A Head-to-Head Comparison Between [18F]Fluorodeoxyglucose ([18F]FDG) Positron Emission Tomography/Computed Tomography (PET/CT) and 99mTechnetium-Hexamethylpropylene Amine Oxime (HMPAO)-Labeled Leukocyte Scintigraphy in a Case Series of Patients with Suspected Vascular Prosthesis Infection: To Trust Is Good, but to Check Is Better
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Population
2.2. Exclusion Criteria
2.3. Imaging Equipment and Radiopharmaceuticals [18F]FDG PET/CT
2.4. Radiolabeling of Autologous Leukocytes and WBC Scintigraphy
2.5. Interpretation Criteria
2.6. Data Analysis
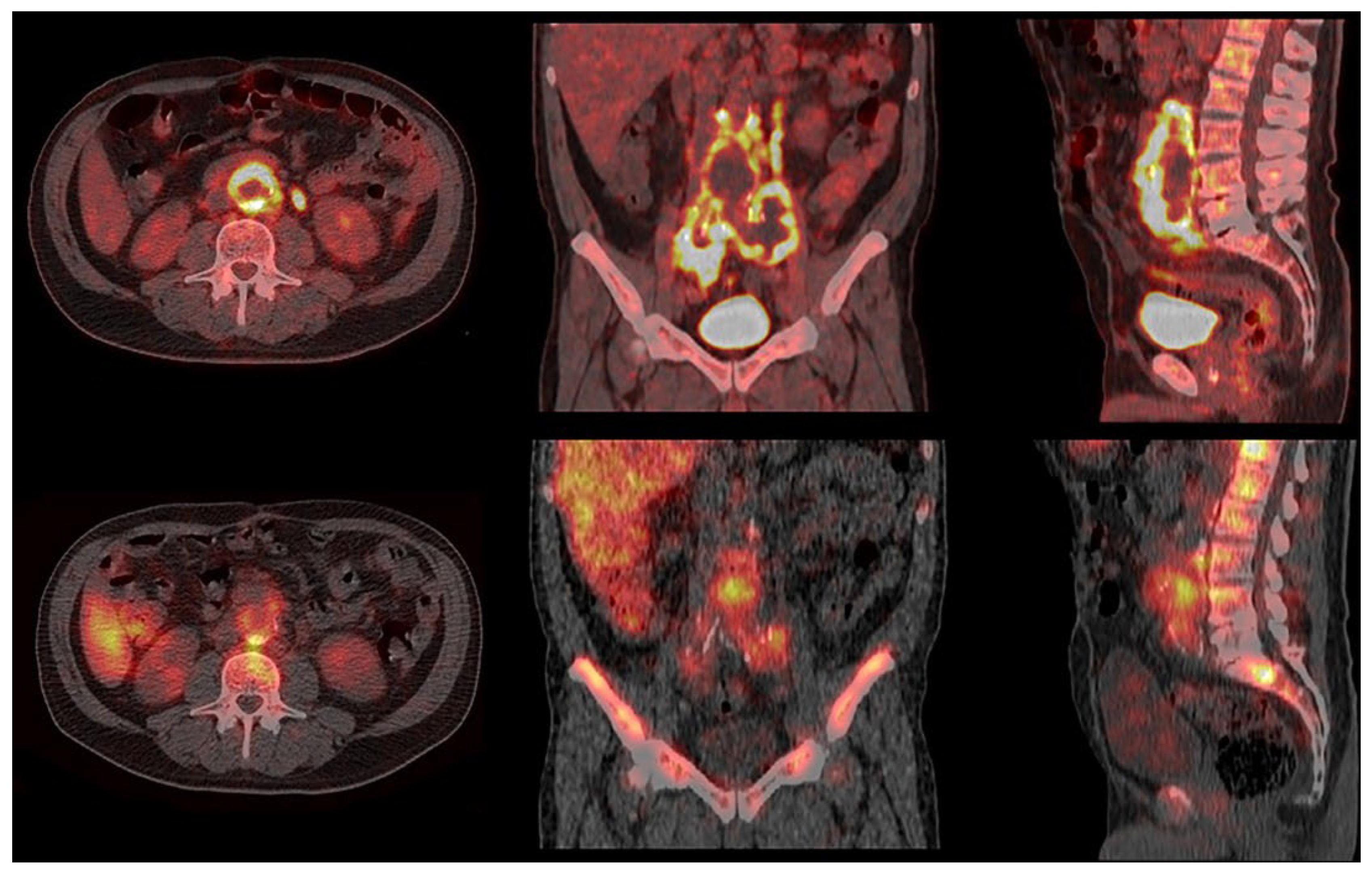
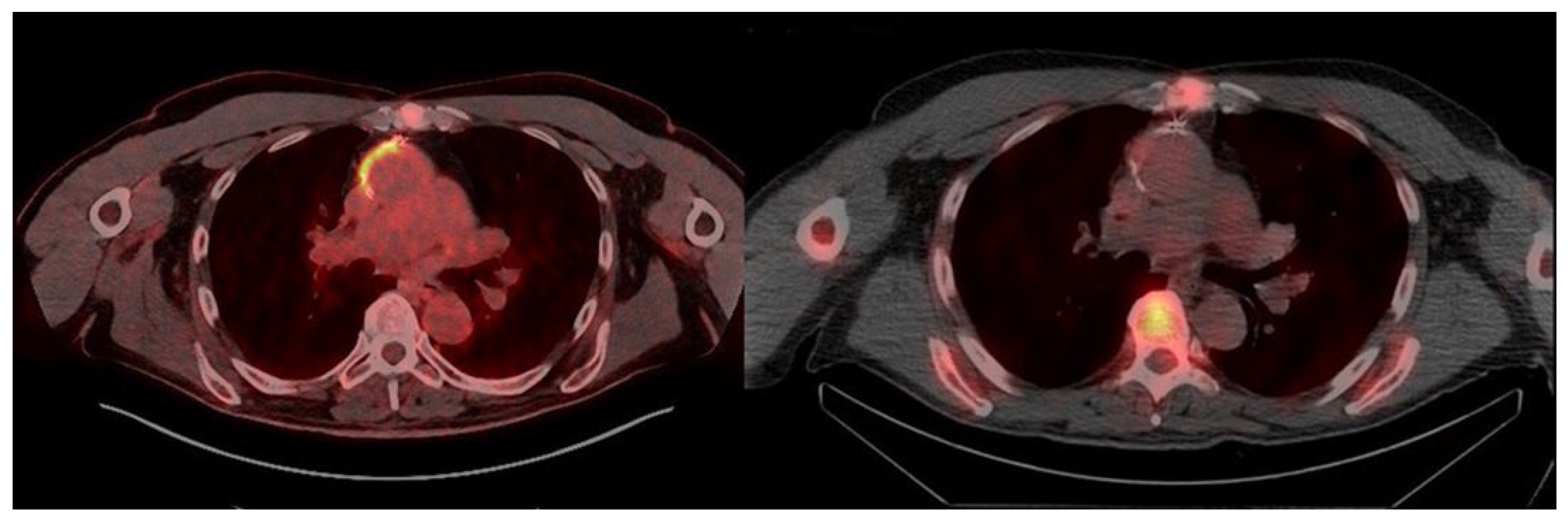
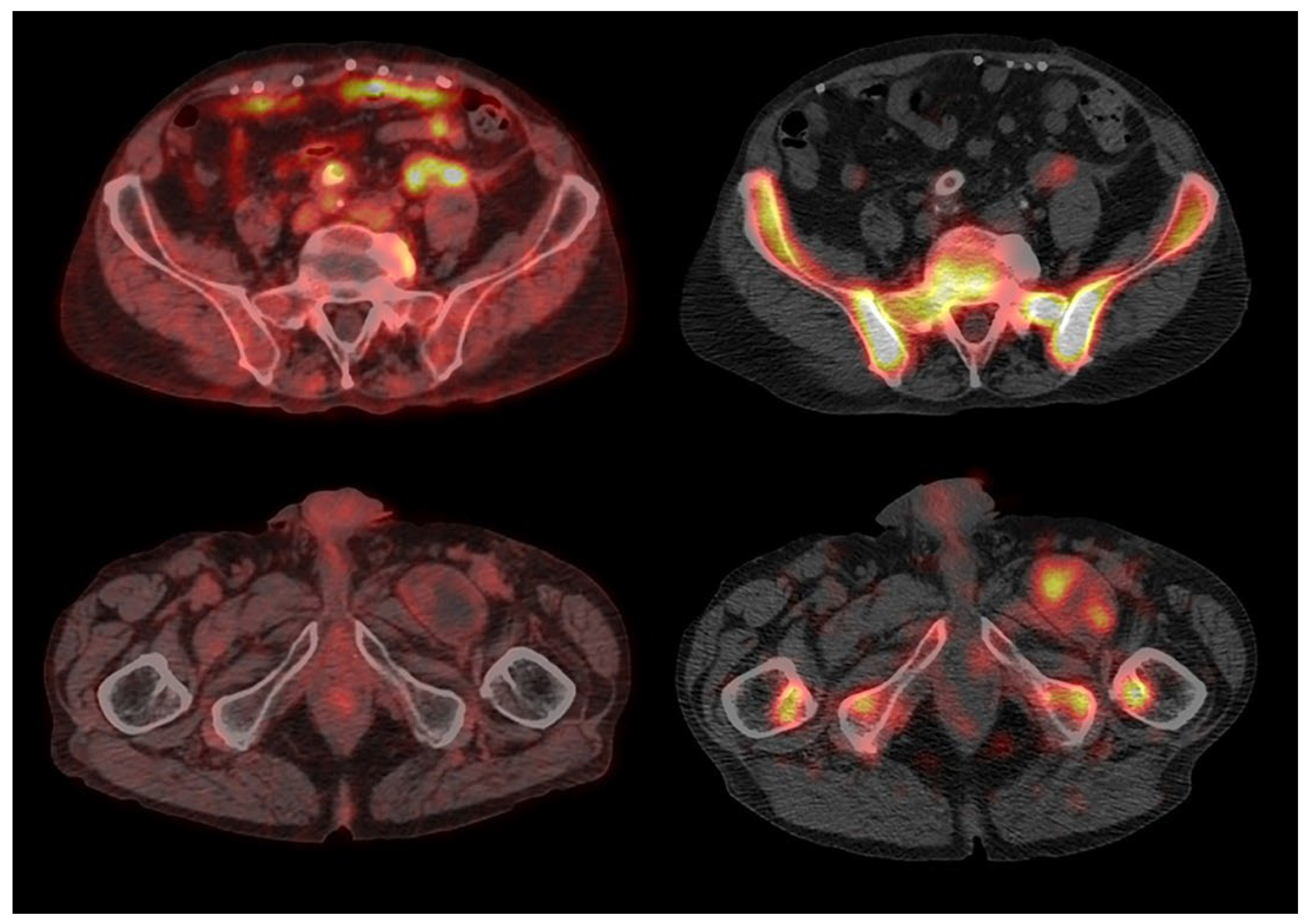
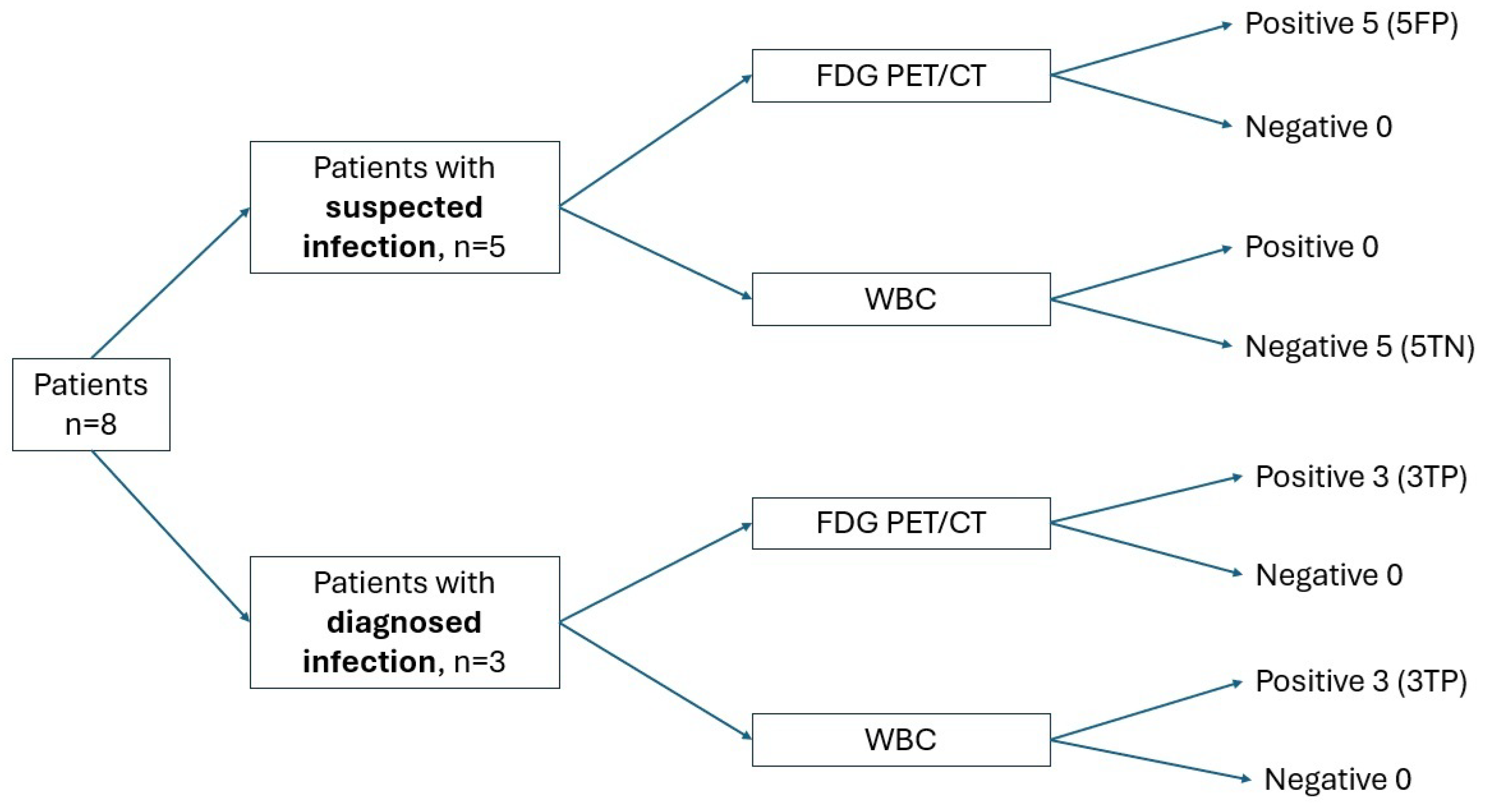
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| PVGI | Prosthetic vascular graft infection |
| CTA | CT angiography |
| [18F]FDG | Glucose analog 2-deoxy-2-[18F]fluoro-D-glucose |
| WBC | White blood cells |
| MAGIC | Management of Aortic Graft Infection Collaboration |
| MSSA | Methicillin-sensitive Staphylococcus aureus |
| PTFE | Polytetrafluoroethylene |
References
- Van Hemelrijck, M.; Sromicki, J.; Husmann, L.; Rancic, Z.; Hasse, B.; Carrel, T.P. Vascular Graft Infections. Vessel. Plus 2022, 6, 47. [Google Scholar] [CrossRef]
- Lauri, C.; Iezzi, R.; Rossi, M.; Tinelli, G.; Sica, S.; Signore, A.; Posa, A.; Tanzilli, A.; Panzera, C.; Taurino, M.; et al. Imaging Modalities for the Diagnosis of Vascular Graft Infections: A Consensus Paper amongst Different Specialists. J. Clin. Med. 2020, 9, 1510. [Google Scholar] [CrossRef] [PubMed]
- Folmer, E.I.R.; Von Meijenfeldt, G.C.; Van der Laan, M.J.; Glaudemans, A.W.; Slart, R.H.; Saleem, B.R.; Zeebregts, C.J. Diagnostic Imaging in Vascular Graft Infection: A Systematic Review and Meta-Analysis. Eur. J. Vasc. Endovasc. Surg. 2018, 56, 719–729. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.W. Aortic Endograft Infection: Diagnosis and Management. Vasc. Spéc. Int. 2023, 39, 26. [Google Scholar] [CrossRef]
- Signore, A.; Jamar, F.; Israel, O.; Buscombe, J.; Martin-Comin, J.; Lazzeri, E. Clinical indications, image acquisition and data interpretation for white blood cells and anti-granulocyte monoclonal antibody scintigraphy: An EANM procedural guideline. Eur. J. Nucl. Med. 2018, 45, 1816–1831. [Google Scholar] [CrossRef]
- Chakfé, N.; Diener, H.; Lejay, A.; Assadian, O.; Berard, X.; Caillon, J.; Fourneau, I.; Glaudemans, A.W.; Koncar, I.; Lindholt, J.; et al. Editor’s Choice—European Society for Vascular Surgery (ESVS) 2020 Clinical Practice Guidelines on the Management of Vascular Graft and Endograft Infections. Eur. J. Vasc. Endovasc. Surg. 2020, 59, 339–384. [Google Scholar] [CrossRef]
- Lauri, C.; Signore, A.; Glaudemans, A.W.J.M.; Treglia, G.; Gheysens, O.; Slart, R.H.J.A.; Iezzi, R.; Prakken, N.H.J.; Debus, E.S.; Honig, S.; et al. Evidence-based guideline of the European Association of Nuclear Medicine (EANM) on imaging infection in vascular grafts. Eur. J. Nucl. Med. 2022, 49, 3430–3451. [Google Scholar] [CrossRef]
- Adlouni, M.; Sheikh, D.; Dang, V.; Koh, E.Y.; Fong, B.; Nathani, R.; Rahimi, M. Diagnosis of prosthetic vascular graft infection using the management aortic graft infection collaboration (MAGIC) criteria. Vascular 2025, 17085381251326995. [Google Scholar] [CrossRef]
- Puges, M.; Bérard, X.; Ruiz, J.-B.; Debordeaux, F.; Desclaux, A.; Stecken, L.; Pereyre, S.; Hocquelet, A.; Bordenave, L.; Pinaquy, J.-B.; et al. Retrospective Study Comparing WBC scan and 18F-FDG PET/CT in Patients with Suspected Prosthetic Vascular Graft Infection. Eur. J. Vasc. Endovasc. Surg. 2019, 57, 876–884. [Google Scholar] [CrossRef]
- Agius, C.; Rakotonirina, H.; Lacoeuille, F.; Bouchet, F.; Vervueren, L.; Le Jeune, J.-J.; Couturier, O. Infection de prothèse vasculaire: 18 TEP-FDG vs scintigraphie aux leucocytes marqués (planaires et TEMP/TDM). Med. Nucleaire-Imagerie Fonct. Metab. 2011, 35, 628–640. [Google Scholar] [CrossRef][Green Version]
- Costa, D.; Andreucci, M.; Ielapi, N.; Serraino, G.F.; Mastroroberto, P.; Bracale, U.M.; Serra, R. Infection of Vascular Prostheses: A Comprehensive Review. Prosthesis 2023, 5, 148–166. [Google Scholar] [CrossRef]
- de Vries, E.F.; Roca, M.; Jamar, F.; Israel, O.; Signore, A. Guidelines for the labelling of leucocytes with (99m)Tc-HMPAO. Inflammation/Infection Taskgroup of the European Association of Nuclear Medicine. Eur. J. Nucl. Med. Mol. Imaging 2010, 37, 842–848. [Google Scholar] [CrossRef] [PubMed]
- Badarna, M.; Keidar, Z.; Arnon-Sheleg, E. FDG PET/CT in vascular graft infection: A pictorial review. Q. J. Nucl. Med. Mol. Imaging 2025, 69, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Bowles, H.; Ambrosioni, J.; Mestres, G.; Hernández-Meneses, M.; Sánchez, N.; Llopis, J.; Yugueros, X.; Almela, M.; Moreno, A.; Riambau, V.; et al. Diagnostic yield of 18F-FDG PET/CT in suspected diagnosis of vascular graft infection: A prospective cohort study. J. Nucl. Cardiol. 2018, 27, 294–302. [Google Scholar] [CrossRef]
- Haidar, G.; Singh, N. Fever of Unknown Origin. N. Engl. J. Med. 2022, 386, 463–477. [Google Scholar] [CrossRef]
- Rutherford, R.B.; Baker, J.; Ernst, C.; Johnston, K.; Porter, J.M.; Ahn, S.; Jones, D.N. Recommended standards for reports dealing with lower extremity ischemia: Revised version. J. Vasc. Surg. 1997, 26, 517–538. [Google Scholar] [CrossRef]
- Dong, W.; Li, Y.; Zhu, J.; Xia, J.; He, L.; Yun, M.; Jiao, J.; Zhu, G.; Hacker, M.; Wei, Y.; et al. Detection of aortic prosthetic graft infection with 18 F-FDG PET/CT imaging, concordance with consensus MAGIC graft infection criteria. J. Nucl. Cardiol. 2020, 28, 1005–1016. [Google Scholar] [CrossRef]
- Li, H.L.; Chan, Y.C.; Cheng, S.W. Current Evidence on Management of Aortic Stent-graft Infection: A Systematic Review and Meta-Analysis. Ann. Vasc. Surg. 2018, 51, 306–313. [Google Scholar] [CrossRef]
- Post, I.C.J.H.; Vos, C.G. Systematic Review and Meta-Analysis on the Management of Open Abdominal Aortic Graft Infections. Eur. J. Vasc. Endovasc. Surg. 2019, 58, 258–281. [Google Scholar] [CrossRef]
- Chrapko, B.E.; Chrapko, M.; Nocuń, A.; Zubilewicz, T.; Stefaniak, B.; Mitura, J.; Wolski, A.; Terelecki, P. Patterns of vascular graft infection in 18F-FDG PET/CT. Nucl. Med. Rev. 2020, 23, 63–70. [Google Scholar] [CrossRef]
- Folmer, E.I.R.; von Meijenfeldt, G.C.; Scholten, R.S.T.R.O.G.; van der Laan, M.J.; Glaudemans, A.W.; Slart, R.H.; Zeebregts, C.J.; Saleem, B.R. A systematic review and meta-analysis of 18 F-fluoro-d-deoxyglucose positron emission tomography interpretation methods in vascular graft and endograft infection. J. Vasc. Surg. 2020, 72, 2174–2185.e2. [Google Scholar] [CrossRef] [PubMed]
- Rojoa, D.; Kontopodis, N.; Antoniou, S.A.; Ioannou, C.V.; Antoniou, G.A. 18F-FDG PET in the Diagnosis of Vascular Prosthetic Graft Infection: A Diagnostic Test Accuracy Meta-Analysis. Eur. J. Vasc. Endovasc. Surg. 2018, 57, 292–301. [Google Scholar] [CrossRef] [PubMed]
- Tsuda, K.; Washiyama, N.; Takahashi, D.; Natsume, K.; Ohashi, Y.; Hirano, M.; Takeuchi, Y.; Shiiya, N. 18-Fluorodeoxyglucose positron emission tomography in the diagnosis of prosthetic aortic graft infection: The difference between open and endovascular repair. Eur. J. Cardio-Thoracic Surg. 2022, 63, ezac542. [Google Scholar] [CrossRef] [PubMed]
- de la Rubia-Marcos, M.; García-Alonso, P.; Mena-Melgar, C.; Tagliatori-Nogueira, B.; Herrero-Muñoz, A.; Sandoval-Moreno, C.; Paniagua-Correa, C.; Castillejos-Rodríguez, L.; Ortega-Valle, A.; Balsa-Bretón, M. TC-white blood cell scintigraphy with SPECT/CT in the diagnosis of vascular graft infection. Rev. Espanola Med. Nucl. Imagen Mol. (Engl. Ed.) 2020, 39, 347–352. (In Spanish) [Google Scholar] [CrossRef]
- Lauri, C.; Campagna, G.; Aloisi, F.; Posa, A.; Iezzi, R.; Sirignano, P.; Taurino, M.; Signore, A. How to combine CTA, 99m Tc-WBC SPECT/CT, and [18 F]FDG PET/CT in patients with suspected abdominal vascular endograft infections? Eur. J. Nucl. Med. 2023, 50, 3235–3250. [Google Scholar] [CrossRef]
- Sollini, M.; Bartoli, F.; Boni, R.; Zanca, R.; Colli, A.; Levantino, M.; Menichetti, F.; Ferrari, M.; Berchiolli, R.; Lazzeri, E.; et al. Role of Multimodal Imaging in Patients With Suspected Infections After the Bentall Procedure. Front. Cardiovasc. Med. 2021, 8, 745556. [Google Scholar] [CrossRef]
- Cohort, T.V.; Husmann, L.; Huellner, M.W.; Ledergerber, B.; Anagnostopoulos, A.; Stolzmann, P.; Sah, B.-R.; Burger, I.A.; Rancic, Z.; Hasse, B. Comparing diagnostic accuracy of 18 F-FDG-PET/CT, contrast enhanced CT and combined imaging in patients with suspected vascular graft infections. Eur. J. Nucl. Med. 2018, 46, 1359–1368. [Google Scholar] [CrossRef]
- Rahimi, M.; Adlouni, M.; Ahmed, A.I.; Alnabelsi, T.; Chinnadurai, P.; Al-Mallah, M.H. Diagnostic Accuracy of FDG PET for the Identification of Vascular Graft Infection. Ann. Vasc. Surg. 2022, 87, 422–429. [Google Scholar] [CrossRef]
- Mahmoodi, Z.; Salarzaei, M.; Sheikh, M. Prosthetic vascular graft infection: A systematic review and meta-analysis on diagnostic accuracy of 18FDG PET/CT. Gen. Thorac. Cardiovasc. Surg. 2021, 70, 219–229. [Google Scholar] [CrossRef]


| Patient No. | Age | Comorbidities | Site of the Graft | Microorganisms | CTA | Final Diagnosis |
|---|---|---|---|---|---|---|
| 1 | 69 | // | Thoracic aorta | // | Not performed (allergy to c.m.) | Not infected |
| 2 | 72 | // | Abdominal aorta | E. coli | Not diagnostic | Not infected |
| 3 | 57 | // | Aorto-bisiliac | S. warneri and S. epidermidis | + | Infected |
| 4 | 63 | Marfan syndrome | Thoracic aorta | MSSA | + | Infected |
| 5 | 59 | // | Thoracic aorta | // | Not performed | Not infected |
| 6 | 69 | // | Lower limb | S. capitis | Not performed (renal failure) | Not infected |
| 7 | 77 | // | Lower limb | MSSA and S. aureus OXAs | Not performed (allergy to c.m.) | Not infected |
| 8 | 71 | Diabetes mellitus | Aorto-left femoral | S. epidermidis erythromycin/clindamycin resistant | + | Infected |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Scarpuzza, M.; Ambrogio, A.; Leo, A.; Suardi, L.R.; Marconi, M.; Falcone, M.; Berchiolli, R.; Lazzeri, E. A Head-to-Head Comparison Between [18F]Fluorodeoxyglucose ([18F]FDG) Positron Emission Tomography/Computed Tomography (PET/CT) and 99mTechnetium-Hexamethylpropylene Amine Oxime (HMPAO)-Labeled Leukocyte Scintigraphy in a Case Series of Patients with Suspected Vascular Prosthesis Infection: To Trust Is Good, but to Check Is Better. J. Clin. Med. 2025, 14, 4352. https://doi.org/10.3390/jcm14124352
Scarpuzza M, Ambrogio A, Leo A, Suardi LR, Marconi M, Falcone M, Berchiolli R, Lazzeri E. A Head-to-Head Comparison Between [18F]Fluorodeoxyglucose ([18F]FDG) Positron Emission Tomography/Computed Tomography (PET/CT) and 99mTechnetium-Hexamethylpropylene Amine Oxime (HMPAO)-Labeled Leukocyte Scintigraphy in a Case Series of Patients with Suspected Vascular Prosthesis Infection: To Trust Is Good, but to Check Is Better. Journal of Clinical Medicine. 2025; 14(12):4352. https://doi.org/10.3390/jcm14124352
Chicago/Turabian StyleScarpuzza, Marina, Alice Ambrogio, Andrea Leo, Lorenzo Roberto Suardi, Michele Marconi, Marco Falcone, Raffaella Berchiolli, and Elena Lazzeri. 2025. "A Head-to-Head Comparison Between [18F]Fluorodeoxyglucose ([18F]FDG) Positron Emission Tomography/Computed Tomography (PET/CT) and 99mTechnetium-Hexamethylpropylene Amine Oxime (HMPAO)-Labeled Leukocyte Scintigraphy in a Case Series of Patients with Suspected Vascular Prosthesis Infection: To Trust Is Good, but to Check Is Better" Journal of Clinical Medicine 14, no. 12: 4352. https://doi.org/10.3390/jcm14124352
APA StyleScarpuzza, M., Ambrogio, A., Leo, A., Suardi, L. R., Marconi, M., Falcone, M., Berchiolli, R., & Lazzeri, E. (2025). A Head-to-Head Comparison Between [18F]Fluorodeoxyglucose ([18F]FDG) Positron Emission Tomography/Computed Tomography (PET/CT) and 99mTechnetium-Hexamethylpropylene Amine Oxime (HMPAO)-Labeled Leukocyte Scintigraphy in a Case Series of Patients with Suspected Vascular Prosthesis Infection: To Trust Is Good, but to Check Is Better. Journal of Clinical Medicine, 14(12), 4352. https://doi.org/10.3390/jcm14124352







