biventricular failure to optimize risk stratification of chronic heart failure. ESC Heart Fail. 2022, 9, 476–485. [Google Scholar] [CrossRef]
- Pierucci, N.; La Fazia, V.M.; Gianni, C.; Mohanty, S.; Lavalle, C.; Cishek, M.B.; Canby, R.C.; Natale, A. Cardiac contractility modulation in a patient with refractory systolic heart failure following orthotopic heart transplant. HeartRhythm Case Rep. 2024, 10, 33–37. [Google Scholar] [CrossRef]

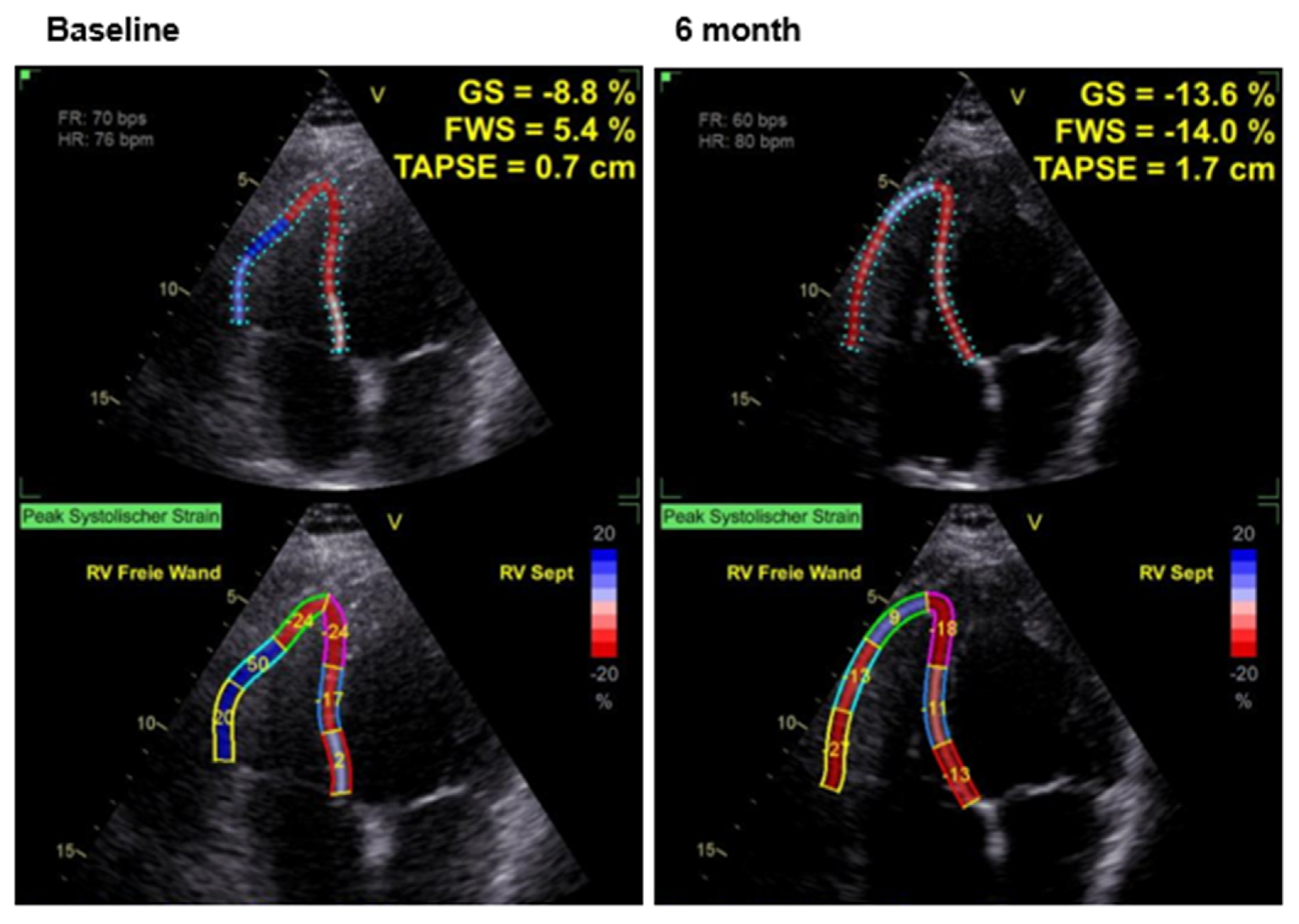
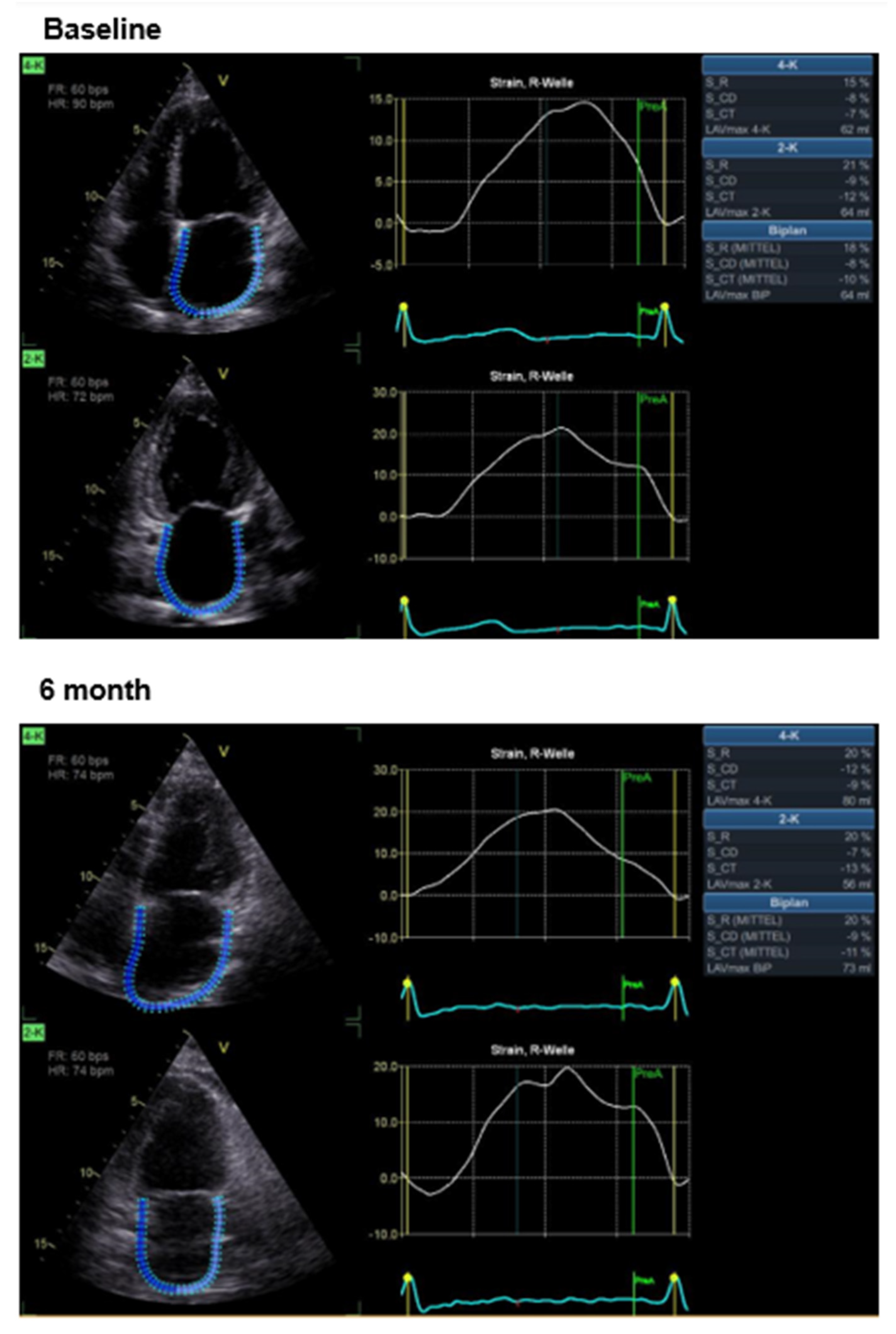
| Parameter | Mean (All Patients; N = 22) | Ischemic Cardiomyopathy [11] | Non-Ischemic Cardiomyopathy [11] |
|---|---|---|---|
| Male sex | 16 (73%) | 9 (81.8%) | 8 (63.6%) |
| Age [years] | 69.6 ± 6.4 | 69.0 ± 7.7 | 70.3 ± 5.1 |
| Hight [cm] | 170.6 ± 10.0 | 174.1 ± 9.1 | 167.1 ± 10.1 |
| Weight [kg] | 95.5 ± 21.9 | 100.0 ± 23.2 | 91.0 ± 20.5 |
| GFR [ml/min*1.73 m2] | 62.4 ± 17.5 | 60.3 ± 13.0 | 64.4 ± 21.5 |
| NT-pro BP [pg/mL] | 2669.2 ± 3716.8 | 1528.5 ± 1809.0 | 3810.0 ± 4781.2 |
| NYHA stage | |||
| NYHA I | 0.0 (0%) | 0 (0.0%) | 0 (0.0%) |
| NYHA II | 4 (18.18%) | 4 (36.4%) | 0 (0.0%) |
| NYHA III | 12 (54.6%) | 6 (54.5%) | 6 (54.5%) |
| NYHA IV | 6 (27.3%) | 1 (9.1%) | 5 (45.5%) |
| Hypertension | 18 (82.8%) | 9 (81.8%) | 9 (81.8%) |
| Diabetes mellitus | 14 (64.6%) | 9 (81.8%) | 5 (45.5%) |
| Coronary heart disease | 11 (50.0%) | 11 (100.0%) | 0 (0.0%) |
| History of PCI | 11 (50.0%) | 11 (100.0%) | 0 (0.0%) |
| History of CABG | 1 (4.6%) | 1 (9.1%) | 0 (0.0%) |
| History of heart value surgery | 6 (27.3%) | 3 (27.3%) | 3 (27.3%) |
| Antiplatelet therapy (APT) | |||
| Single APT | 9 (40.9%) | 6 (54.5%) | 3 (27.3%) |
| Dual APT | 2 (9.1%) | 1 (9.1%) | 1 (9.1%) |
| Non | 11 (50.0%) | 4 (36.4%) | 7 (63.6%) |
| (D)OAC | 11 (50.0%) | 6 (54.5%) | 5 (45.5%) |
| ß-blockers | 19 (86.4%) | 10 (90.9%) | 9 (81.8%) |
| ACE inhibitors | 3 (13.6%) | 2 (18.2%) | 1 (9.1%) |
| ARB | 1 (4.6%) | 1 (9.1%) | 0 (0.0%) |
| Sacubitril/Valsartan | 18 (81.8%) | 8 (72.7%) | 10 (90.9%) |
| MRA | 15 (68.2%) | 6 (54.5%) | 9 (81.8%) |
| SGLT2 inhibitors | 14 (64.6% | 7 (63.6%) | 7 (63.6%) |
| Diuretic KCCQ | 19 (86.4%) 31.3 ± 16.8 | 10 (90.9%) 37.7 ± 19.0 | 9 (81.8%) 24.9 ± 11.8 |
| Parameter | CCM Active (N = 22) | No Active CCM (N = 39) | p-Value |
|---|---|---|---|
| RV GS [%] | −13.7 ± 4.5 | −10.1 ± 5.0 | <0.05 |
| FWS [%] | −14.6 ± 7.3 | −10.3 ± 10.2 | <0.05 |
| LA S_R [%] | 19.7 ± 1.0 | 15.3 ± 10.2 | <0.05 |
| LA S_CD [%] | −9.0 ± 5.0 | −8.1 ± 5.4 | n.s. |
| LA S_CT [%] | −11.5 ± 7.0 | −7.1 ± 8.5 | <0.05 |
| TAPSE [mm] | 18.8 ± 5.1 | 17.6 ± 5.6 | n.s. |
| Trvmax [m/s] | 2.6± 0.4 | 2.9 ± 1.3 | n.s. |
| RVDd [mm] | 39.2 ± 16.9 | 38.8± 7.5 | n.s. |
| RVDs [mm] | 30.2 ± 17.5 | 30.3 ± 7.0 | n.s. |
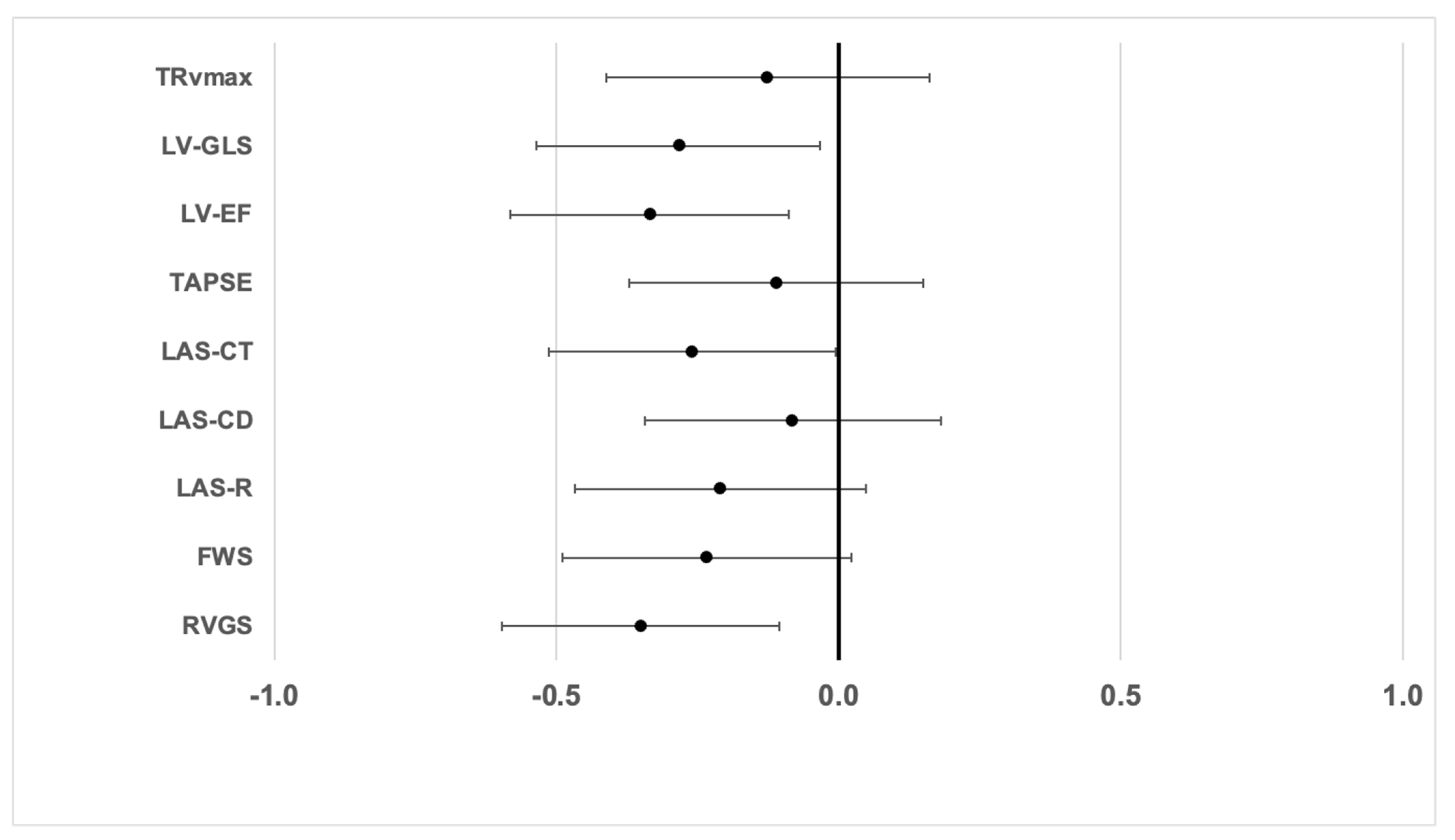
| Variable | Standardized Beta | p-Value |
|---|---|---|
| Age | −0.112 | 0.39 |
| LVEF | 0.163 | 0.210 |
| LV GLS | 0.055 | 0.673 |
| TRVmax | −0.173 | 0.23 |
| RV GS | −0.147 | 0.261 |
| FWS | −0.070 | 0.597 |
| LAS_R | 0.270 | 0.037 |
| LAS_CD | −0.081 | 0.536 |
| LAS_CT | −0.321 | 0.012 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Raab, C.; Roehl, P.; Wiora, M.; Ebelt, H. Effects of Cardiac Contractility Modulation on Right Ventricular and Left Atrial Strain in Patients with Chronic Heart Failure. J. Clin. Med. 2025, 14, 4484. https://doi.org/10.3390/jcm14134484
Raab C, Roehl P, Wiora M, Ebelt H. Effects of Cardiac Contractility Modulation on Right Ventricular and Left Atrial Strain in Patients with Chronic Heart Failure. Journal of Clinical Medicine. 2025; 14(13):4484. https://doi.org/10.3390/jcm14134484
Chicago/Turabian StyleRaab, Cornelia, Peter Roehl, Matthias Wiora, and Henning Ebelt. 2025. "Effects of Cardiac Contractility Modulation on Right Ventricular and Left Atrial Strain in Patients with Chronic Heart Failure" Journal of Clinical Medicine 14, no. 13: 4484. https://doi.org/10.3390/jcm14134484
APA StyleRaab, C., Roehl, P., Wiora, M., & Ebelt, H. (2025). Effects of Cardiac Contractility Modulation on Right Ventricular and Left Atrial Strain in Patients with Chronic Heart Failure. Journal of Clinical Medicine, 14(13), 4484. https://doi.org/10.3390/jcm14134484






