Impact of Age at Narcolepsy Onset on Sleep-Onset REM Periods in the Multiple Sleep Latency Test
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BMI | body mass index |
| CSF | cerebrospinal fluid |
| EDS | excessive daytime sleepiness |
| ESS | Epworth Sleepiness Scale |
| MSLT | multiple sleep latency test |
| NT1 | narcolepsy type 1 |
| NT2 | narcolepsy type 2 |
| PSG | polysomnography |
| PSQI | Pittsburgh Sleep Quality Index |
| REM | rapid eye movement |
| SOREMP | sleep-onset rapid eye movement period |
References
- Scammell, T.E. Narcolepsy. N. Engl. J. Med. 2015, 373, 2654–2662. [Google Scholar] [CrossRef] [PubMed]
- American Academy of Sleep Medicine. International Classification of Sleep Disorders, 3rd ed.; American Academy of Sleep Medicine: Darien, IL, USA, 2014. [Google Scholar]
- Kornum, B.R.; Knudsen, S.; Ollila, H.M.; Pizza, F.; Jennum, P.J.; Dauvilliers, Y.; Overeem, S. Narcolepsy. Nat. Rev. Dis. Primers 2017, 3, 16100. [Google Scholar] [CrossRef] [PubMed]
- Shin, Y.K.; Yoon, I.Y.; Han, E.K.; No, Y.M.; Hong, M.C.; Yun, Y.D.; Jung, B.K.; Chung, S.H.; Choi, J.B.; Cyn, J.G.; et al. Prevalence of narcolepsy-cataplexy in Korean adolescents. Acta Neurol. Scand. 2008, 117, 273–278. [Google Scholar] [CrossRef] [PubMed]
- Ohayon, M.M.; Ferini-Strambi, L.; Plazzi, G.; Smirne, S.; Castronovo, V. How age influences the expression of narcolepsy. J. Psychosom. Res. 2005, 59, 399–405. [Google Scholar] [CrossRef]
- Dauvilliers, Y.; Montplaisir, J.; Molinari, N.; Carlander, B.; Ondze, B.; Besset, A.; Billiard, M. Age at onset of narcolepsy in two large populations of patients in France and Quebec. Neurology 2001, 57, 2029–2033. [Google Scholar] [CrossRef]
- Park, H.R.; Song, P.; Lee, S.Y.; Epidemiology Committee of Korean Sleep Research. National Estimates of Narcolepsy in Korea. J. Clin. Neurol. 2023, 19, 83–89. [Google Scholar] [CrossRef]
- Almeneessier, A.S.; Alballa, N.S.; Alsalman, B.H.; Aleissi, S.; Olaish, A.H.; BaHammam, A.S. A 10-Year Longitudinal Observational Study of Cataplexy in a Cohort of Narcolepsy Type 1 Patients. Nat. Sci. Sleep 2019, 11, 231–239. [Google Scholar] [CrossRef]
- Dauvilliers, Y.; Gosselin, A.; Paquet, J.; Touchon, J.; Billiard, M.; Montplaisir, J. Effect of age on MSLT results in patients with narcolepsy-cataplexy. Neurology 2004, 62, 46–50. [Google Scholar] [CrossRef]
- Nevsimalova, S.; Buskova, J.; Kemlink, D.; Sonka, K.; Skibova, J. Does age at the onset of narcolepsy influence the course and severity of the disease? Sleep Med. 2009, 10, 967–972. [Google Scholar] [CrossRef]
- Krahn, L.E.; Arand, D.L.; Avidan, A.Y.; Davila, D.G.; DeBassio, W.A.; Ruoff, C.M.; Harrod, C.G. Recommended protocols for the Multiple Sleep Latency Test and Maintenance of Wakefulness Test in adults: Guidance from the American Academy of Sleep Medicine. J. Clin. Sleep Med. 2021, 17, 2489–2498. [Google Scholar] [CrossRef]
- Luppi, P.H.; Clement, O.; Sapin, E.; Gervasoni, D.; Peyron, C.; Leger, L.; Salvert, D.; Fort, P. The neuronal network responsible for paradoxical sleep and its dysfunctions causing narcolepsy and rapid eye movement (REM) behavior disorder. Sleep Med. Rev. 2011, 15, 153–163. [Google Scholar] [CrossRef] [PubMed]
- Roman, A.; Meftah, S.; Arthaud, S.; Luppi, P.H.; Peyron, C. The inappropriate occurrence of rapid eye movement sleep in narcolepsy is not due to a defect in homeostatic regulation of rapid eye movement sleep. Sleep 2018, 41, zsy046. [Google Scholar] [CrossRef] [PubMed]
- Suominen, K.; Mantere, O.; Valtonen, H.; Arvilommi, P.; Leppamaki, S.; Paunio, T.; Isometsa, E. Early age at onset of bipolar disorder is associated with more severe clinical features but delayed treatment seeking. Bipolar Disord. 2007, 9, 698–705. [Google Scholar] [CrossRef] [PubMed]
- Mendez, M.F. Early-Onset Alzheimer Disease. Neurol. Clin. 2017, 35, 263–281. [Google Scholar] [CrossRef]
- Zisook, S.; Lesser, I.; Stewart, J.W.; Wisniewski, S.R.; Balasubramani, G.K.; Fava, M.; Gilmer, W.S.; Dresselhaus, T.R.; Thase, M.E.; Nierenberg, A.A.; et al. Effect of age at onset on the course of major depressive disorder. Am. J. Psychiatry 2007, 164, 1539–1546. [Google Scholar] [CrossRef]
- Lividini, A.; Pizza, F.; Filardi, M.; Vandi, S.; Ingravallo, F.; Antelmi, E.; Bruni, O.; Cosentino, F.I.I.; Ferri, R.; Guarnieri, B.; et al. Narcolepsy type 1 features across the life span: Age impact on clinical and polysomnographic phenotype. J. Clin. Sleep Med. 2021, 17, 1363–1370. [Google Scholar] [CrossRef]
- Cairns, A.; Trotti, L.M.; Bogan, R. Demographic and nap-related variance of the MSLT: Results from 2498 suspected hypersomnia patients: Clinical MSLT variance. Sleep Med. 2019, 55, 115–123. [Google Scholar] [CrossRef]
- Zhang, Z.; Dauvilliers, Y.; Plazzi, G.; Mayer, G.; Lammers, G.J.; Santamaria, J.; Partinen, M.; Overeem, S.; Del Rio Villegas, R.; Sonka, K.; et al. Idling for Decades: A European Study on Risk Factors Associated with the Delay Before a Narcolepsy Diagnosis. Nat. Sci. Sleep 2022, 14, 1031–1047. [Google Scholar] [CrossRef]
- Ohayon, M.M.; Thorpy, M.J.; Carls, G.; Black, J.; Cisternas, M.; Pasta, D.J.; Bujanover, S.; Hyman, D.; Villa, K.F. The Nexus Narcolepsy Registry: Methodology, study population characteristics, and patterns and predictors of narcolepsy diagnosis. Sleep Med. 2021, 84, 405–414. [Google Scholar] [CrossRef]
- Jiang, R.Y.; Rochart, R.; Chu, I.; Duka, S.; Vendrame, M. The Lehigh Valley Health Network Narcolepsy Cohort: Clinical and polysomnographic analysis of 304 cases. J. Clin. Sleep Med. 2025, 21, 479–491. [Google Scholar] [CrossRef]
- Akyildiz, U.O.; Tezer, F.I.; Koc, G.; Ismailogullari, S.; Demir, A.B.; Ak, A.K.; Sunter, G.; Kara, K.A.; Berktas, D.T.; Sahin, A.; et al. The REM-sleep-related characteristics of narcolepsy: A nation-wide multicenter study in Turkey, the REMCON study. Sleep Med. 2022, 94, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Mayer, G.; Dauvilliers, Y.; Plazzi, G.; Pizza, F.; Fronczek, R.; Santamaria, J.; Partinen, M.; Overeem, S.; Peraita-Adrados, R.; et al. Exploring the clinical features of narcolepsy type 1 versus narcolepsy type 2 from European Narcolepsy Network database with machine learning. Sci. Rep. 2018, 8, 10628. [Google Scholar] [CrossRef]
- Sansa, G.; Falup-Pecurariu, C.; Salamero, M.; Iranzo, A.; Santamaria, J. Non-random temporal distribution of sleep onset REM periods in the MSLT in narcolepsy. J. Neurol. Sci. 2014, 341, 136–138. [Google Scholar] [CrossRef] [PubMed]
- Bes, F.W.; Jobert, M.; Cordula Muller, L.; Schulz, H. The diurnal distribution of sleep propensity: Experimental data about the interaction of the propensities for slow-wave sleep and REM sleep. J. Sleep Res. 1996, 5, 90–98. [Google Scholar] [CrossRef] [PubMed]
- Charles, A.C.; Janet, C.Z.; Joseph, M.R.; Martin, C.M.-E.; Elliot, D.W. Timing of REM Sleep is Coupled to the Circadian Rhythm of Body Temperature in Man. Sleep 1979, 2, 329–346. [Google Scholar] [CrossRef]
- Aurora, R.N.; Caffo, B.; Crainiceanu, C.; Punjabi, N.M. Correlating subjective and objective sleepiness: Revisiting the association using survival analysis. Sleep 2011, 34, 1707–1714. [Google Scholar] [CrossRef]
- Punjabi, N.M.; O’Hearn, D.J.; Neubauer, D.N.; Nieto, F.J.; Schwartz, A.R.; Smith, P.L.; Bandeen-Roche, K. Modeling hypersomnolence in sleep-disordered breathing. A novel approach using survival analysis. Am. J. Respir. Crit. Care Med. 1999, 159, 1703–1709. [Google Scholar] [CrossRef]
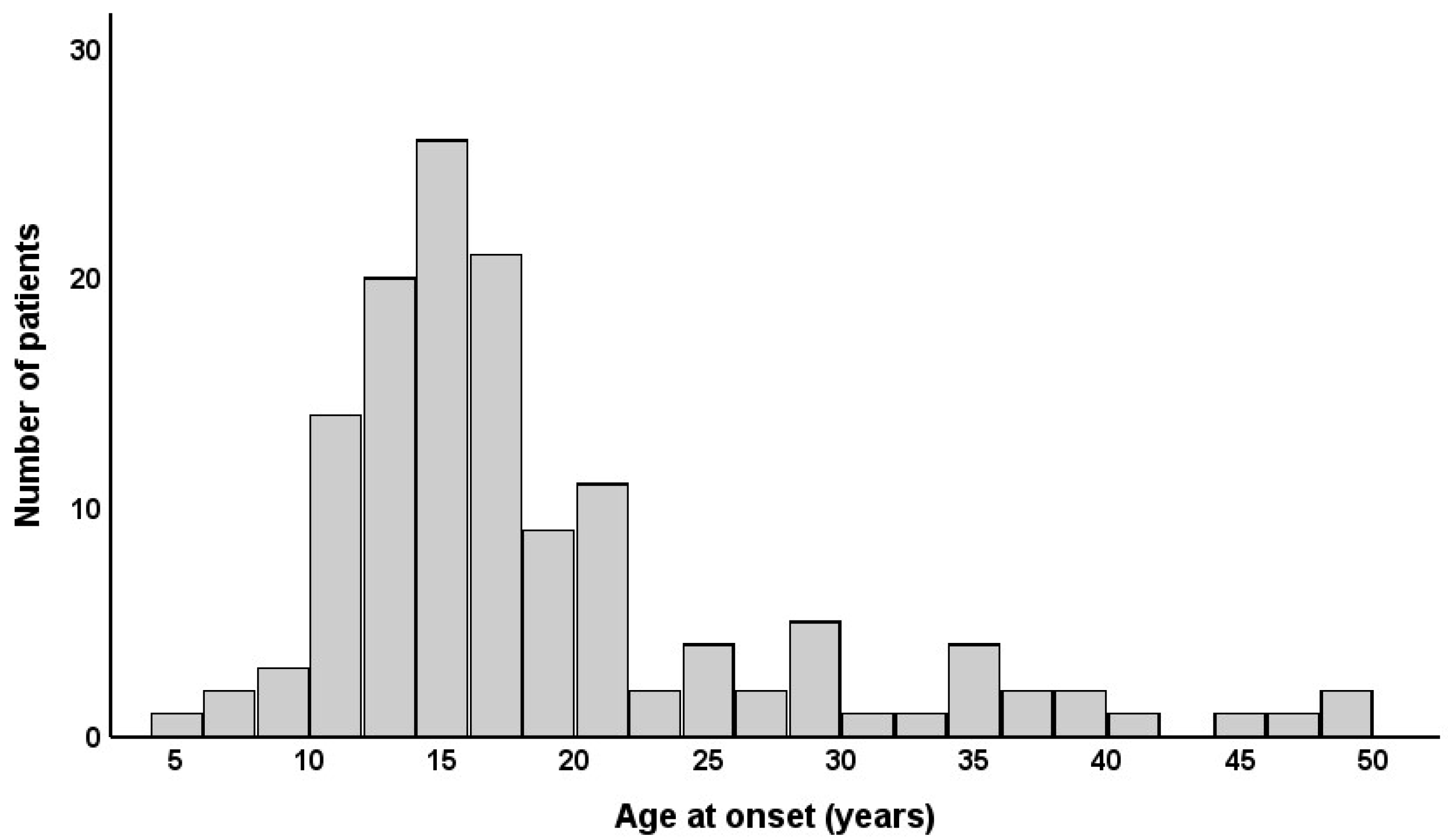

| Variables | Total (n = 135) | Early Onset (n = 105) | Late Onset (n = 30) | p |
|---|---|---|---|---|
| Age at onset, y | 18.3 ± 8.8 | 14.4 ± 3.3 | 32.0 ± 8.1 | <0.001 |
| Age at diagnosis, y | 28.1 ± 13.2 | 24.8 ±12.0 | 39.7 ± 10.2 | <0.001 |
| Sex, male | 79 (58.5%) | 61 (58.1%) | 18 (60.0%) | 0.852 |
| Narcolepsy type | 0.141 | |||
| NT1 | 70 (51.9%) | 58 (55.2%) | 12 (40.0%) | |
| NT2 | 65 (48.1%) | 47 (44.8%) | 18 (60.0%) | |
| BMI, kg/m2 | 25.1 ± 4.5 | 25.2 ± 4.8 | 24.9 ± 3.5 | 0.930 |
| Current smoker (n = 134) | 39 (29.1%) | 29 (27.6%) | 10 (34.5%) | 0.471 |
| Coffee (n = 126) | 78 (61.9%) | 58 (58.6%) | 20 (74.1%) | 0.142 |
| Caffeine drink (n = 76) | 20 (26.3%) | 15 (25.9%) | 5 (27.8%) | 1 |
| ESS score (n = 132) | 15.8 ± 3.9 | 15.7 ± 3.8 | 16.1 ± 4.5 | 0.689 |
| Nap (n = 129) | 113 (87.6%) | 88 (88.0%) | 25 (86.2%) | 0.756 |
| PSQI score (n = 104) | 10.7 ± 5.3 | 11.1 ± 5.5 | 9.3 ± 4.5 | 0.136 |
| PSQI score > 5 | 93 (89.4%) | 73 (91.3%) | 20 (83.3%) | 0.273 |
| Cataplexy | 70 (51.9%) | 58 (55.2%) | 12 (40.0%) | 0.141 |
| Hallucination (n = 114) | 46 (40.4%) | 39 (43.8%) | 7 (28.0%) | 0.154 |
| Sleep paralysis (n = 113) | 52 (46.0%) | 41 (47.1%) | 11 (42.3%) | 0.665 |
| Tetrad | 24 (17.8%) | 20 (19.0%) | 4 (13.3%) | 0.470 |
| Triad | 31 (23.0%) | 26 (24.8%) | 5 (16.7%) | 0.353 |
| Variables | Total (n = 135) | Early Onset (n = 105) | Late Onset (n = 30) | p |
|---|---|---|---|---|
| MSLT | ||||
| Mean sleep latency, min | 2.8 ± 2.1 | 2.9 ± 2.2 | 2.8 ± 1.7 | 0.518 |
| No. of SOREMPs | 3.4 ± 1.1 | 3.4 ± 1.1 | 3.5 ± 1.1 | 0.732 |
| Positive SOREMP | ||||
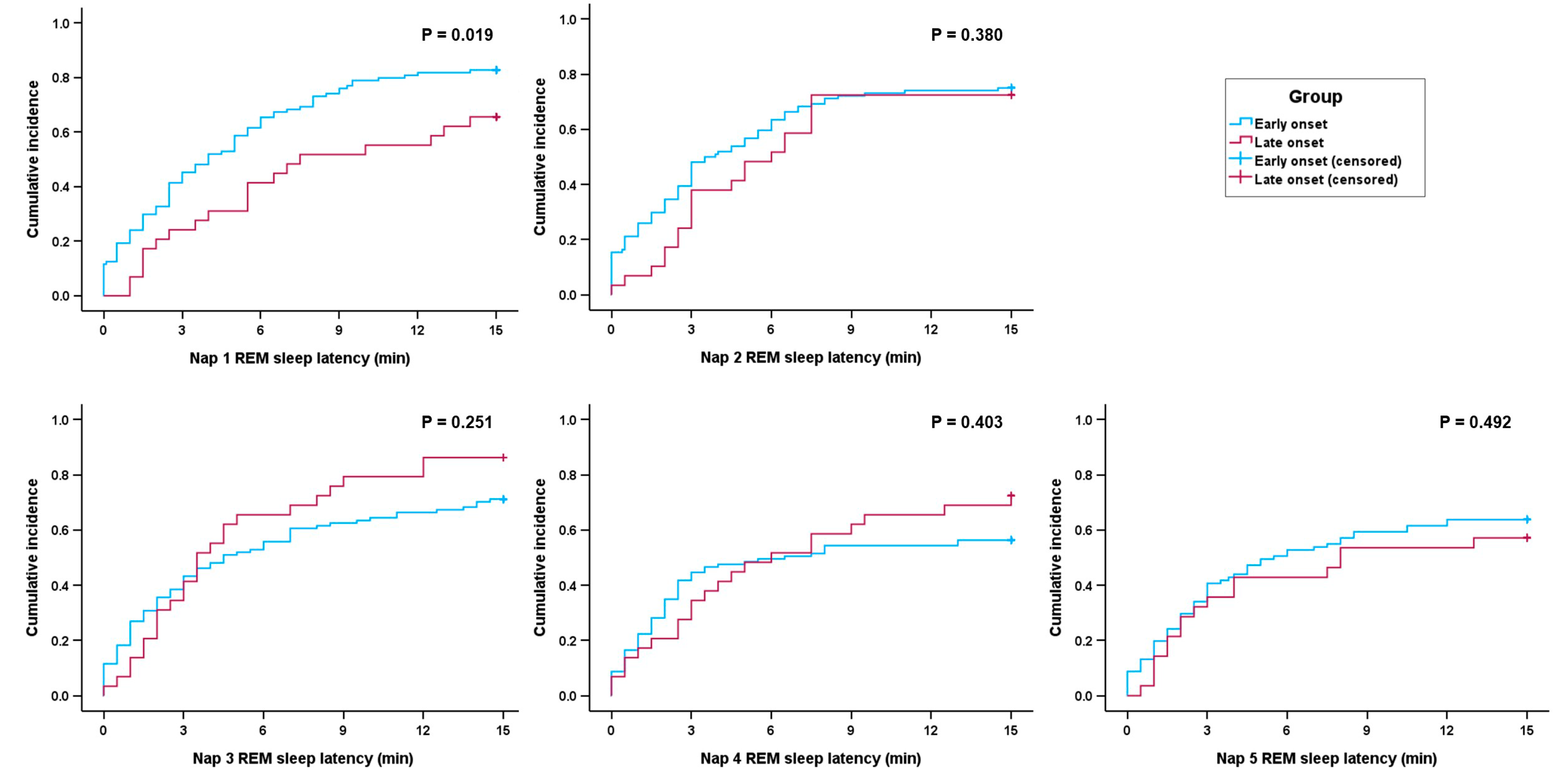
| 1st nap | 105 (77.8%) | 86 (81.9%) | 19 (63.3%) | 0.031 |
| 2nd nap | 99 (73.3%) | 78 (74.3%) | 21 (70.0%) | 0.640 |
| 3rd nap | 99 (73.3%) | 74 (70.5%) | 25 (83.3%) | 0.160 |
| 4th nap | 79 (58.5%) | 58 (55.2%) | 21 (70.0%) | 0.148 |
| 5th nap (n = 123) | 74 (60.2%) | 58 (61.7%) | 16 (55.2%) | 0.530 |
| REM sleep latency, min (n = 133) | 4.1 ± 3.0 | 3.9 ± 2.9 | 4.8 ± 3.0 | 0.111 |
| PSG | ||||
| TST, min | 459.3 ± 58.8 | 461.4 ± 58.7 | 452.1 ± 59.5 | 0.383 |
| Sleep latency, min | 5.5 ± 8.8 | 6.0 ± 9.6 | 3.7 ± 4.9 | 0.033 |
| REM sleep latency, min | 61.3 ± 70.0 | 60.2 ± 65.4 | 65.1 ± 85.2 | 0.683 |
| Nocturnal SOREMP | 55 (40.7%) | 43 (41.0%) | 12 (40.0%) | 0.537 |
| Sleep efficiency, % | 90.3 ± 6.7 | 90.4 ± 6.8 | 89.9 ± 6.3 | 0.715 |
| WASO, min | 47.1 ± 43.9 | 47.8 ± 47.3 | 44.3 ± 29.3 | 0.617 |
| N1, % | 17.7 ± 12.2 | 16.8 ± 11.5 | 20.7 ± 14.1 | 0.242 |
| N2, % | 50.7 ± 11.7 | 49.9 ± 11.9 | 53.5 ± 10.6 | 0.165 |
| N3, % | 9.5 ± 9.1 | 10.8 ± 9.1 | 5.0 ± 7.4 | <0.001 |
| REM, % | 21.7 ± 6.9 | 22.0 ± 6.6 | 20.9 ± 8.2 | 0.454 |
| Arousal index, /h | 15.7 ± 12.0 | 14.7 ± 12.2 | 19.5 ± 10.6 | 0.016 |
| AHI, /h | 6.6 ± 9.7 | 6.2 ± 10.5 | 7.8 ± 6.5 | 0.023 |
| Mean SpO2, % | 95.7 ± 1.8 | 95.8 ± 1.9 | 95.5 ± 1.6 | 0.258 |
| Minimum SpO2, % | 89.8 ± 4.3 | 90.2 ± 4.5 | 88.7 ± 3.6 | 0.016 |
| PLMI, /h (n = 129) | 5.2 ± 12.8 | 4.6 ± 12.3 | 7.5 ± 14.2 | 0.973 |
| Variables | Coefficient * | p |
|---|---|---|
| MSLT | ||
| REM sleep latency, min | 0.241 | 0.005 |
| Mean sleep latency, min | 0.099 | 0.255 |
| No. of SOREMPs | −0.047 | 0.590 |
| PSG | ||
| TST, min | −0.144 | 0.095 |
| Sleep latency, min | −0.041 | 0.640 |
| REM sleep latency, min | −0.058 | 0.505 |
| Sleep efficiency, % | −0.030 | 0.729 |
| WASO, min | −0.018 | 0.837 |
| N1, % | 0.090 | 0.299 |
| N2, % | 0.089 | 0.306 |
| N3, % | −0.359 | <0.001 |
| REM, % | 0.059 | 0.494 |
| Arousal index, /h | 0.200 | 0.020 |
| AHI, /h | 0.127 | 0.142 |
| PLMI, /h | 0.007 | 0.936 |
| Clinical | ||
| BMI, kg/m2 | 0.054 | 0.534 |
| ESS score | 0.043 | 0.622 |
| PSQI score | −0.050 | 0.612 |
| Univariate | Multivariate | |||||
|---|---|---|---|---|---|---|
| Variables | β | 95% CI | p | β | 95% CI | p |
| Age at onset, y | 0.069 | 0.011–0.127 | 0.021 | 0.049 | 0.004–0.095 | 0.033 |
| Female (vs. male) | 0.320 | −0.716–1.357 | 0.542 | 0.249 | −0.564–1.063 | 0.545 |
| BMI, kg/m2 | −0.174 | −0.283–−0.066 | 0.022 | −0.106 | −0.194–−0.018 | 0.018 |
| NT1 (vs. NT2) | −2.742 | −3.649–−1.834 | <0.001 | −1.083 | −1.968–−0.199 | 0.017 |
| MSLT | ||||||
| No. of SOREMPs | −1.475 | −1.864–−1.085 | <0.001 | −0.921 | −1.335–−0.507 | <0.001 |
| Mean sleep latency, min | 0.644 | 0.427–0.861 | <0.001 | 0.258 | 0.044–0.472 | 0.018 |
| PSG | ||||||
| Sleep efficiency, % | 0.134 | 0.061–0.207 | <0.001 | 0.058 | −0.003–0.119 | 0.064 |
| WASO, min | −0.018 | −0.029–−0.007 | 0.002 | |||
| N1, % | −0.082 | −0.122–−0.043 | <0.001 | |||
| Univariate | Multivariate | |||||
|---|---|---|---|---|---|---|
| Variables | HR | 95% CI | p | HR | 95% CI | p |
| Age at onset, y | 0.966 | 0.941–0.991 | 0.009 | 0.955 | 0.929–0.982 | 0.001 |
| Female (vs. male) | 0.792 | 0.534–1.175 | 0.792 | 0.868 | 0.582–1.296 | 0.490 |
| BMI, kg/m2 | 1.033 | 0.991–1.077 | 0.122 | |||
| NT1 (vs. NT2) | 1.718 | 1.166–2.532 | 0.006 | 1.093 | 0.718–1.665 | 0.678 |
| ESS score | 0.998 | 0.950–1.048 | 0.938 | |||
| PSG sleep efficiency, % | 0.997 | 0.969–1.025 | 0.829 | |||
| No. of SOREMPs | 2.121 | 1.719–2.617 | <0.001 | 2.155 | 1.707–2.720 | <0.001 |
| Mean sleep latency, min | 0.858 | 0.771–0.954 | 0.005 | 0.983 | 0.878–1.100 | 0.764 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sunwoo, J.-S.; Ji, K.-H.; Kim, D.; Kim, K.M.; Choi, Y.H.; Cho, J.W.; Kim, H.; Lee, W.; Jung, Y.J.; Koo, D.L.; et al. Impact of Age at Narcolepsy Onset on Sleep-Onset REM Periods in the Multiple Sleep Latency Test. J. Clin. Med. 2025, 14, 4379. https://doi.org/10.3390/jcm14124379
Sunwoo J-S, Ji K-H, Kim D, Kim KM, Choi YH, Cho JW, Kim H, Lee W, Jung YJ, Koo DL, et al. Impact of Age at Narcolepsy Onset on Sleep-Onset REM Periods in the Multiple Sleep Latency Test. Journal of Clinical Medicine. 2025; 14(12):4379. https://doi.org/10.3390/jcm14124379
Chicago/Turabian StyleSunwoo, Jun-Sang, Ki-Hwan Ji, Daeyoung Kim, Kyung Min Kim, Yun Ho Choi, Jae Wook Cho, Hyeyun Kim, Wonwoo Lee, Yu Jin Jung, Dae Lim Koo, and et al. 2025. "Impact of Age at Narcolepsy Onset on Sleep-Onset REM Periods in the Multiple Sleep Latency Test" Journal of Clinical Medicine 14, no. 12: 4379. https://doi.org/10.3390/jcm14124379
APA StyleSunwoo, J.-S., Ji, K.-H., Kim, D., Kim, K. M., Choi, Y. H., Cho, J. W., Kim, H., Lee, W., Jung, Y. J., Koo, D. L., Im, H.-J., & Yang, K. I. (2025). Impact of Age at Narcolepsy Onset on Sleep-Onset REM Periods in the Multiple Sleep Latency Test. Journal of Clinical Medicine, 14(12), 4379. https://doi.org/10.3390/jcm14124379









