Multidisciplinary En-Bloc Resection of Sacral Chordoma: A Narrative Review and Illustrative Case
Abstract
1. Introduction
2. Materials and Methods
3. Results: Narrative Review
3.1. Multidisciplinary Surgical Management
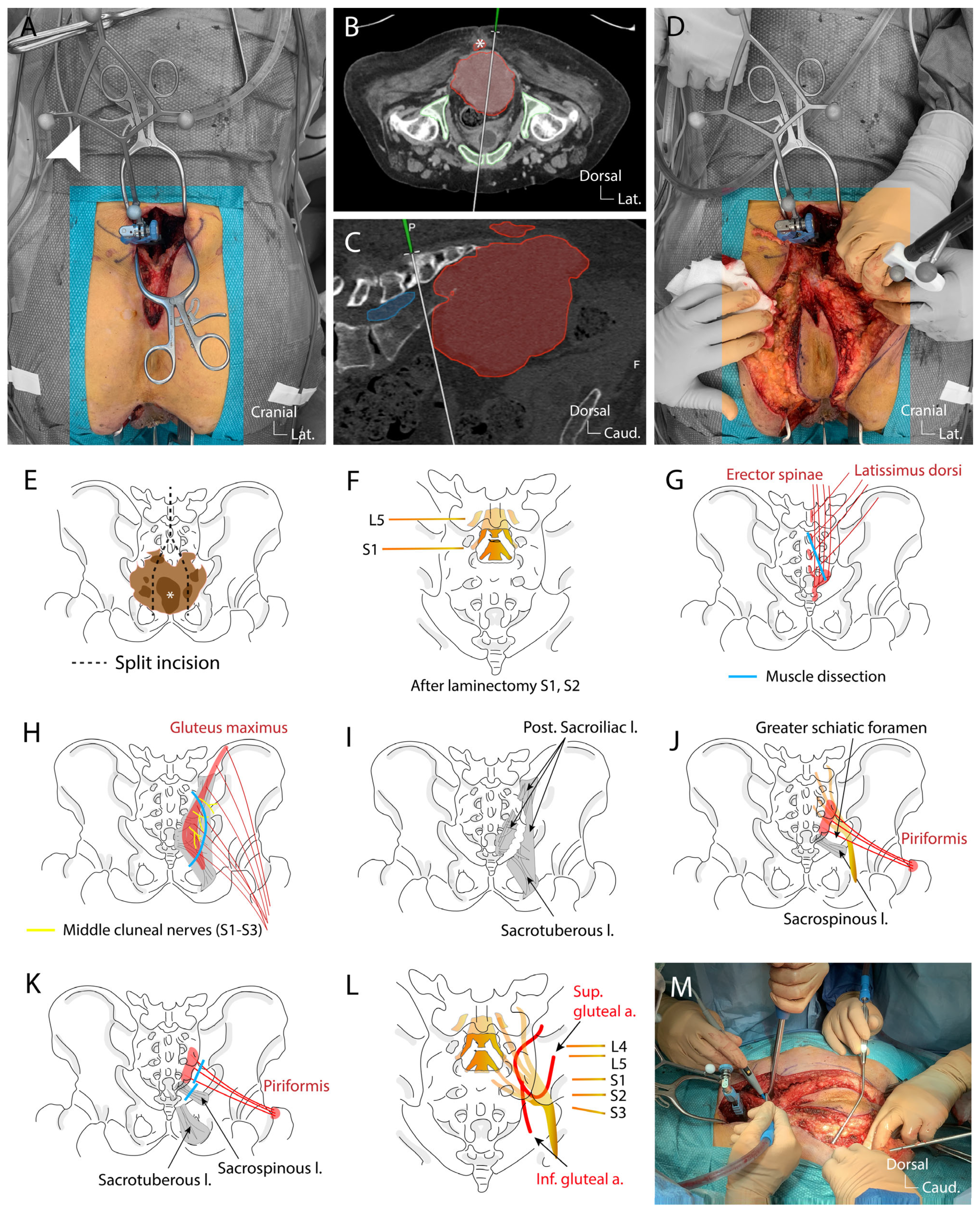
3.2. Surgical Technique
3.3. Trade-Off Between Oncological Goal and Neurological Function
3.4. Sacrectomy and Mechanical Stability
3.5. Reconstruction Strategies for Sacral Defects
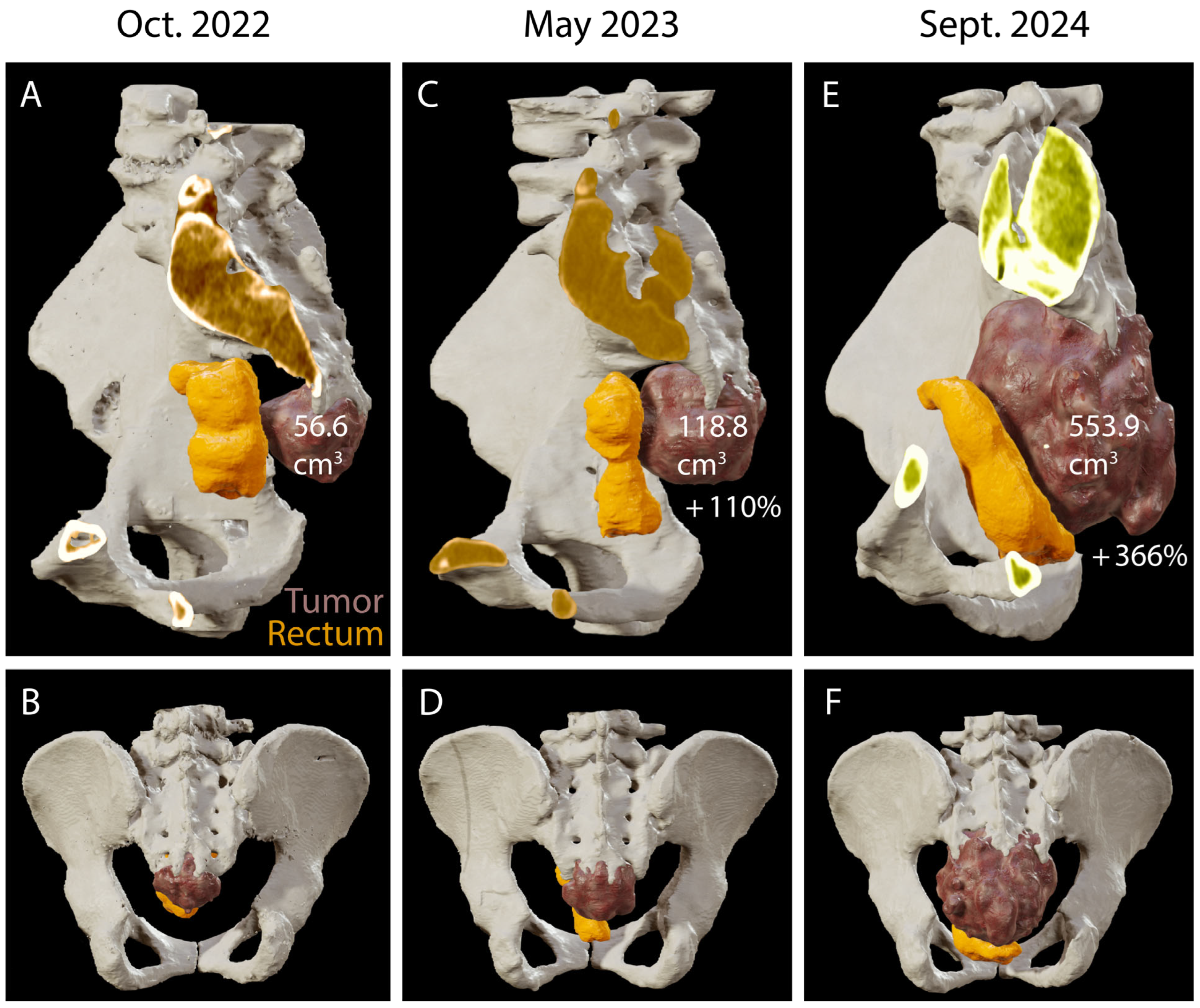
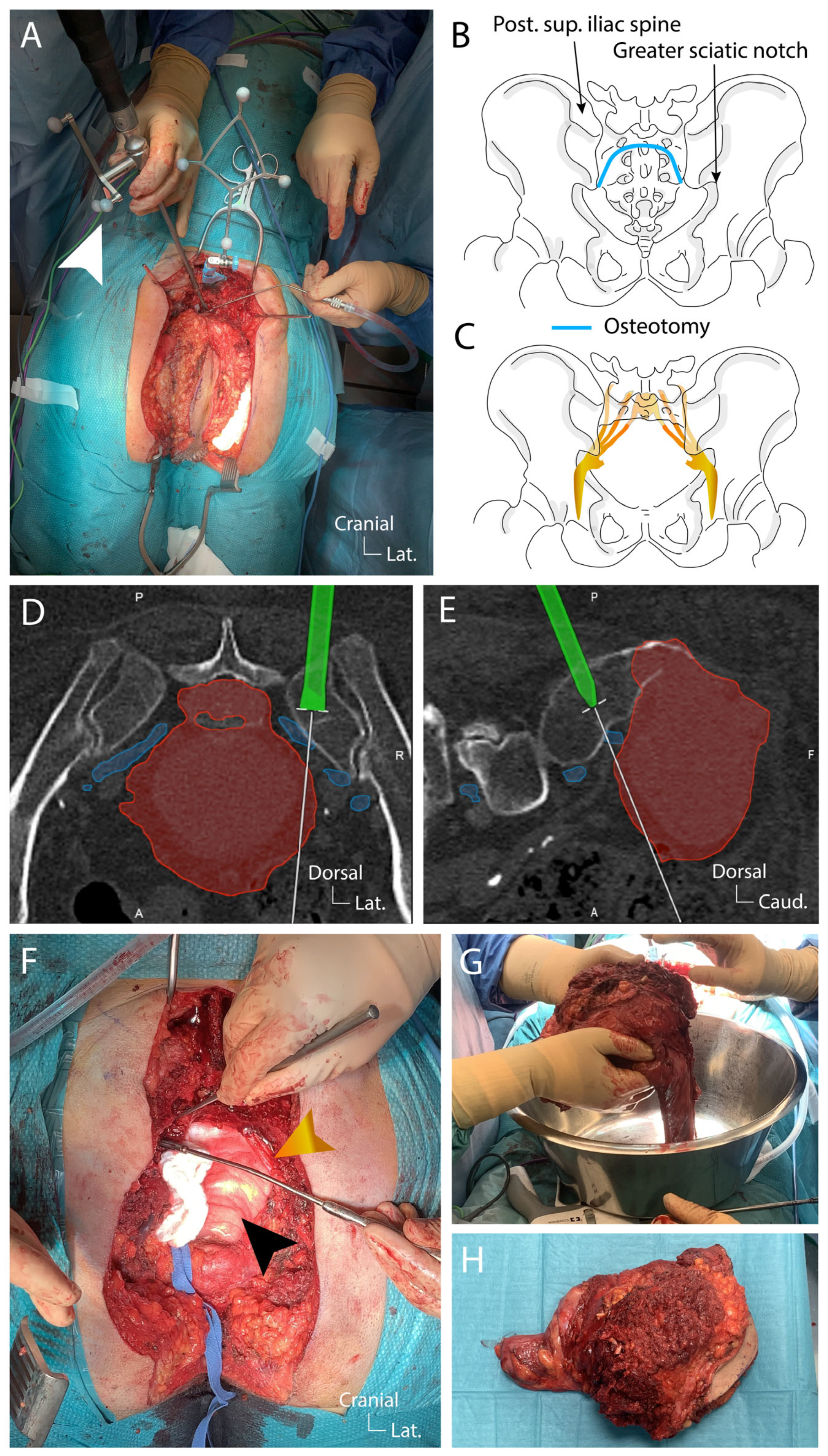
4. Results: Illustrative Case
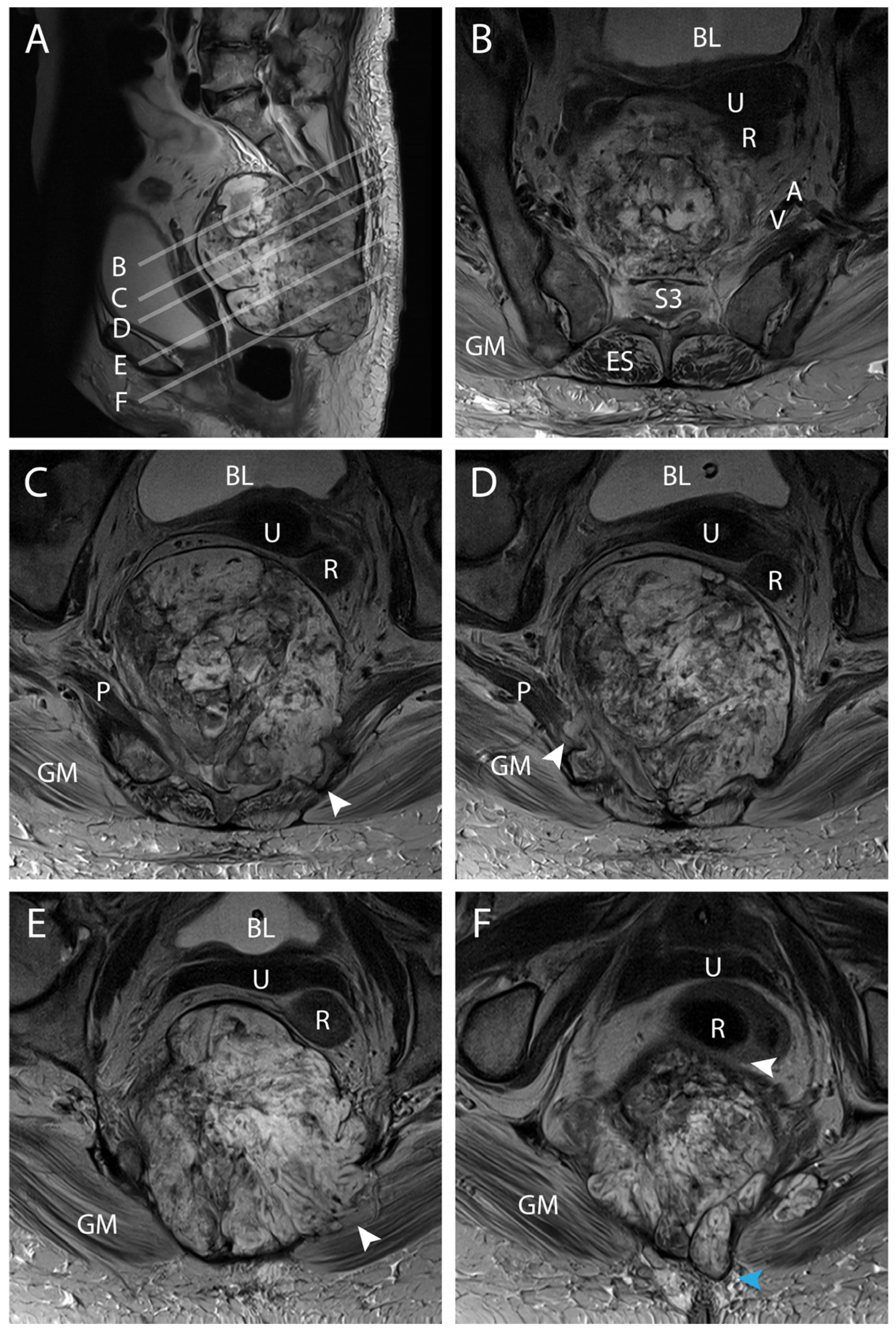
4.1. Clinical Elements
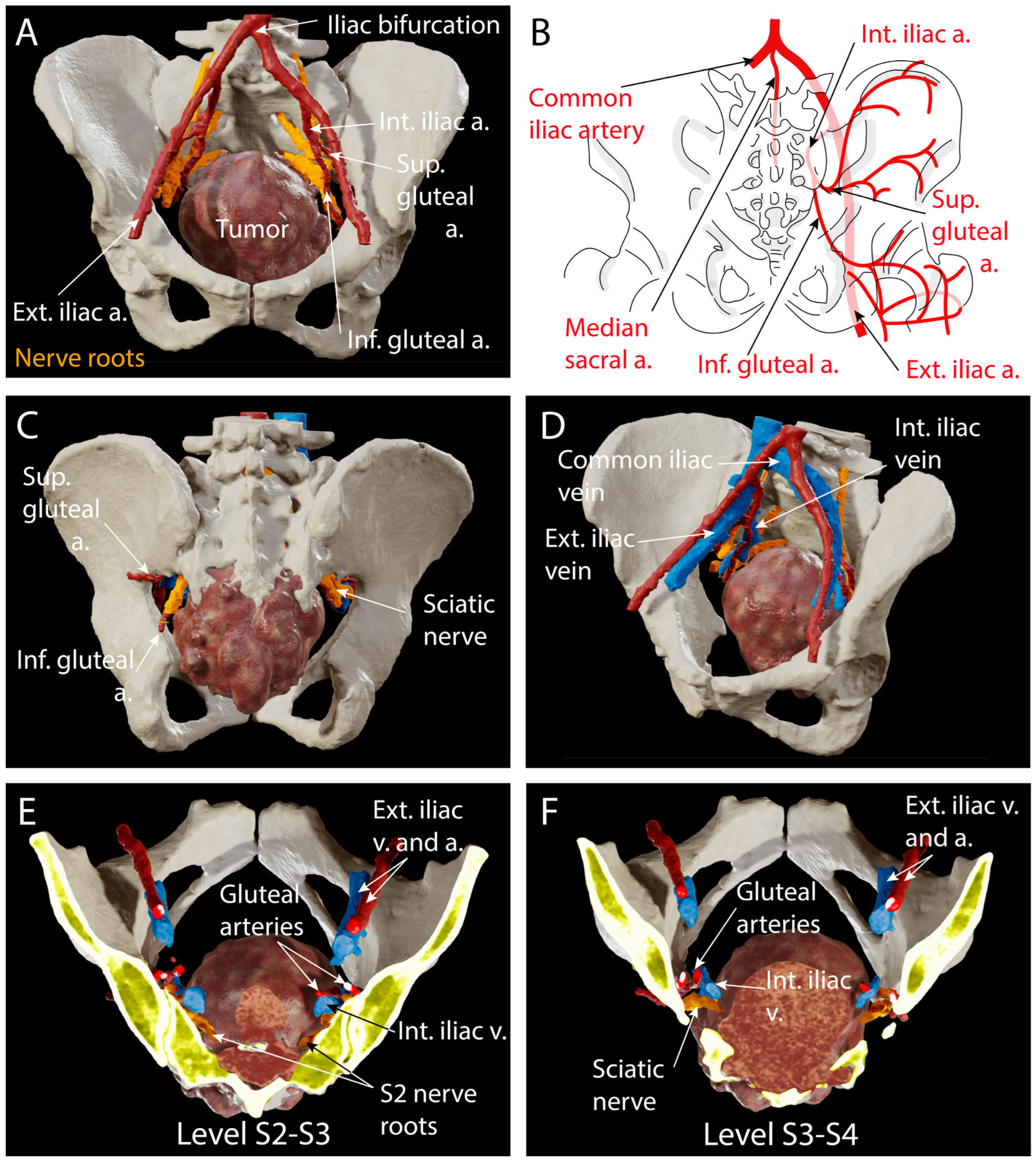
4.2. Preoperative Planning
4.3. Surgical Technique
4.4. Postoperative Course
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| PBRT | Proton beam radiation therapy |
| VRAM | Vertical rectus abdominis myocutaneous flap |
| VAC | Vacuum-assisted closure |
References
- Schroeder, C.; de Lomba, W.C.; Leary, O.P.; De la Garza Ramos, R.; Gillette, J.S.; Miner, T.J.; Woo, A.S.; Fridley, J.S.; Gokaslan, Z.L.; Zadnik Sullivan, P.L. Multidisciplinary surgical considerations for en bloc resection of sacral chordoma: Review of recent advances and a contemporary single-center series. Neurosurg. Focus 2024, 56, E7. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, P.C.; Xu, R.; Sciubba, D.M.; McGirt, M.J.; Nelson, C.; Witham, T.F.; Wolinksy, J.P.; Gokaslan, Z.L. Long-term clinical outcomes following en bloc resections for sacral chordomas and chondrosarcomas: A series of twenty consecutive patients. Spine 2009, 34, 2233–2239. [Google Scholar] [CrossRef] [PubMed]
- Colangeli, S.; Muratori, F.; Bettini, L.; Frenos, F.; Totti, F.; D’Arienzo, A.; Campo, F.R.; Scoccianti, G.; Beltrami, G.; Campanacci, D.A.; et al. Surgical treatment of sacral chordoma: En bloc resection with negative margins is a determinant of the long-term outcome. Surg. Technol. Int. 2018, 33, 343–348. [Google Scholar] [PubMed]
- Fourney, D.R.; Rhines, L.D.; Hentschel, S.J.; Skibber, J.M.; Wolinsky, J.P.; Weber, K.L.; Suki, D.; Gallia, G.L.; Garonzik, I.; Gokaslan, Z.L. En bloc resection of primary sacral tumors: Classification of surgical approaches and outcome. J. Neurosurg. Spine 2005, 3, 111–122. [Google Scholar] [CrossRef]
- El-Hajj, V.G.; Ghaith, A.K.; Hoang, H.; Nguyen, R.H.; Al-Saidi, N.N.; Graepel, S.P.; Atallah, E.; Elmi-Terander, A.; Lehrer, E.J.; Brown, P.D.; et al. Impact of proton versus photon adjuvant radiotherapy on overall survival in the management of skull base and spinal chordomas: A National Cancer Database analysis. J. Neurosurg. 2024, 142, 239–247. [Google Scholar] [CrossRef]
- Ghaith, A.K.; Nguyen, R.; El-Hajj, V.G.; Montaser, A.; De Biase, G.; Ravindran, K.; Perez-Vega, C.; Lee, S.J.; Dominari, A.; Battistin, U.; et al. Proton versus photon adjuvant radiotherapy: A multicenter comparative evaluation of recurrence following spinal chordoma resection. Neurosurg. Focus 2024, 56, E9. [Google Scholar] [CrossRef]
- Walser, M.; Bojaxhiu, B.; Kawashiro, S.; Tran, S.; Beer, J.; Leiser, D.; Pica, A.; Bachtiary, B.; Weber, D.C. Clinical Outcome of Sacral Chordoma Patients Treated with Pencil Beam Scanning Proton Therapy. Clin. Oncol. 2021, 33, e578–e585. [Google Scholar] [CrossRef]
- Ozger, H.; Eralp, L.; Sungur, M.; Atalar, A.C. Surgical management of sacral chordoma. Acta Orthop. Belg. 2010, 76, 243–253. [Google Scholar]
- Klein, L.; Lenga, P.; Dao Trong, P.; Kleineidam, H.; Krieg, S.M.; Ishak, B. Tailored surgical approaches for spinal chordomas: A multidisciplinary perspective. Acta Neurochir. 2024, 166, 393. [Google Scholar] [CrossRef]
- Terterov, S.; Diaz-Aguilar, D.; Scharnweber, R.; Tucker, A.; Niu, T.; Woodard, J.; Brara, H.; Poh, M.; Merna, C.; Wang, S.; et al. Surgical nuances of partial sacrectomy for chordoma. Surg. Neurol. Int. 2017, 8, 277. [Google Scholar]
- Zoccali, C.; Skoch, J.; Patel, A.S.; Walter, C.M.; Maykowski, P.; Baaj, A.A. Residual neurological function after sacral root resection during en-bloc sacrectomy: A systematic review. Eur. Spine J. 2016, 25, 3925–3931. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.Y.; Thongtrangan, I.; Balabhadra, R.S.V.; Murovic, J.A.; Kim, D.H. Surgical techniques for total sacrectomy and spino pelvic reconstruction. Neurosurg. Focus 2003, 15, E5. [Google Scholar] [CrossRef] [PubMed]
- Ramamurthy, R.; Bose, J.C.; Muthusamy, V.; Natarajan, M.; Kun-jithapatham, D. Staged sacrectomy—An adaptive approach. J. Neurosurg. Spine 2009, 11, 285–294. [Google Scholar] [CrossRef]
- Clarke, M.J.; Dasenbrock, H.; Bydon, A.; Sciubba, D.M.; McGirt, M.J.; Hsieh, P.C.; Yassari, R.; Gokaslan, Z.L.; Wolinsky, J.P. Posterior-only approach for en bloc sacrectomy: Clinical outcomes in 36 consecutive patients. Neurosurgery 2012, 71, 357–364. [Google Scholar] [CrossRef]
- Moran, D.; Zadnik, P.L.; Taylor, T.; Groves, M.L.; Yurter, A.; Wolinsky, J.P.; Witham, T.F.; Bydon, A.; Gokaslan, Z.L.; Sciubba, D.M. Maintenance of bowel, bladder, and motor functions after sacrectomy. Spine J. 2015, 15, 222–229. [Google Scholar] [CrossRef]
- Tang, O.Y.; Sullivan, P.Z.; Tubre, T.; Feler, J.; Shao, B.; Hart, J.; Gokaslan, Z.L. Navigation-assisted resection of tumoral calcinosis of the lumbosacral spine: Illustrative case. J. Neurosurg. Case Lessons 2022, 4, CASE22213. [Google Scholar] [CrossRef]
- Shuman, W.H.; Valliani, A.A.; Chapman, E.K.; Martini, M.L.; Neifert, S.N.; Baron, R.B.; Schupper, A.J.; Steinberger, J.M.; Caridi, J.M. Intraoperative navigation in spine surgery: Effects on complications and reoperations. World Neurosurg. 2022, 160, e404–e411. [Google Scholar] [CrossRef]
- Landriel, F.; Albergo, J.I.; Farfalli, G.; Yampolsky, C.; Ayerza, M.; Aponte-Tinao, L.; Teixeira, W.; Ritacco, L.; Hem, S. Navigated multipla-nar osteotomies for spinal primary bone tumors. Surg. Neurol. Int. 2022, 13, 58. [Google Scholar] [CrossRef]
- Massaad, E.; Shankar, G.M.; Shin, J.H. Novel Applications of Spinal Navigation in Deformity and Oncology Surgery-Beyond Screw Placement. Oper. Neurosurg. 2021, 21 (Suppl. S1), S23–S38. [Google Scholar] [CrossRef]
- Sommer, F.; Hussain, I.; Kirnaz, S.; Goldberg, J.; McGrath, L.; Navarro-Ramirez, R.; Waterkeyn, F.; Schmidt, F.; Gadjradj, P.S.; Härtl, R. Safety and feasibility of augmented reality assistance in minimally invasive and open resection of benign intradural extramedullary tumors. Neurospine 2022, 19, 501–512. [Google Scholar] [CrossRef]
- Carl, B.; Bopp, M.; Saß, B.; Voellger, B.; Nimsky, C. Implementation of augmented reality support in spine surgery. Eur. Spine J. 2019, 28, 1697–1711. [Google Scholar] [CrossRef] [PubMed]
- Carl, B.; Bopp, M.; Saß, B.; Pojskic, M.; Nimsky, C. Augmented reality in intradural spinal tumor surgery. Acta Neurochir. 2019, 161, 2181–2193. [Google Scholar] [CrossRef] [PubMed]
- Leary, O.P.; Crozier, J.; Liu, D.D.; Niu, T.; Pertsch, N.J.; Camara-Quintana, J.Q.; Svokos, K.A.; Syed, S.; Telfeian, A.E.; Oyelese, A.A.; et al. Three-dimensional printed anatomic modeling for surgical planning and real-time operative guidance in complex primary spinal column tumors: Single-center experience and case series. World Neurosurg. 2021, 145, e116–e126. [Google Scholar] [CrossRef] [PubMed]
- Paun, L.; Lavé, A.; Molliqaj, G.; Haemmerli, J.; Oranges, C.M.; Dominguez, D.E.; Buchs, N.; Vargas, M.I.; Tessitore, E. Three-dimensional virtual reality-assisted surgical planning for neuronavigated sacrectomy of a chordoma: A technical note. Int. Orthop. 2024, 48, 2931–2939. [Google Scholar] [CrossRef]
- Jahangiri, F.R.; Al Eissa, S.; Jahangiri, A.F.; Al-Habib, A. Intraoperative neurophysiological monitoring during sacrectomy procedures. Neurodiagn J. 2013, 53, 312–322. [Google Scholar]
- Schilling, A.; Pennington, Z.; Ehresman, J.; Hersh, A.; Srivastava, S.; Hung, B.; Botros, D.; Cottrill, E.; Lubelski, D.; Goodwin, C.R.; et al. Impact of Multidisciplinary Intraoperative Teams on Thirty-Day Complications After Sacral Tumor Resection. World Neurosurg. 2021, 152, e558–e566. [Google Scholar] [CrossRef]
- Housari, G.; González, M.; Calero, P.; Beni, R.; Lobo, E. Sacral chordoma: Management of a rare disease in a tertiary hospital Gada Housari. Clin. Transl. Oncol. 2013, 15, 327–330. [Google Scholar] [CrossRef]
- Pillai, S.; Govender, S. Sacral chordoma: A review of literature. J. Orthop. 2018, 15, 679–684. [Google Scholar] [CrossRef]
- Yu, E.; Koffer, P.P.; DiPetrillo, T.A.; Kinsella, T.J. Incidence, Treatment, and Survival Patterns for Sacral Chordoma in the United States, 1974–2011. Front. Oncol. 2016, 6, 203. [Google Scholar] [CrossRef]
- Prabhakaran Chandrashekar, M.; Vijayakumar, K. Sacral chordomas: A 10-year study. Australas. Radiol. 1998, 56, 42–46. [Google Scholar] [CrossRef]
- Dea, N.; Fisher, C.G.; Reynolds, J.J.; Schwab, J.H.; Rhines, L.D.; Gokaslan, Z.L.; Bettegowda, C.; Sahgal, A.; Lazáry, Á.; Luzzati, A.; et al. Current treatment strategy for newly diagnosed chordoma of the mobile spine and sacrum: Results of an international survey. J. Neurosurg. Spine 2018, 30, 119–125. [Google Scholar] [CrossRef] [PubMed]
- Walcott, B.P.; Nahed, B.V.; Mohyeldin, A.; Coumans, J.-V.; Kahle, K.T.; Ferreira, M.J. Chordoma: Current concepts, management, and future directions. Lancet Oncol. 2012, 13, e69–e76. [Google Scholar] [CrossRef] [PubMed]
- Bergh, P.; Kindblom, L.G.; Gunterberg, B.; Remotti, F.; Ryd, W.; Meis-Kindblom, J.M. Prognostic factors in chordoma of the sacrum and mobile spine: A study of 39 patients. Cancer 2000, 88, 2122–2134. [Google Scholar] [CrossRef]
- Asavamongkolkul, A.; Waikakul, S. Wide resection of sacral chordoma via a posterior approach. Int. Orthop. 2012, 36, 607–612. [Google Scholar] [CrossRef]
- Akiyama, T. Juxtacortical chordoma of the sacrum. J. Orthop. Sci. 2008, 13, 476–480. [Google Scholar] [CrossRef]
- Ji, T.; Guo, W.; Yang, R.; Tang, X.; Wang, Y.; Huang, L. What Are the Conditional Survival and Functional Outcomes After Surgical Treatment of 115 Patients with Sacral Chordoma? Clin. Orthop. Relat. Res. 2017, 475, 620–630. [Google Scholar] [CrossRef]
- Yang, Y.; Niu, X.; Liu, W.; Xu, H. Recurrence and survival factors analysis of 171 cases of sacral chordoma in a single institute. Eur. Spine J. 2017, 26, 1910–1916. [Google Scholar] [CrossRef]
- Sciubba, D.M.; Petteys, R.J.; Garces-Ambrossi, G.L.; Noggle, J.C.; McGirt, M.J.; Wolinsky, J.P.; Witham, T.F.; Gokaslan, Z.L. Diagnosis and management of sacral tumors. J. Neurosurg. Spine 2009, 10, 244–256. [Google Scholar] [CrossRef]
- Zuckerman, S.; Bilsky, M.; Laufer, I. Chordomas of the skull base, mobile spine, and sacrum: An epidemiologic investigation of presentation, treatment, and survival. World Neurosurg. 2018, 1016, E618–E627. [Google Scholar] [CrossRef]
- Zuckerman, S.L.; Lee, S.H.; Chang, G.J.; Walsh, G.L.; Mehran, R.J.; Gokaslan, Z.L.; Rao, G.; Tatsui, C.E.; Rhines, L.D. Outcomes of Surgery for Sacral Chordoma and Impact of Complications: A Report of 50 Consecutive Patients with Long-Term Follow-Up. Glob. Spine J. 2021, 11, 740–750. [Google Scholar] [CrossRef]
- Park, L.; Delaney, T.F.; Liebsch, N.J.; Hornicek, F.J.; Goldberg, S.; Mankin, H.; Rosenberg, A.E.; Rosenthal, D.I.; Suit, H.D. Therapeutic strategies for mobile spine chordoma: En bloc Versus intralesional surgery with adjuvant charged-particle therapy. J. Neurooncol. 2025, 171, 229–240. [Google Scholar] [CrossRef]
- Park, L.; Delaney, T.F.; Liebsch, N.J.; Hornicek, F.J.; Goldberg, S.; Mankin, H.; Rosenberg, A.E.; Rosenthal, D.I.; Suit, H.D. Sacral chordomas: Impact of high-dose proton/photon-beam radiation therapy combined with or without surgery for primary versus recurrent tumor. Radiat. Oncol. 2006, 65, 1514–1521. [Google Scholar] [CrossRef] [PubMed]
- Radaelli, S.; Stacchiotti, S.; Ruggieri, P.; Donati, D.; Casali, P.G.; Palmerini, E.; Collini, P.; Gambarotti, M.; Porcu, L.; Boriani, S.; et al. Sacral Chordoma: Long-term Outcome of a Large Series of Patients Surgically Treated at Two Reference Centers. Spine 2016, 41, 1049–1057. [Google Scholar] [CrossRef] [PubMed]
- Goumenos, S.; Kakouratos, G.; Trikoupis, I.; Gavriil, P.; Gerasimidis, P.; Soultanis, K.; Patapis, P.; Kontogeorgakos, V.; Papagelopoulos, P. Clinical Outcome after Surgical Treatment of Sacral Chordomas: A Single-Center Retrospective Cohort of 27 Patients. Cancers 2024, 16, 973. [Google Scholar] [CrossRef]
- Kerekes, D.; Goodwin, C.R.; Ahmed, A.K.; Verlaan, J.J.; Bettegowda, C.; Abu-Bonsrah, N.; Sciubba, D.M. Local and Distant Recurrence in Resected Sacral Chordomas: A Systematic Review and Pooled Cohort Analysis. Glob. Spine J. 2019, 9, 191–201. [Google Scholar] [CrossRef]
- van Wulfften Palthe, O.D.R.; Tromp, I.; Ferreira, A.; Fiore, A.; Bramer, J.A.M.; van Dijk, N.C.; DeLaney, T.F.; Schwab, J.H.; Hornicek, F.J. Sacral chordoma: A clinical review of 101 cases with 30-year experience in a single institution. Spine J. 2019, 19, 869–879. [Google Scholar] [CrossRef]
- Nagra, I. A 45-year-old woman with a pre-sacral mass lesion: Diagnosis and discussion. Skelet. Radiol. 2010, 39, 199–200. [Google Scholar] [CrossRef]
- Kayani, B. Prognostic factors in the operative management of dedifferentiated sacral chordomas. Neurosurgery 2014, 75, 269–275. [Google Scholar] [CrossRef]
- Ishil, K. Local recurrence after S2–3 sacrectomy in sacral chordoma. Report of four cases. J. Neurosurg. 2002, 97, 98–101. [Google Scholar] [CrossRef]
- Osaka, S.; Osaka, E.; Kojima, T.; Yoshida, Y.; Tokuhashi, Y. Long term outcome following surgical treatment of sacral chordoma. J. Surg. Oncol. 2014, 109, 184–188. [Google Scholar] [CrossRef]
- Cheng, E.Y.; Ozerdemoglu, R.A.; Transfeldt, E.E.; Thompson, R.C., Jr. Lumbosacral chordoma. Progn. Factors Treat. Spine 1999, 24, 1639–1645. [Google Scholar] [CrossRef] [PubMed]
- Zuckerman, S.L.; Amini, B.; Lee, S.H.; Rao, G.; Tatsui, C.E.; Rhines, L.D. Predictive Value of Preoperative Magnetic Resonance Imaging Findings for Survival and Local Recurrence in Patients Undergoing En Bloc Resection of Sacral Chordomas. Neurosurgery 2019, 85, 834–842. [Google Scholar] [CrossRef] [PubMed]
- Yanagawa, T.; Okamoto, M.; Ohno, T.; Chikuda, H. How to Prevent Local Recurrence of Sacral Chordoma Treated with Carbon-Ion Radiotherapy: An Analysis of the Risk Factors of Local Failure and an Adequate Disease Margin. Oncology 2025, 103, 30–36. [Google Scholar] [CrossRef]
- Farsad, K.; Kattapuram, S.V.; Sacknoff, R.; Ono, J.; Nielsen, G.P. Sacral Chordoma. RadioGraphics 2009, 29, 1525–1530. [Google Scholar] [CrossRef]
- Atalar, H. Management of sacrococcygeal chordomas. Int. Orthop. 2006, 30, 514–518. [Google Scholar] [CrossRef]
- Sung, M.S. Sacrococcygeal chordoma: MR imaging in 30 patients. Skelet. Radiol. 2005, 34, 87–94. [Google Scholar] [CrossRef]
- Rao, B.S.S. Sacral chordoma—A report of two cases. Indian J. Surg. 2005, 67, 207–209. [Google Scholar]
- Weidlich, A.; Schaser, K.D.; Weitz, J.; Kirchberg, J.; Fritzmann, J.; Reeps, C.; Schwabe, P.; Melcher, I.; Disch, A.; Dragu, A.; et al. Surgical and Oncologic Outcome following Sacrectomy for Primary Malignant Bone Tumors and Locally Recurrent Rectal Cancer. Cancers 2024, 16, 2334. [Google Scholar] [CrossRef]
- Luksanapruksa, P.; Singhatanadgige, W.; Rose, P.C.; Bumpass, D.B. Management of spinal giant cell tumors. Spine J. 2016, 16, 259–269. [Google Scholar] [CrossRef]
- Houdek, M.T.; Bakri, K.; Tibbo, M.E.; Wagner, E.R.; Rose, P.S.; Sim, F.H.; Moran, S.L. Outcome and complications following vertical rectus abdominis myocutaneous flap surgery to reconstruct sacrectomy defects. Plast. Reconstr. Surg. 2018, 142, 1327–1335. [Google Scholar] [CrossRef]
- Leary, O.P.; Liu, D.D.; Boyajian, M.K.; Syed, S.; Camara-Quintana, J.Q.; Niu, T.; Svokos, K.A.; Crozier, J.; Oyelese, A.A.; Liu, P.Y.; et al. Complex wound closure by plastic surgery following resection of spinal neoplasms minimizes post-operative wound complications in high-risk patients. J. Neurosurg. Spine 2020, 33, 77–86. [Google Scholar] [CrossRef] [PubMed]
- Koh, P.K.; Tan, B.K.; Hong, S.W.; Tan, M.H.; Tay, A.G.; Song, C.; Tan, K.C. The gluteus maximus muscle flap for reconstruction of sacral chordoma defects. Ann. Plast. Surg. 2004, 53, 44–49. [Google Scholar] [CrossRef] [PubMed]
- Miles, W.K.; Chang, D.W.; Kroll, S.S.; Miller, M.J.; Langstein, H.N.; Reece, G.P.; Evans, G.R.; Robb, G.L. Reconstruction of large sacral defects following total sacrectomy. Plast. Reconstr. Surg. 2000, 105, 2387–2394. [Google Scholar] [CrossRef] [PubMed]
- Garofalo, F.; di Summa, P.G.; Christoforidis, D.; Pracht, M.; Laudato, P.; Cherix, S.; Bouchaab, H.; Raffoul, W.; Demartines, N.; Matter, M. Multidisciplinary approach of lumbo-sacral chordoma: From oncological treatment to reconstructive surgery. J. Surg. Oncol. 2015, 112, 544–554. [Google Scholar] [CrossRef]
- Triolo, J.C.; Buchs, N.C.; Tessitore, E.; Hannouche, D.; Dominguez, D.E.; Kalbermatten, D.F.; Oranges, C.M. Sacral Defect Reconstruction Using Double Pedicled Gracilis Muscle Flap combined with Gluteal Fasciocutaneous Rotation Flap. Plast. Reconstr. Surg. Glob. Open 2022, 10, e4329. [Google Scholar] [CrossRef]
- Sciubba, D.M.; Nelson, C.; Gok, B.; McGirt, M.J.; McLoughlin, G.S.; Noggle, J.C.; Wolinsky, J.P.; Witham, T.F.; Bydon, A.; Gokaslan, Z.L. Evaluation of factors associated with postoperative infection following sacral tumor resection. J. Neurosurg. Spine 2008, 9, 593–599. [Google Scholar] [CrossRef]
- Ter Gunne, A.F.P.; Cohen, D.B. Incidence, prevalence, and analysis of risk factors for surgical site infection following adult spinal surgery. Spine 2009, 34, 1422–1428. [Google Scholar] [CrossRef]
- Sebaaly, A.; Shedid, D.; Boubez, G.; Zairi, F.; Kanhonou, M.; Yuh, S.J.; Wang, Z. Surgicalsite infection in spinal metastasis: Incidence risk factors. Spine J. 2018, 18, 1382–1387. [Google Scholar] [CrossRef]
- Ruggieri, P.; Angelini, A.; Pala, E.; Mercuri, M. Infections in surgery of primary tumors of the sacrum. Spine 2012, 37, 420–428. [Google Scholar] [CrossRef]
- Abraham, J.A.; Kenneally, B.; Amer, K.; Geller, D.S. Can Navigation-assisted Surgery Help Achieve Negative Margins in Resection of Pelvic and Sacral Tumors? Clin. Orthop. Relat. Res. 2018, 476, 499–508. [Google Scholar] [CrossRef]
- Farshad, M.; Selman, F.; Burkhard, M.D.; Müller, D.; Spirig, J.M. Partial sacrectomy with patient-specific osteotomy guides. N. Am. Spine Soc. J. 2021, 8, 100090. [Google Scholar] [CrossRef] [PubMed]
- van Kessel, C.S.; Waller, J.; Steffens, D.; Lee, P.J.; Austin, K.K.S.; Stalley, P.D.; Solomon, M.J. Surgical Outcomes in Pelvic Exenteration Surgery: Comparison of Prone Sacrectomy with Anterior Cortical Sacrectomy Techniques. Ann. Surg. 2023, 278, 945–953. [Google Scholar] [CrossRef]
- Kaiser, R.; Khadanovich, A.; Benes, M.; Reynolds, J.; Mawhinney, G.; Giele, H.; Kachlik, D. Clinical Anatomy of the Sacral Nerve Roots and Its Relevance to Their Reconstruction After Sacrectomy. Neurosurgery 2025, 96, 505–513. [Google Scholar] [CrossRef]
- Biagini, R.; Ruggieri, P.; Mercuri, M.; Capanna, R.; Briccoli, A.; Perin, S.; Orsini, U.; Demitri, S.; Arlecchini, S. Neurologic deficit after resection of the sacrum. Chir. Organi Mov. 1997, 82, 357–372. [Google Scholar]
- Stener, B.; Gutenberg, B. High amputation of the sacrum for extirpation of tumors: Principles and technique. Spine 1978, 3, 351–366. [Google Scholar] [CrossRef]
- Gunterberg, B.; Norlen, L.; Stener, B.; Sundin, T. Neurourologic evaluation after resection of the sacrum. Investig. Urol. 1975, 13, 183–188. [Google Scholar]
- Guo, Y.; Palmer, J.L.; Shen, L.; Kaur, G.; Willey, J.; Zhang, T.; Bruera, E.; Wolinsky, J.P.; Gokaslan, Z.L. Bowel and bladder continence, wound healing, and functional outcomes in patients who underwent sacrectomy. J. Neurosurg. Spine 2005, 3, 106–110. [Google Scholar] [CrossRef]
- Berra, L.V.; Armocida, D.; Palmieri, M.; Di Norcia, V.; D’Angelo, L.; Mongardini, M.; Vigliotta, M.; Maccari, E.; Santoro, A. Sacral Nerves Reconstruction After Surgical Resection of a Large Sacral Chordoma Restores the Urinary and Sexual Function and the Anal Continence. Neurospine 2022, 19, 155–162. [Google Scholar] [CrossRef]
- Fuchs, B.; Dickey, I.; Yaszemski, M.; Inwards, C.; Sim, F. Operative management of sacral chordoma. J. Bone Jt. Surg. Am. 2005, 87, 2211–2216. [Google Scholar]
- Imai, R.; Kamada, T.; Araki, N. Working group for bone and soft tissue sarcomas. Carbon ion radiation therapy for unresectable sacral chordoma: An analysis of 188 cases. Int. J. Radiat. Oncol. Biol. Phys. 2016, 95, 322–327. [Google Scholar] [CrossRef]
- Unni, K. Dahlin’s Bone Tumors: General Aspects and Data on 11,087 Cases, 5th ed.; Lippincott-Raven: Philadelphia, PA, USA, 1996. [Google Scholar]
- Baratti, D.; Gronchi, A.; Pennacchioli, E.; Lozza, L.; Colecchia, M.; Fiore, M.; Santinami, M. Chordoma: Natural history and results in 28 patients treated at a single institution. Ann. Surg. Oncol. 2003, 10, 291–296. [Google Scholar] [CrossRef] [PubMed]
- Uhl, M.; Welzel, T.; Jensen, A.; Ellerbrock, M.; Haberer, T.; Jäkel, O.; Herfarth, K.; Debus, J. Carbon ion beam treatment in patients with primary and recurrent sacrococcygeal chordoma. Strahlenther. Onkol. 2015, 191, 597–603. [Google Scholar] [CrossRef] [PubMed]
- Akimaya, T.; Ogura, K.; Gokita, T.; Tsukushi, S.; Iwata, S. Analysis of the infiltrative features of chordoma: The relationship between micro-skip metastasis and postoperative outcomes. Ann. Surg. Oncol. 2018, 25, 6268–6296. [Google Scholar] [CrossRef]
- Hugate, R.R., Jr.; Dickey, I.D.; Phimolsarnti, R.; Yaszemski, M.J.; Sim, F.H. Mechanical effects of partial sacrectomy: When is reconstruction necessary? Clin. Orthop. Relat. Res. 2006, 450, 82–88. [Google Scholar] [CrossRef]
- Yu, B.; Zheng, Z.; Zhuang, X.; Chen, H.; Xie, D.; Luk, K.D.; Lu, W.W. Biomechanical effects of transverse partial sacrectomy on the sacroiliac joints: An in vitro human cadaveric investigation of the borderline of sacroiliac joint instability. Spine 2009, 34, 1370–1375. [Google Scholar] [CrossRef]
- Mikula, A.L.; Pennington, Z.; Lakomkin, N.; Prablek, M.; Amini, B.; Karim, S.M.; Patel, S.S.; Lubelski, D.; Sciubba, D.M.; Alvarez-Breckenridge, C.; et al. Risk factors for sacral fracture following en bloc chordoma resection. J. Neurosurg. Spine 2023, 39, 611–617. [Google Scholar] [CrossRef] [PubMed]
- Gottfried, O.N.; Omeis, I.; Mehta, V.A.; Solakoglu, C.; Gokaslan, Z.L.; Wolinsky, J.P. Sacral tumor resection and the impact on pelvic incidence. J. Neurosurg. Spine 2011, 14, 78–84. [Google Scholar] [CrossRef]
- Court, C.; Briand, S.; Mir, O.; Le Péchoux, C.; Lazure, T.; Missenard, G.; Bouthors, C. Management of chordoma of the sacrum and mobile spine. Orthop. Traumatol. Surg. Res. 2022, 108, 103169. [Google Scholar] [CrossRef]
- Tang, X.; Yang, R.; Qu, H.; Cai, Z.; Guo, W. Factors Associated with Spinopelvic Fixation Mechanical Failure After Total Sacrectomy. Spine 2018, 43, 1268–1274. [Google Scholar] [CrossRef]
- Lv, Z.; Li, J.; Yang, Z.; Li, X.; Yang, Q.; Li, Z. A novel three dimensional-printed biomechanically evaluated patient-specific sacral implant in spinopelvic reconstruction after total en bloc sacrectomy. Front. Bioeng. Biotechnol. 2023, 11, 1153801. [Google Scholar] [CrossRef]
- Gong, T.; Lu, M.; Wang, Y.; Li, Z.; He, X.; Luo, Y.; Zhou, Y.; Tu, C.; Min, L. Is 3D-printed self-stabilizing endoprosthesis reconstruction without supplemental fixation following total sacrectomy a viable approach for sacral tumours? Eur. Spine J. 2024, 33, 4316–4324. [Google Scholar] [CrossRef]
- Turbucz, M.; Pokorni, A.J.; Hajnal, B.; Koch, K.; Szoverfi, Z.; Varga, P.P.; Lazary, A.; Eltes, P.E. The biomechanical effect of lumbopelvic distance reduction on reconstruction after total sacrectomy: A comparative finite element analysis of four techniques. Spine J. 2024, 24, 1981–1992. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Yu, Y.; Zhu, R.; Lv, H.; Jia, Y.; Zeng, Z.; Chen, B.; Ding, Z. Structural stability of different reconstruction techniques following total sacrectomy: A biomechanical study. Clin. Biomech. 2011, 26, 977–981. [Google Scholar] [CrossRef] [PubMed]
- Marmouset, D.; Haseny, B.; Dukan, R.; Saint-Etienne, A.; Missenard, G.; Court, C.; Bouthors, C. Characteristics, survivals and risk factors of surgical site infections after En Bloc sacrectomy for primary malignant sacral tumors at a single center. Orthop. Traumatol. Surg. Res. 2022, 108, 103197. [Google Scholar] [CrossRef]
- Chen, K.W.; Yang, H.L.; Lu, J.; Wang, G.L.; Ji, Y.M.; Bao, Z.H.; Wu, G.Z.; Gu, Y.; Sun, Z.Y.; Zhu, R.F. Risk factors for postoperative wound infections of sacral chordoma after surgical excision. J. Spinal Disord. Tech. 2011, 24, 230–234. [Google Scholar] [CrossRef]
- Ishida, W.; Elder, B.D.; Lo, S.-F.L.; Witham, T.F. Spinopelvic reconstruction following lumbosacral tumor resection. Spinopelvic Reconstr. Spinal Tumors 2016, 1, 25–30. [Google Scholar]
- Asaad, M.; Rajesh, A.; Wahood, W.; Vyas, K.S.; Houdek, M.T.; Rose, P.S.; Moran, S.L. Flap reconstruction for sacrectomy defects: A systematic review and meta-analysis. J. Plast. Reconstr. Aesthet. Surg. 2020, 73, 255–268. [Google Scholar] [CrossRef]
- Kim, J.E.; Pang, J.; Christensen, J.M.; Coon, D.; Zadnik, P.L.; Wolinsky, J.P.; Gokaslan, Z.L.; Bydon, A.; Sciubba, D.M.; Witham, T.; et al. Soft-tissue reconstruction after total en bloc sacrectomy. J. Neurosurg. Spine 2015, 22, 571–581. [Google Scholar] [CrossRef]
- Glatt, B.S.; Disa, J.J.; Mehrara, B.J.; Pusic, A.L.; Boland, P.; Cordeiro, P.G. Reconstruction of extensive partial or total sacrectomy defects with a transabdominal vertical rectus abdominis myocutaneous flap. Ann. Plast. Surg. 2006, 56, 526–531. [Google Scholar] [CrossRef]
- Chang, D.W.; Friel, M.T.; Youssef, A.A. Reconstructive strategies in soft tissue reconstruction after resection of spinal neoplasms. Spine 2007, 32, 1101–1106. [Google Scholar] [CrossRef]
- Chieng, L.O.; Hubbard, Z.; Salgado, C.J.; Levi, A.D.; Chim, H. Reconstruction of open wounds as a complication of spinal surgery with flaps: A systematic review. Neurosurg. Focus 2015, 39, E17. [Google Scholar] [CrossRef] [PubMed]
- Cohen, L.E.; Fullerton, N.; Mundy, L.R.; Weinstein, A.L.; Fu, K.M.; Ketner, J.J.; Härtl, R.; Spector, J.A. Optimizing successful outcomes in complex spine reconstruction using local muscle flaps. Plast. Reconstr. Surg. 2016, 137, 295–301. [Google Scholar] [CrossRef] [PubMed]
- Garvey, P.B.; Rhines, L.D.; Dong, W.; Chang, D.W. Immediate soft-tissue reconstruction for complex defects of the spine following surgery for spinal neoplasms. Plast. Reconstr. Surg. 2010, 125, 1460–1466. [Google Scholar] [CrossRef]
- Inglesby, D.C.; Young, Z.T.; Alshareef, M.; Ritter, A.; Gunasekaran, A.; Kalhorn, S.P.; Lance Tavana, M. Paraspinous muscle flaps for the treatment of complex spinal wounds. Spine 2020, 45, 599–604. [Google Scholar] [CrossRef]
- Wright, M.A.; Weinstein, A.L.; Bernstein, J.L.; Franck, P.; Lara, D.O.; Samadi, A.; Cohen, L.E.; Härtl, R.; Baaj, A.A.; Spector, J.A. Muscle flap closure following complex spine surgery: A decade of experience. Plast Reconstr Surg. 2020, 146, 642e–650e. [Google Scholar] [CrossRef]
- Maricevich, M.; Maricevich, R.; Chim, H.; Moran, S.L.; Rose, P.S.; Mardini, S. Reconstruction following partial and total sacrectomy defects: An analysis of outcomes and complications. J. Plast. Reconstr. Aesthet. Surg. 2014, 67, 1257–1266. [Google Scholar] [CrossRef]
- Knol, J.; Keller, D.S. Total Mesorectal Excision Technique-Past, Present, and Future. Clin. Colon. Rectal Surg. 2020, 33, 134–143. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kiss-Bodolay, D.; Ris, F.; Lavalley, A.; Nouri, A.; Oranges, C.M.; Meurette, G.; Schaller, K.; Tessitore, E.; Molliqaj, G. Multidisciplinary En-Bloc Resection of Sacral Chordoma: A Narrative Review and Illustrative Case. J. Clin. Med. 2025, 14, 4480. https://doi.org/10.3390/jcm14134480
Kiss-Bodolay D, Ris F, Lavalley A, Nouri A, Oranges CM, Meurette G, Schaller K, Tessitore E, Molliqaj G. Multidisciplinary En-Bloc Resection of Sacral Chordoma: A Narrative Review and Illustrative Case. Journal of Clinical Medicine. 2025; 14(13):4480. https://doi.org/10.3390/jcm14134480
Chicago/Turabian StyleKiss-Bodolay, Daniel, Frederic Ris, Adrien Lavalley, Aria Nouri, Carlo M. Oranges, Guillaume Meurette, Karl Schaller, Enrico Tessitore, and Granit Molliqaj. 2025. "Multidisciplinary En-Bloc Resection of Sacral Chordoma: A Narrative Review and Illustrative Case" Journal of Clinical Medicine 14, no. 13: 4480. https://doi.org/10.3390/jcm14134480
APA StyleKiss-Bodolay, D., Ris, F., Lavalley, A., Nouri, A., Oranges, C. M., Meurette, G., Schaller, K., Tessitore, E., & Molliqaj, G. (2025). Multidisciplinary En-Bloc Resection of Sacral Chordoma: A Narrative Review and Illustrative Case. Journal of Clinical Medicine, 14(13), 4480. https://doi.org/10.3390/jcm14134480






