Low-Dose Intratympanic Gentamicin Injections for Intractable Meniere’s Disease: How Many Are Optimal?
Abstract
1. Introduction
2. Materials & Methods
2.1. Subjects
2.2. Audiometric Tests
2.3. Vestibular Tests
2.4. Low-Dose Intratympanic Gentamicin Injection (ITGM) Administration
2.5. Exploratory Tympanotomy
2.6. Statistical Analysis
3. Results
3.1. Patient Characteristics
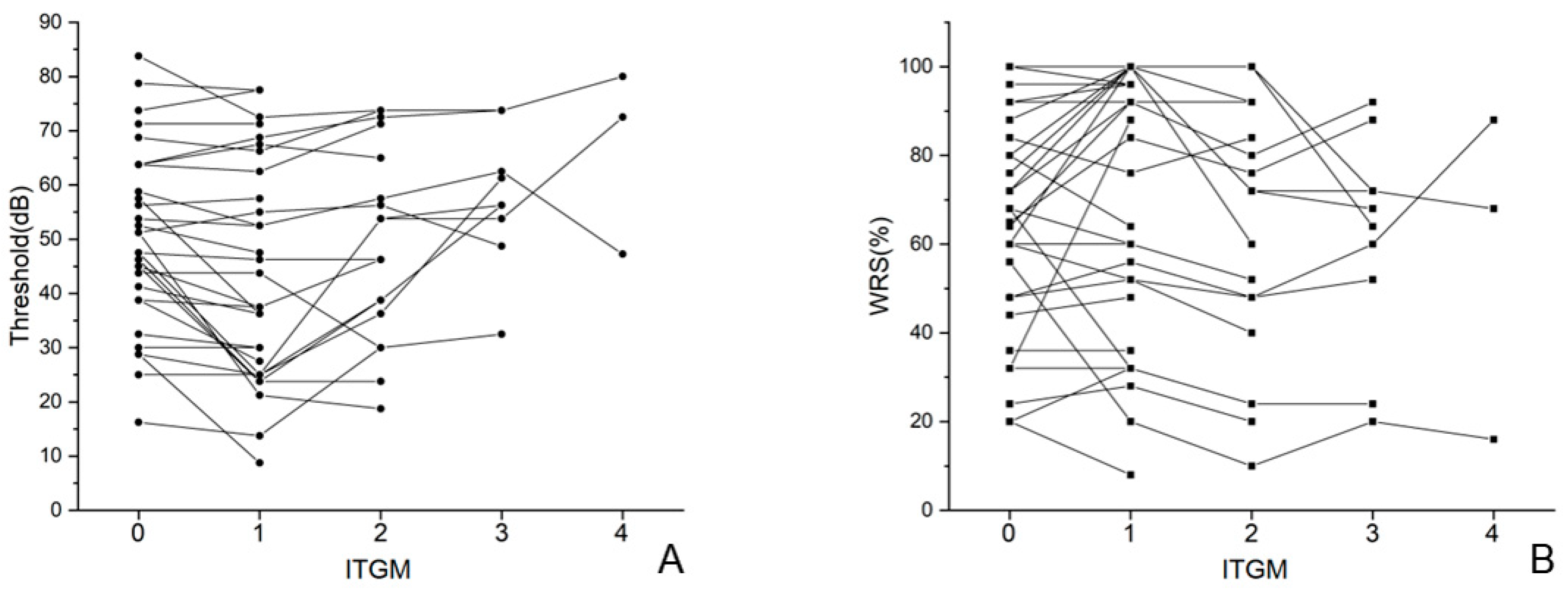
3.2. Audiometric Evaluation in Single Injection Group (SG) and Multiple Injection Group (MG)
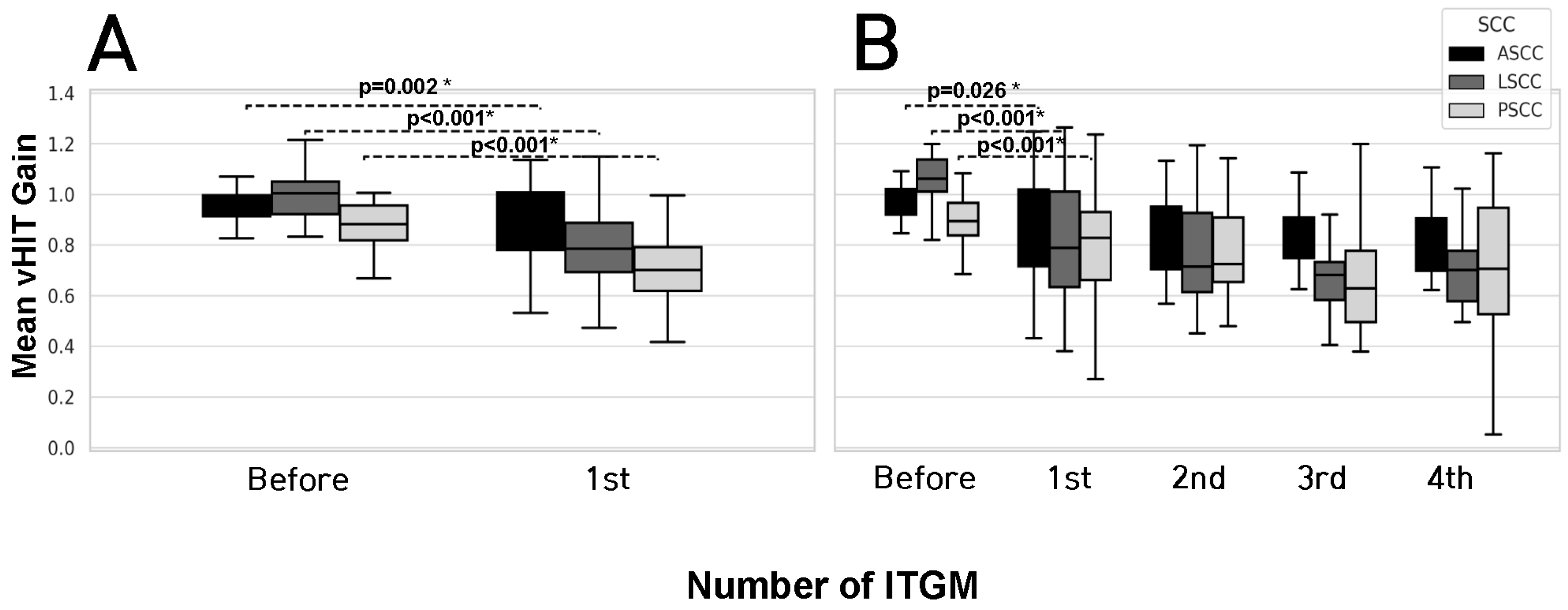
3.3. Vestibular Test Results in Single Injection Group (SG) and Multiple Injection Group (MG)
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| MD | Meniere’s disease |
| ITGM | Intratympanic gentamicin injection |
| SG | Single injection group |
| MG | Multiple injection group |
| PTA | Pure tone audiometry |
| WRS | Word recognition score |
| vHIT | Video head impulse test |
| SCCs | Semicircular canals |
| CP | Canal paresis |
References
- Basura, G.J.; Adams, M.E.; Monfared, A.; Schwartz, S.R.; Antonelli, P.J.; Burkard, R.; Bush, M.L.; Bykowski, J.; Colandrea, M.; Derebery, J.; et al. Clinical Practice Guideline: Ménière’s Disease. Otolaryngol. Head. Neck Surg. 2020, 162 (Suppl. S2), S1–S55. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Escamez, J.A.; Carey, J.; Chung, W.H.; Goebel, J.A.; Magnusson, M.; Mandalà, M.; Newman-Toker, D.E.; Strupp, M.; Suzuki, M.; Trabalzini, F.; et al. Diagnostic criteria for Menière’s disease. J. Vestib. Res. 2015, 25, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Lyu, Y.; Guo, J.; Li, X.; Jian, H.; Li, Y.; Wang, J.; Fan, Z.; Wang, H.; Zhang, D. Long-term efficacy of dexamethasone treatment via tympanic antrum catheterization for intractable Meniere’s disease. Front. Neurol. 2022, 13, 1056724. [Google Scholar] [CrossRef] [PubMed]
- Benjamin, T.; Gillard, D.; Abouzari, M.; Djalilian, H.R.; Sharon, J.D. Vestibular and auditory manifestations of migraine. Curr. Opin. Neurol. 2022, 35, 84–89. [Google Scholar] [CrossRef]
- Chen, J.Y.; Guo, Z.Q.; Wang, J.; Liu, D.; Tian, E.; Guo, J.Q.; Kong, W.J.; Zhang, S.L. Vestibular migraine or Meniere’s disease: A diagnostic dilemma. J. Neurol. 2023, 270, 1955–1968. [Google Scholar] [CrossRef]
- Peng, A.; Hu, J.; Wang, Q.; Pan, X.; Zhang, Z.; Jiang, W.; Chen, Y.; Huang, C. A comparison of endolymphatic duct blockage, endolymphatic sac drainage and endolymphatic sac decompression surgery in reversing endolymphatic hydrops in Meniere’s disease. J. Otolaryngol. Head Neck Surg. 2021, 50, 70. [Google Scholar] [CrossRef]
- Lyford-Pike, S.; Vogelheim, C.; Chu, E.; Della Santina, C.C.; Carey, J.P. Gentamicin is primarily localized in vestibular type I hair cells after intratympanic administration. J. Assoc. Res. Otolaryngol. 2007, 8, 497–508. [Google Scholar] [CrossRef]
- Junet, P.; Karkas, A.; Dumas, G.; Quesada, J.L.; Schmerber, S. Vestibular results after intratympanic gentamicin therapy in disabling Menière’s disease. Eur. Arch. Otorhinolaryngol. 2016, 273, 3011–3018. [Google Scholar] [CrossRef]
- Webster, K.E.; Galbraith, K.; Lee, A.; Harrington-Benton, N.A.; Judd, O.; Kaski, D.; Maarsingh, O.R.; MacKeith, S.; Ray, J.; Van Vugt, V.A.; et al. Intratympanic gentamicin for Ménière’s disease. Cochrane Database Syst. Rev. 2023, 2, Cd015246. [Google Scholar]
- Söderman, A.C.; Bagger-Sjöbäck, D.; Bergenius, J.; Langius, A. Factors influencing quality of life in patients with Ménière’s disease, identified by a multidimensional approach. Otol. Neurotol. 2002, 23, 941–948. [Google Scholar] [CrossRef]
- Leng, Y.; Liu, B.; Zhou, R.; Liu, J.; Liu, D.; Zhang, S.L.; Kong, W.J. Repeated courses of intratympanic dexamethasone injection are effective for intractable Meniere’s disease. Acta Otolaryngol. 2017, 137, 154–160. [Google Scholar] [CrossRef] [PubMed]
- Nicolas, S.; Kmeid, M.; Mansour, C.; Fraysse, B.; Deguine, O.; Marx, M.; Fraysse, M.E. Long-term Vertigo Control and Vestibular Function After Low-dose On-demand Transtympanic Gentamicin for Refractory Menière’s Disease. Otol. Neurotol. 2019, 40, 218–225. [Google Scholar] [CrossRef] [PubMed]
- Casani, A.P.; Piaggi, P.; Cerchiai, N.; Seccia, V.; Franceschini, S.S.; Dallan, I. Intratympanic treatment of intractable unilateral Meniere disease: Gentamicin or dexamethasone? A randomized controlled trial. Otolaryngol. Head Neck Surg. 2012, 146, 430–437. [Google Scholar] [CrossRef]
- Casani, A.P.; Cerchiai, N.; Navari, E.; Dallan, I.; Piaggi, P.; Sellari-Franceschini, S. Intratympanic gentamicin for Meniere’s disease: Short- and long-term follow-up of two regimens of treatment. Otolaryngol. Head Neck Surg. 2014, 150, 847–852. [Google Scholar] [CrossRef]
- Guan, Y.; Chari, D.A.; Liu, Y.H.; Rauch, S.D. Efficacy and Durability of Intratympanic Gentamicin Treatment for Meniere’s Disease. Front. Neurol. 2021, 12, 765208. [Google Scholar] [CrossRef] [PubMed]
- Gurgel, R.K.; Jackler, R.K.; Dobie, R.A.; Popelka, G.R. A new standardized format for reporting hearing outcome in clinical trials. Otolaryngol. Head Neck Surg. 2012, 147, 803–807. [Google Scholar] [CrossRef]
- Lou, Z.; Lou, Z.; Lv, T.; Chen, Z. Role of topical antibiotic ointment in the lateral graft following underlay myringoplasty: A prospective randomised study. J. Otolaryngol. Head Neck Surg. 2023, 52, 80. [Google Scholar] [CrossRef]
- Kim, J.; Lee, J.; Lee, K.W.; Bahng, J.; Lee, J.H.; Choi, C.H.; Cho, S.J.; Shin, E.Y.; Park, J. Test-Retest Reliability of Word Recognition Score Using Korean Standard Monosyllabic Word Lists for Adults as a Function of the Number of Test Words. J. Audiol. Otol. 2015, 19, 68–73. [Google Scholar] [CrossRef]
- Lee, J.Y.; Kim, M.B. Effect of Aging and Direction of Impulse in Suppression Head Impulse Test. Otol. Neurotol. 2020, 41, e1231–e1236. [Google Scholar] [CrossRef]
- Lee, J.Y.; Kim, C.H.; Park, J.S.; Kim, M.B. Peripheral Vestibulopathy Presenting as Acute Vertigo and Spontaneous Nystagmus with Negative Video Head Impulse Test. Otolaryngol. Head Neck Surg. 2019, 160, 894–901. [Google Scholar] [CrossRef]
- Lee, J.Y.; Kim, M.B. Change of VOR gain and pure-tone threshold after single low-dose intratympanic gentamicin injection in Meniere’s disease. Acta Otolaryngol. 2020, 140, 314–318. [Google Scholar] [CrossRef] [PubMed]
- Postema, R.J.; Kingma, C.M.; Wit, H.P.; Albers, F.W.; Van Der Laan, B.F. Intratympanic gentamicin therapy for control of vertigo in unilateral Menire’s disease: A prospective, double-blind, randomized, placebo-controlled trial. Acta Otolaryngol. 2008, 128, 876–880. [Google Scholar] [CrossRef]
- Stokroos, R.; Kingma, H. Selective vestibular ablation by intratympanic gentamicin in patients with unilateral active Ménière’s disease: A prospective, double-blind, placebo-controlled, randomized clinical trial. Acta Otolaryngol. 2004, 124, 172–175. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Pyykkö, I.; Zhang, Q.; Yang, J.; Duan, M. Consensus on intratympanic drug delivery for Menière’s disease. Eur. Arch. Otorhinolaryngol. 2022, 279, 3795–3799. [Google Scholar] [CrossRef] [PubMed]
- Fehrmann, M.L.A.; Huinck, W.J.; Thijssen, M.E.G.; Haer-Wigman, L.; Yntema, H.G.; Rotteveel, L.J.C.; Widdershoven, J.C.C.; Goderie, T.; van Dooren, M.F.; Hoefsloot, E.H.; et al. Stable long-term outcomes after cochlear implantation in subjects with TMPRSS3 associated hearing loss: A retrospective multicentre study. J. Otolaryngol. Head Neck Surg. 2023, 52, 82. [Google Scholar] [CrossRef] [PubMed]
- Cao, Z.; Yue, F.; Huang, W.; Rajenderkumar, D.; Zhao, F. Different medications for the treatment of Ménière’s disease by intratympanic injection: A systematic review and network meta-analysis. Clin. Otolaryngol. 2019, 44, 619–627. [Google Scholar] [CrossRef]
- Patel, M.; Agarwal, K.; Arshad, Q.; Hariri, M.; Rea, P.; Seemungal, B.M.; Golding, J.F.; Harcourt, J.P.; Bronstein, A.M. Intratympanic methylprednisolone versus gentamicin in patients with unilateral Ménière’s disease: A randomised, double-blind, comparative effectiveness trial. Lancet 2016, 388, 2753–2762. [Google Scholar] [CrossRef]
- Naples, J.G.; Henry, L.; Brant, J.A.; Eliades, S.J.; Ruckenstein, M.J. Intratympanic Therapies in Ménière Disease: Evaluation of Outcomes and Early Vertigo Control. Laryngoscope 2019, 129, 216–221. [Google Scholar] [CrossRef]
- Crane, B.T.; Minor, L.B.; Della Santina, C.C.; Carey, J.P. Middle ear exploration in patients with Ménière’s disease who have failed outpatient intratympanic gentamicin therapy. Otol. Neurotol. 2009, 30, 619–624. [Google Scholar] [CrossRef]
- Silverstein, H.; Rowan, P.T.; Olds, M.J.; Rosenberg, S.I. Inner ear perfusion and the role of round window patency. Am. J. Otol. 1997, 18, 586–589. [Google Scholar]
- Marques, P.S.; Dias, C.C.; Perez-Fernandez, N.; Spratley, J. Instrumental head impulse test changes after intratympanic gentamicin for unilateral definite Ménière’s disease: A systematic review and meta-analysis. Auris Nasus Larynx 2018, 45, 943–951. [Google Scholar] [CrossRef] [PubMed]
- Büki, B.; Jünger, H. Intratympanal gentamicin in Meniere’s disease: Effects on individual semicircular canals. Auris Nasus Larynx 2018, 45, 39–44. [Google Scholar] [CrossRef] [PubMed]
- Marques, P.; Manrique-Huarte, R.; Perez-Fernandez, N. Single intratympanic gentamicin injection in Ménière’s disease: VOR change and prognostic usefulness. Laryngoscope 2015, 125, 1915–1920. [Google Scholar] [CrossRef] [PubMed]
- Martin-Sanz, E.; Diaz, J.Y.; Esteban-Sanchez, J.; Sanz-Fernández, R.; Perez-Fernandez, N. Delayed Effect and Gain Restoration After Intratympanic Gentamicin for Menière’s Disease. Otol. Neurotol. 2019, 40, 79–87. [Google Scholar] [CrossRef]
- Yetişer, S. Intratympanic Gentamicin for Intractable Ménière’s Disease—A Review and Analysis of Audiovestibular Impact. Int. Arch. Otorhinolaryngol. 2018, 22, 190–194. [Google Scholar] [CrossRef]
- Molnár, A.; Maihoub, S.; Gáborján, A.; Tamás, L.; Szirmai, Á. Intratympanic gentamycine for Ménière’s disease: Is there a selective vestibulotoxic effect? Eur. Arch. Otorhinolaryngol. 2020, 277, 1949–1954. [Google Scholar] [CrossRef]
- MacKeith, S.A.; Whiteside, O.J.; Mawby, T.; Bottrill, I.D. Middle ear gentamicin-soaked pledgets in the treatment of Ménière’s disease. Otol. Neurotol. 2014, 35, 305–309. [Google Scholar] [CrossRef]
- Roth, S.M.; Williams, S.M.; Jiang, L.; Menon, K.S.; Jeka, J.J. Susceptibility genes for gentamicin-induced vestibular dysfunction. J. Vestib. Res. 2008, 18, 59–68. [Google Scholar] [CrossRef]
- Ahmed, R.M.; MacDougall, H.G.; Halmagyi, G.M. Unilateral vestibular loss due to systemically administered gentamicin. Otol. Neurotol. 2011, 32, 1158–1162. [Google Scholar] [CrossRef]


| Single Injection Group (N = 14) | Multiple Injection Group (N = 19) | p Value | |
|---|---|---|---|
| Age (years) | 56.7 ± 14.6 | 56.8 ± 11.6 | 0.489 |
| Gender | |||
| Male/Female | 6 (42.9%)/8 (57.1%) | 6 (31.6%)/13 (68.4%) | 0.716 |
| Site | |||
| Right/Left | 11 (78.6%)/3 (21.4%) | 10 (52.6%)/9 (47.4%) | 0.160 |
| Initial average hearing threshold (dB) | 51.3 ± 16.7 | 50.3 ± 15.9 | 0.873 |
| Initial WRS (%) | 66.3 ± 29.2 | 66.8 ± 22.2 | 0.955 |
| Initial vHIT gains | |||
| ASCC | 0.95 ± 0.13 | 0.96 ± 0.07 | 0.763 |
| LSCC | 1.01 ± 0.12 | 1.02 ± 0.1 | 0.576 |
| PSCC | 0.91 ± 0.17 | 0.88 ± 0.12 | 0.591 |
| Initial canal paresis (%) | 25.1 ± 12.7 | 27.8 ± 16.1 | 0.734 |
| Period from diagnosis to first ITGM | 3.4 ± 2.8 years | 2.5 ± 1.4 years | 0.541 |
| Period from first to second ITGM | 8.1 ± 6.4 months | ||
| Period from second to third ITGM | 20.4 ± 11.5 months | ||
| Period from third to fourth ITGM | 3.4 ± 2.5 months | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hong, J.-P.; Byun, H.; Kim, M.-B. Low-Dose Intratympanic Gentamicin Injections for Intractable Meniere’s Disease: How Many Are Optimal? J. Clin. Med. 2025, 14, 4342. https://doi.org/10.3390/jcm14124342
Hong J-P, Byun H, Kim M-B. Low-Dose Intratympanic Gentamicin Injections for Intractable Meniere’s Disease: How Many Are Optimal? Journal of Clinical Medicine. 2025; 14(12):4342. https://doi.org/10.3390/jcm14124342
Chicago/Turabian StyleHong, Joon-Pyo, Hayoung Byun, and Min-Beom Kim. 2025. "Low-Dose Intratympanic Gentamicin Injections for Intractable Meniere’s Disease: How Many Are Optimal?" Journal of Clinical Medicine 14, no. 12: 4342. https://doi.org/10.3390/jcm14124342
APA StyleHong, J.-P., Byun, H., & Kim, M.-B. (2025). Low-Dose Intratympanic Gentamicin Injections for Intractable Meniere’s Disease: How Many Are Optimal? Journal of Clinical Medicine, 14(12), 4342. https://doi.org/10.3390/jcm14124342






