Visual Quality and Symptomatology Following Implantation of a Non-Diffractive Extended Depth-of-Focus Intraocular Lens
Abstract
1. Introduction
2. Materials and Methods
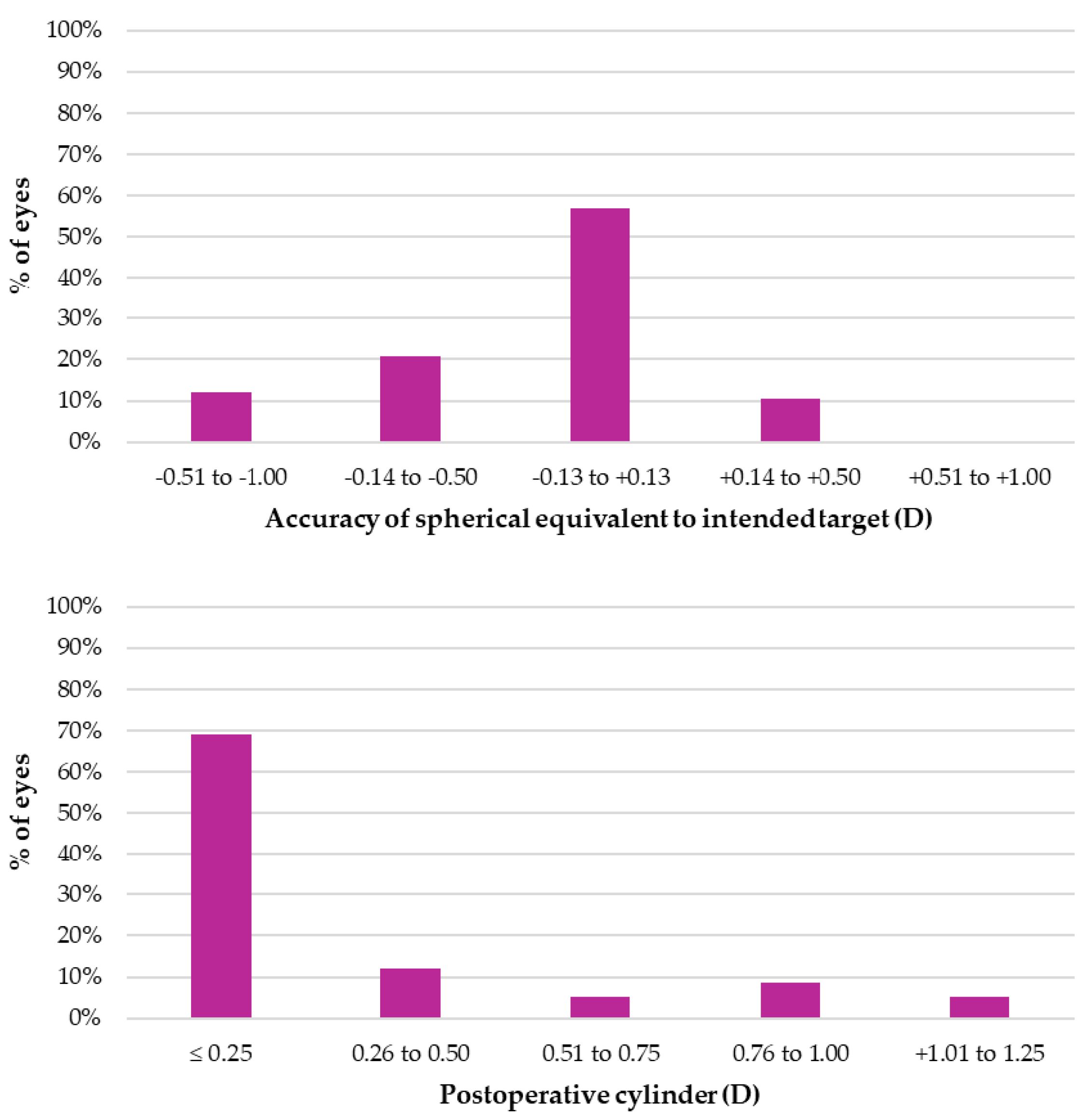
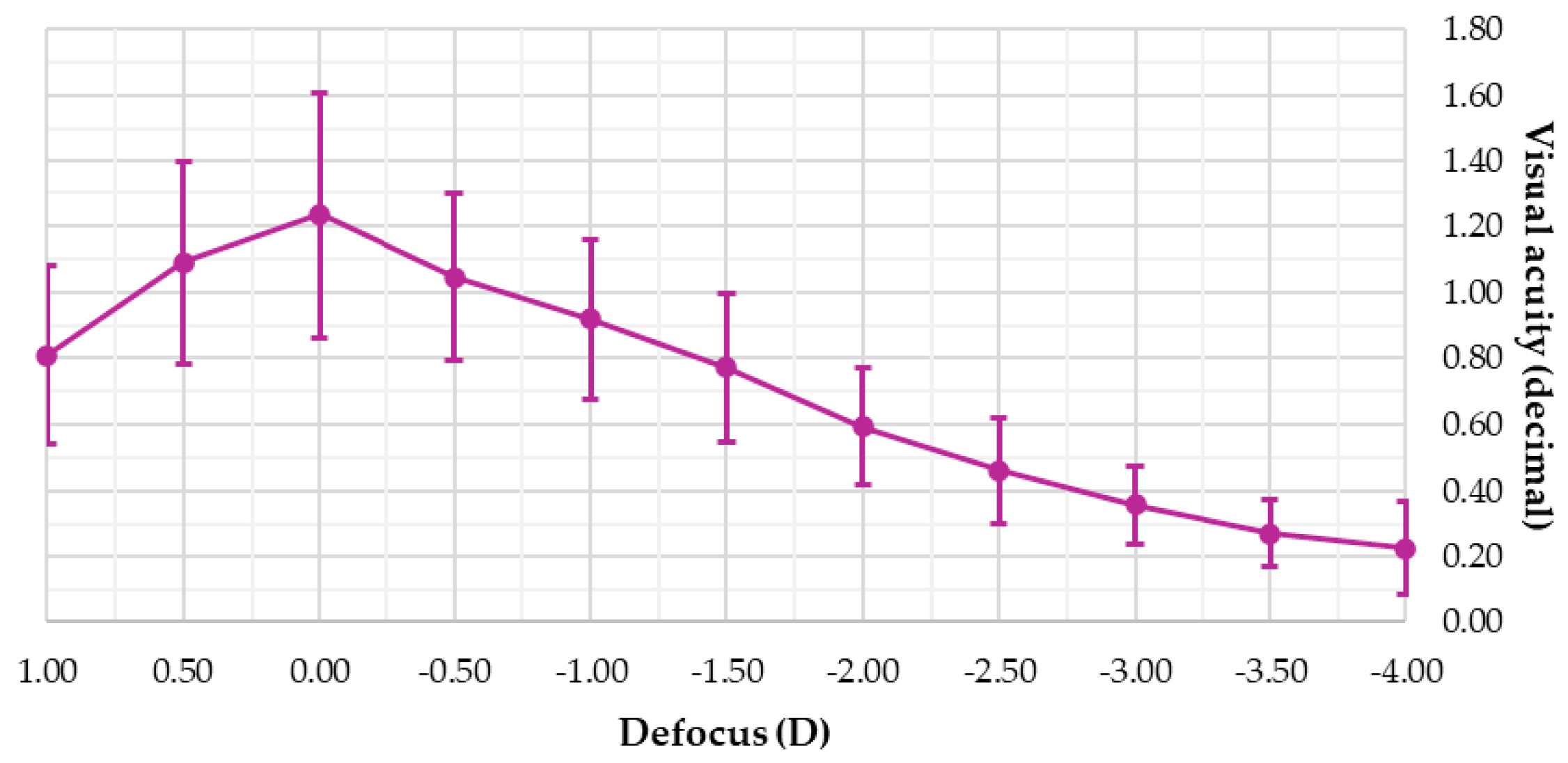
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Schnider, C.; Yuen, L.; Rampat, R.; Zhu, D.; Dhallu, S.; Trinh, T.; Gurnani, B.; Abdelmaksoud, A.; Bhogal-Bhamra, G.; Wolffsohn, J.S.; et al. BCLA CLEAR presbyopia: Management with intraocular lenses. Contact Lens Anterior Eye 2024, 47, 102253. [Google Scholar] [CrossRef] [PubMed]
- Kanclerz, P.; Toto, F.; Grzybowski, A.; Alio, J.L. Extended Depth-of-Field Intraocular Lenses: An Update. Asia Pac. J. Ophthalmol. 2020, 9, 194–202. [Google Scholar] [CrossRef] [PubMed]
- Azor, J.A.; Vega, F.; Armengol, J.; Millan, M.S. Optical Assessment and Expected Visual Quality of Four Extended Range of Vision Intraocular Lenses. J. Refract. Surg. 2022, 38, 688–697. [Google Scholar] [CrossRef]
- Fernández-Núñez, S.; Pérez-Sanz, L.; Gómez-Pedrero, J.A.; García-Montero, M.; Albarrán-Diego, C.; Garzón, N. Optical quality in vitro and in vivo of an extended depth-of-focus intraocular lens with isofocal design. Graefes Arch. Clin. Exp. Ophthalmol. 2024, 262, 3905–3913. [Google Scholar] [CrossRef]
- Rampat, R.; Gatinel, D. Multifocal and Extended Depth-of-Focus Intraocular Lenses in 2020. Ophthalmology 2021, 128, e164–e185. [Google Scholar] [CrossRef] [PubMed]
- Megiddo-Barnir, E.; Alió, J.L. Latest Development in Extended Depth-of-Focus Intraocular Lenses: An Update. Asia Pac. J. Ophthalmol. 2023, 12, 58–79. [Google Scholar] [CrossRef]
- Karam, M.; Alkhowaiter, N.; Alkhabbaz, A.; Aldubaikhi, A.; Alsaif, A.; Shareef, E.; Alazaz, R.; Alotaibi, A.; Koaik, M.; Jabbour, S. Extended Depth of Focus Versus Trifocal for Intraocular Lens Implantation: An Updated Systematic Review and Meta-Analysis. Am. J. Ophthalmol. 2023, 251, 52–70. [Google Scholar] [CrossRef]
- Tavassoli, S.; Ziaei, H.; Yadegarfar, M.E.; Gokul, A.; Kernohan, A.; Evans, J.R.; Ziaei, M. Trifocal versus extended depth of focus (EDOF) intraocular lenses after cataract extraction. Cochrane Database Syst. Rev. 2024, 7, Cd014891. [Google Scholar] [CrossRef]
- Ferrando Gil, J.; Churruca Irazola, A.; Reparaz, I.; Lauzirika, G.; Martínez-Soroa, I.; Mendicute, J. Visual, Refractive, Functional, and Patient Satisfaction Outcomes After Implantation of a New Extended Depth-of-Focus Intraocular Lens. Clin. Ophthalmol. 2024, 18, 3801–3813. [Google Scholar] [CrossRef]
- Kniestedt, C.; Stamper, R.L. Visual acuity and its measurement. Ophthalmol. Clin. N. Am. 2003, 16, 155–170. [Google Scholar] [CrossRef]
- Mäntyjärvi, M.; Laitinen, T. Normal values for the Pelli-Robson contrast sensitivity test. J. Cataract. Refract. Surg. 2001, 27, 261–266. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Vallejo, M.; Burguera, N.; Rocha-de-Lossada, C.; Aramberri, J.; Fernández, J. Refraction and defocus curves in eyes with monofocal and multifocal intraocular lenses. J. Optom. 2023, 16, 236–243. [Google Scholar] [CrossRef] [PubMed]
- McAlinden, C.; Pesudovs, K.; Moore, J.E. The Development of an Instrument to Measure Quality of Vision: The Quality of Vision (QoV) Questionnaire. Investig. Ophthalmol. Vis. Sci. 2010, 51, 5537–5545. [Google Scholar] [CrossRef] [PubMed]
- Monaco, G.; Gari, M.; Di Censo, F.; Poscia, A.; Ruggi, G.; Scialdone, A. Visual performance after bilateral implantation of 2 new presbyopia-correcting intraocular lenses: Trifocal versus extended range of vision. J. Cataract. Refract. Surg. 2017, 43, 737–747. [Google Scholar] [CrossRef]
- Gil, M.A.; Varón, C.; Cardona, G.; Buil, J.A. Visual acuity and defocus curves with six multifocal intraocular lenses. Int. Ophthalmol. 2020, 40, 393–401. [Google Scholar] [CrossRef]
- Webers, V.S.C.; Bauer, N.J.C.; Saelens, I.E.Y.; Creten, O.J.M.; Berendschot, T.; van den Biggelaar, F.; Nuijts, R. Comparison of the intermediate distance of a trifocal IOL with an extended depth-of-focus IOL: Results of a prospective randomized trial. J. Cataract. Refract. Surg. 2020, 46, 193–203. [Google Scholar] [CrossRef]
- Scheepers, M.A.; Bunce, C.B.; Michaelides, M.; Hall, B. Clinical outcomes of a trifocal compared with an extended depth of focus IOL following bilateral cataract surgery. Can. J. Ophthalmol. 2023, 58, 393–400. [Google Scholar] [CrossRef]
- Cochener, B.; Boutillier, G.; Lamard, M.; Auberger-Zagnoli, C. A Comparative Evaluation of a New Generation of Diffractive Trifocal and Extended Depth of Focus Intraocular Lenses. J. Refract. Surg. 2018, 34, 507–514. [Google Scholar] [CrossRef]
- Hirvelä, H.; Koskela, P.; Laatikainen, L. Visual acuity and contrast sensitivity in the elderly. Acta Ophthalmol. Scand. 1995, 73, 111–115. [Google Scholar] [CrossRef]
- Ang, R.E.; Picache, G.C.S.; Rivera, M.C.R.; Lopez, L.R.L.; Cruz, E.M. A Comparative Evaluation of Visual, Refractive, and Patient-Reported Outcomes of Three Extended Depth of Focus (EDOF) Intraocular Lenses. Clin. Ophthalmol. 2020, 14, 2339–2351. Available online: https://www.tandfonline.com/doi/full/10.2147/OPTH.S255285 (accessed on 18 June 2025).
- Gundersen, K.G.; Potvin, R. Comparing Visual Acuity, Low Contrast Acuity and Contrast Sensitivity After Trifocal Toric and Extended Depth of Focus Toric Intraocular Lens Implantation. Clin. Ophthalmol. 2020, 14, 1071–1078. [Google Scholar] [CrossRef] [PubMed]
- Fernández, J.; Ribeiro, F.; Rocha-de-Lossada, C.; Rodríguez-Vallejo, M. Functional Classification of Intraocular Lenses Based on Defocus Curves: A Scoping Review and Cluster Analysis. J. Refract. Surg. 2024, 40, e108–e116. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Dong, Y.; Wang, Y. Efficacy and safety of extended depth of focus intraocular lenses in cataract surgery: A systematic review and meta-analysis. BMC Ophthalmol. 2019, 19, 198. [Google Scholar] [CrossRef] [PubMed]
- Corbett, D.; Black, D.; Roberts, T.V.; Cronin, B.; Gunn, D.; Bala, C.; Versace, P.; Tsai, L.; Papadatou, E.; Alarcon, A.; et al. Quality of vision clinical outcomes for a new fully-refractive extended depth of focus Intraocular Lens. Eye 2024, 38, 9–14. [Google Scholar] [CrossRef]
| Technical name | Elon 877PEY |
| Manufacturer | Medicontur (Zsámbék, Hungary) |
| Material | Hydrophobic acrylic |
| Overall diameter (mm) | 13.00 |
| Optic diameter (mm) | 6.00 |
| Optic design | Biconvex aspheric |
| Haptic design | Posterior vaulting fenestrated C-loop with 0° angulation |
| Refractive index | 1.47 |
| Abbe number | 58 |
| A constant (SRK/T) | 118.9 |
| Standard powers (D) | +8.0 D, +30.0 D (0.5 D steps) |
| Extreme powers (D) | +31.0 D, +35.0 D (1.0 D steps) |
| Filtration | UV, blue light |
| Variable | Mean ± SD | Range |
|---|---|---|
| Participants (n) | 28 | - |
| Eyes analyzed (n) | 56 | - |
| Gender (male, %) | 52% | - |
| Working status (active, %) | 62% | - |
| Age (years) | 64.5 ± 9.5 | [47, 86] |
| UDVA (decimal) | 0.20 ± 0.26 | [0.00, 0.90] |
| CDVA (decimal) | 0.84 ± 0.23 | [0.50, 1.00] |
| Sphere (D) | 1.09 ± 3.17 | [−11.25, +7.75] |
| Cylinder (D) | −0.62 ± 0.45 | [−1.25, 0.00] |
| Variable | Mean ± SD (Decimal) | Range (Decimal) |
|---|---|---|
| UDVA monocular | 0.94 ± 0.26 | [0.40, 1.66] |
| UDVA binocular | 1.11 ± 0.33 | [0.80, 1.66] |
| CDVA monocular | 1.05 ± 0.20 | [0.63, 1.66] |
| CDVA binocular | 1.19 ± 0.22 | [0.90, 1.66] |
| UIVA monocular | 0.79 ± 0.17 | [0.30, 1.00] |
| UIVA binocular | 0.90 ± 0.16 | [0.50, 1.20] |
| CIVA monocular | 0.82 ± 0.18 | [0.40, 1.00] |
| CIVA binocular | 0.88 ± 0.22 | [0.60, 1.20] |
| UNVA monocular | 0.60 ± 0.17 | [0.25, 1.00] |
| UNVA binocular | 0.69 ± 0.14 | [0.50, 1.00] |
| CNVA monocular | 0.58 ± 0.15 | [0.30, 0.90] |
| CNVA binocular | 0.65 ± 0.17 | [0.30, 0.90] |
| CS monocular (log) | 1.61 ± 0.15 | [1.20, 1.80] |
| CS binocular (log) | 1.77 ± 0.14 | [1.50, 1.95] |
| Symptom | Mean ± SD (Score) | ||
|---|---|---|---|
| Frequency | Severity | Bothersome | |
| Glare | 1.1 ± 1.0 | 1.1 ± 1.0 | 0.8 ± 0.9 |
| Starburst | 1.0 ± 1.0 | 0.9 ± 0.9 | 0.7 ± 0.9 |
| Blurred vision | 0.6 ± 0.8 | 0.6 ± 0.7 | 0.4 ± 0.6 |
| Double vision | 0.1 ± 0.3 | 0.1 ± 0.3 | 0.1 ± 0.3 |
| Halos | 1.2 ± 1.2 | 1.0 ± 1.0 | 0.9 ± 0.9 |
| Hazy vision | 0.6 ± 0.7 | 0.6 ± 0.8 | 0.5 ± 0.6 |
| Distortion | 0.2 ± 0.6 | 0.2 ± 0.6 | 0.2 ± 0.6 |
| Vision fluctuation | 0.4 ± 0.6 | 0.3 ± 0.5 | 0.2 ± 0.4 |
| Focusing difficulties | 0.9 ± 0.9 | 0.6 ± 0.8 | 0.6 ± 0.8 |
| Difficult judging distance | 0.1 ± 0.4 | 0.1 ± 0.4 | 0.1 ± 0.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cano-Ortiz, A.; Sánchez-Ventosa, Á.; González-Cruces, T.; Villalba-González, M.; Aguilar-Salazar, F.J.; Prados-Carmona, J.J.; Carpena-Torres, C.; Carracedo, G.; Villarrubia, A. Visual Quality and Symptomatology Following Implantation of a Non-Diffractive Extended Depth-of-Focus Intraocular Lens. J. Clin. Med. 2025, 14, 4460. https://doi.org/10.3390/jcm14134460
Cano-Ortiz A, Sánchez-Ventosa Á, González-Cruces T, Villalba-González M, Aguilar-Salazar FJ, Prados-Carmona JJ, Carpena-Torres C, Carracedo G, Villarrubia A. Visual Quality and Symptomatology Following Implantation of a Non-Diffractive Extended Depth-of-Focus Intraocular Lens. Journal of Clinical Medicine. 2025; 14(13):4460. https://doi.org/10.3390/jcm14134460
Chicago/Turabian StyleCano-Ortiz, Antonio, Álvaro Sánchez-Ventosa, Timoteo González-Cruces, Marta Villalba-González, Francisco Javier Aguilar-Salazar, Juan J. Prados-Carmona, Carlos Carpena-Torres, Gonzalo Carracedo, and Alberto Villarrubia. 2025. "Visual Quality and Symptomatology Following Implantation of a Non-Diffractive Extended Depth-of-Focus Intraocular Lens" Journal of Clinical Medicine 14, no. 13: 4460. https://doi.org/10.3390/jcm14134460
APA StyleCano-Ortiz, A., Sánchez-Ventosa, Á., González-Cruces, T., Villalba-González, M., Aguilar-Salazar, F. J., Prados-Carmona, J. J., Carpena-Torres, C., Carracedo, G., & Villarrubia, A. (2025). Visual Quality and Symptomatology Following Implantation of a Non-Diffractive Extended Depth-of-Focus Intraocular Lens. Journal of Clinical Medicine, 14(13), 4460. https://doi.org/10.3390/jcm14134460







