Efficacy and Safety of a Fixed-Dose Combination of Etoricoxib–Tramadol Biphasic Tablet in Moderate-to-Severe Acute Pain: A Randomized, Double-Blind, Parallel-Group, Active-Controlled Trial
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Study Population and Ethics
2.3. Study Intervention
2.4. Statistical Analysis
3. Results
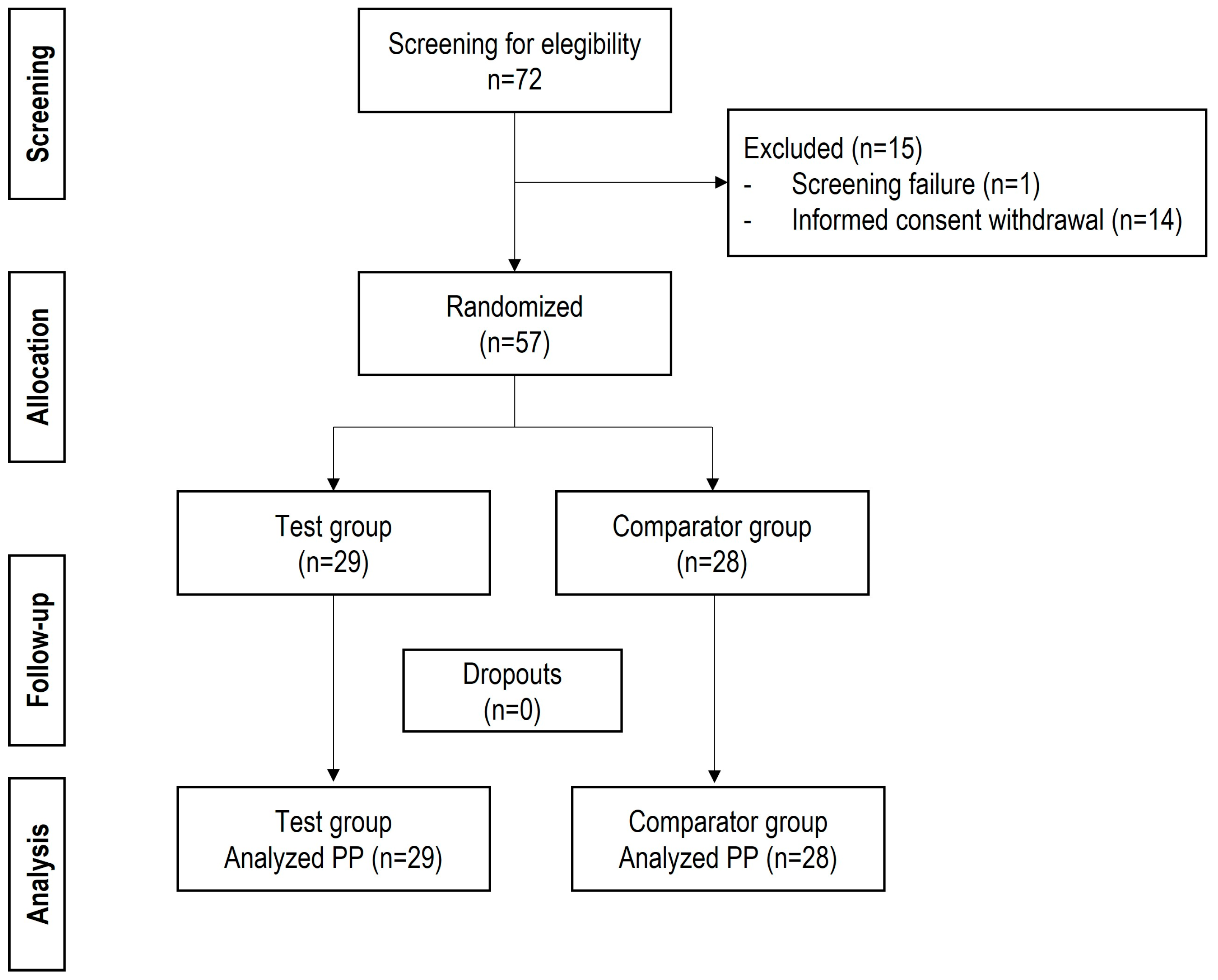
3.1. Population and Surgery Characteristics
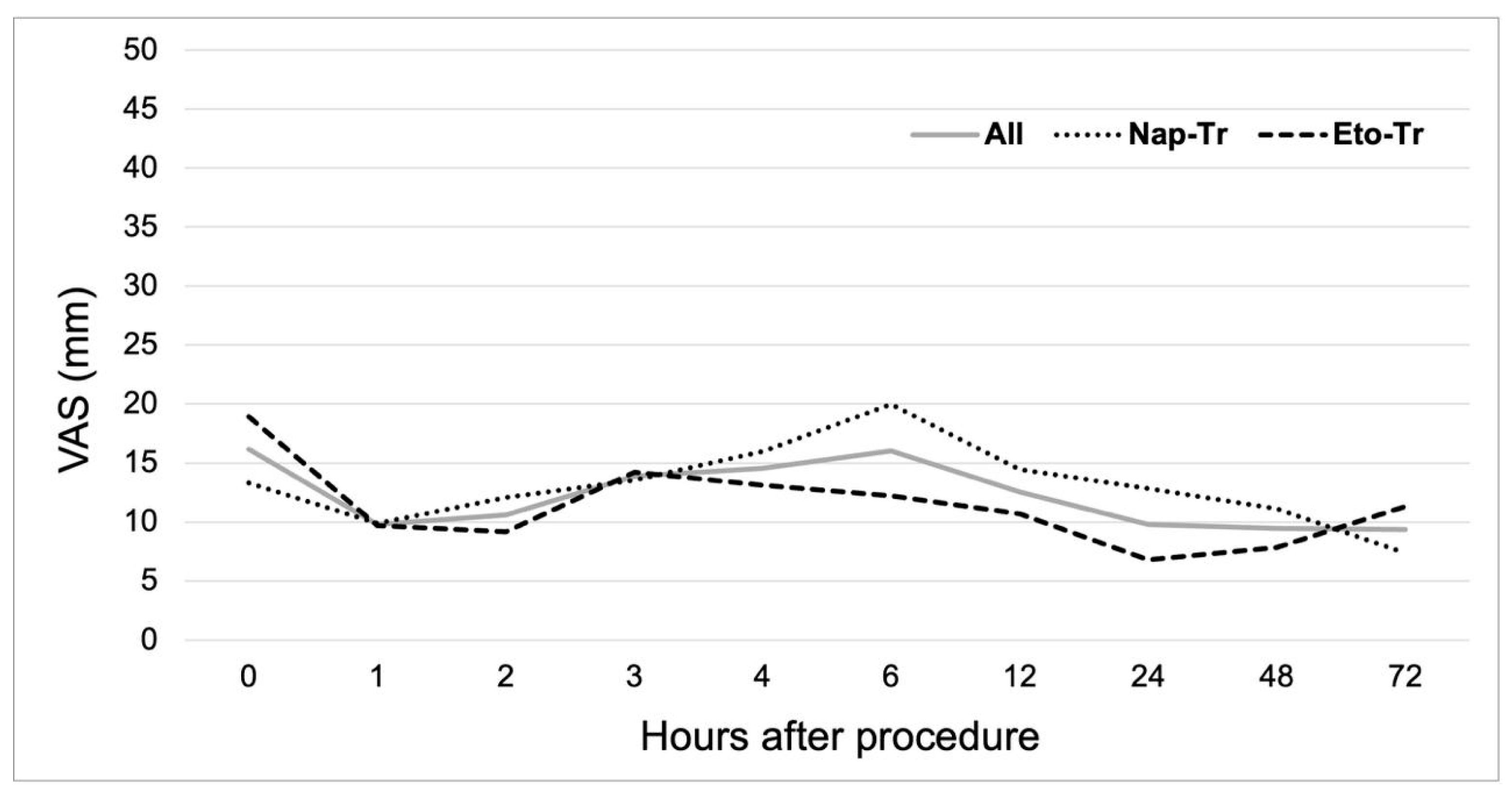
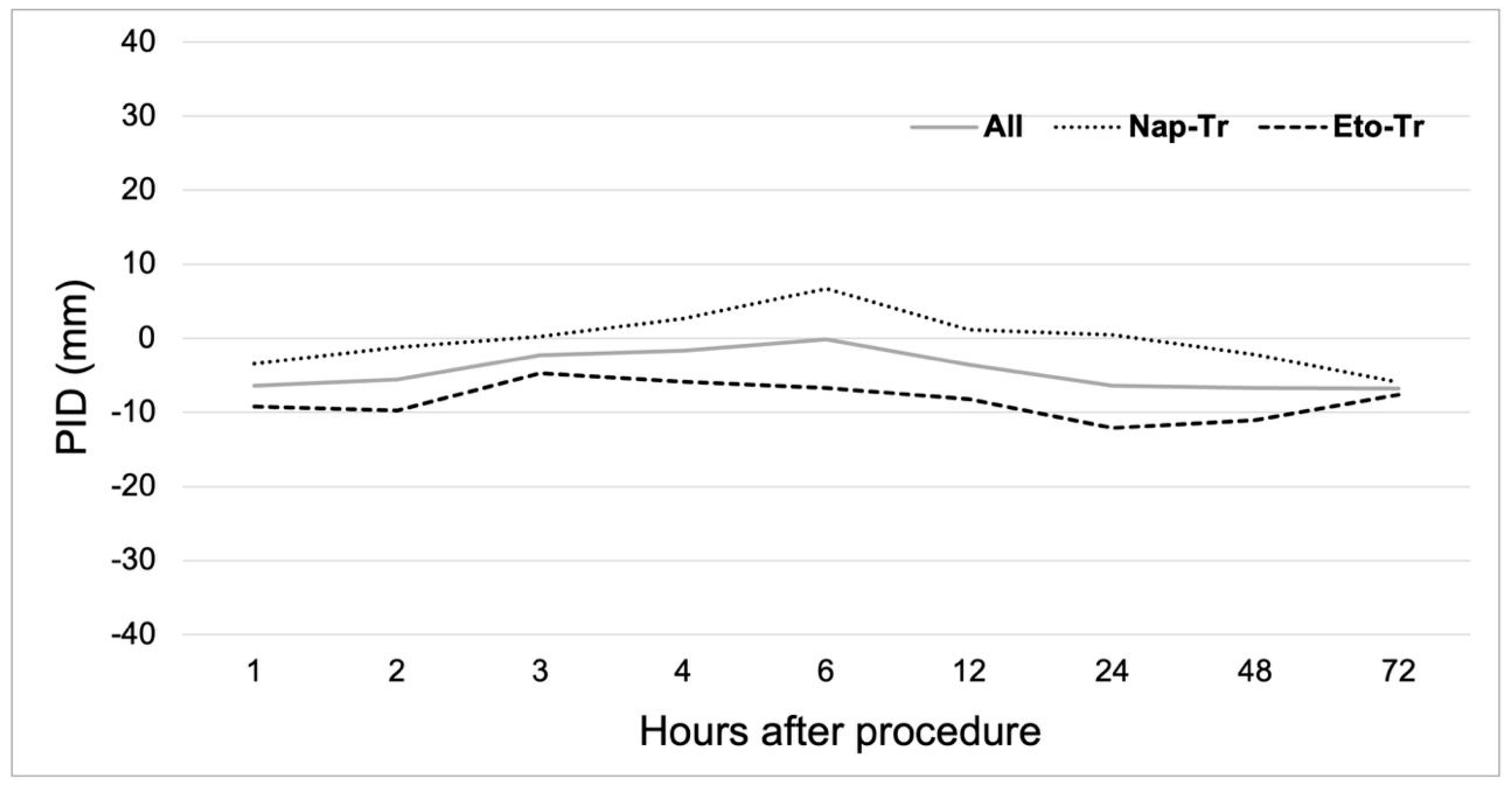
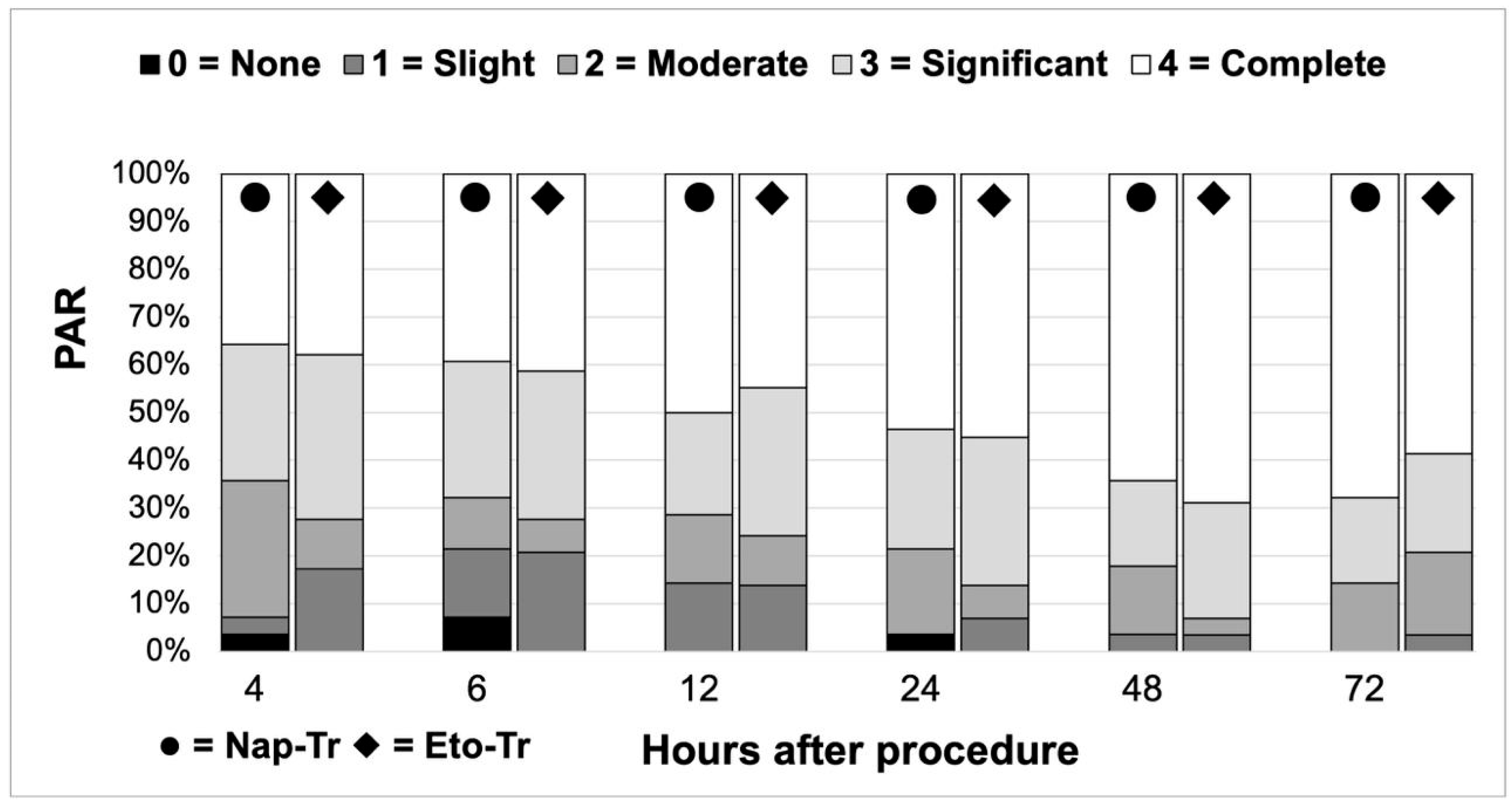
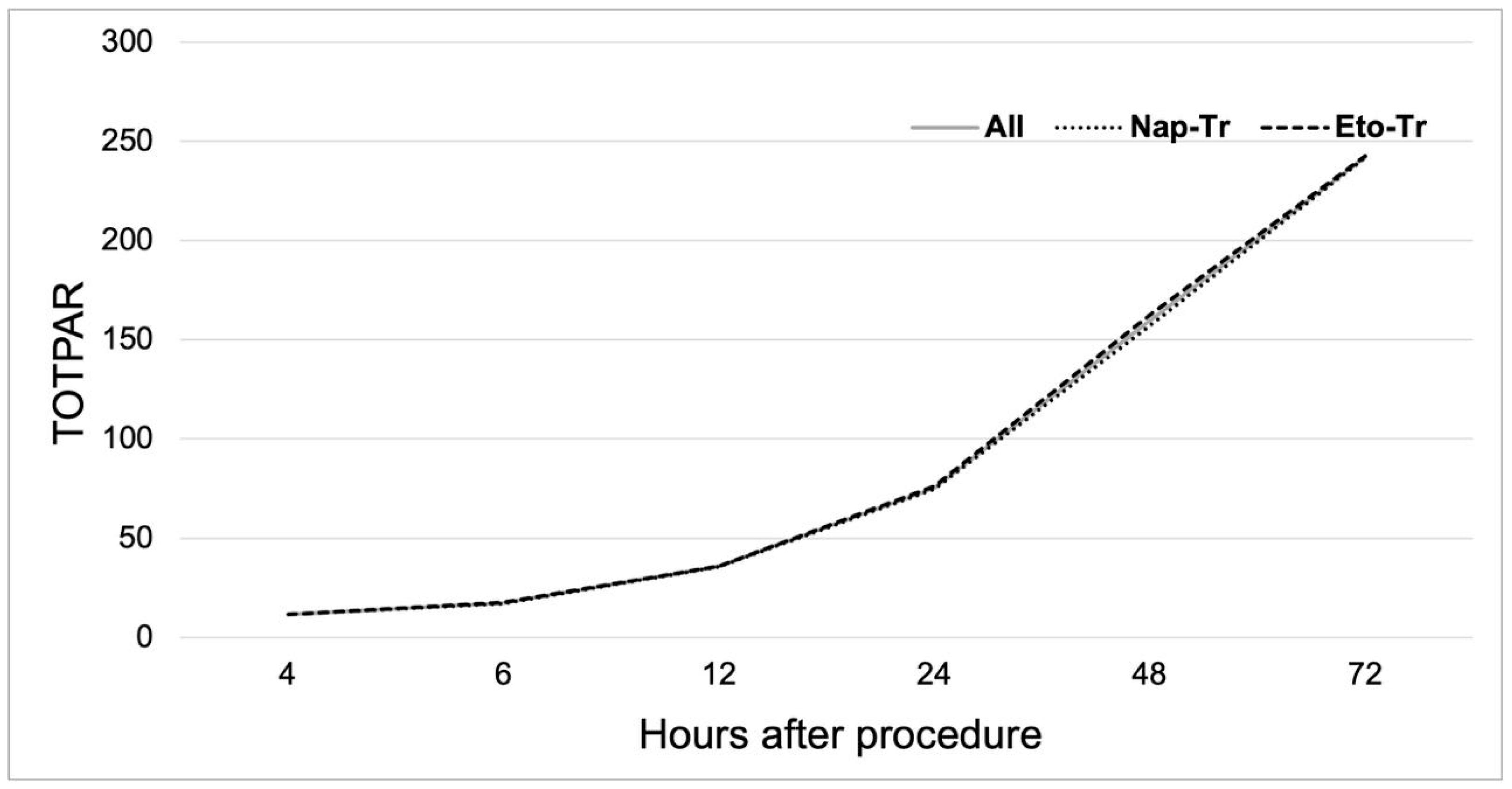
3.2. Analgesic Efficacy
3.3. Trismus Control
3.4. Safety
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AE | Adverse event |
| AUC | Area under the curve plasmatic concentration vs. time |
| CI | Confidence interval |
| Cmax | Maximum plasma concentration |
| COX-2 | Ciclooxynease-2 |
| Eto | Etoricoxib |
| IQR | Interquartile range |
| Nap | Naproxen |
| NSAID | Nonsteroidal anti-inflammatory drugs |
| PAR | Pain relief |
| PID | Pain intensity difference |
| PP | Per protocol analysis |
| T1/2 | Half-life |
| Tmax | Time to peak drug concentration |
| TOTPAR | Total pain relief |
| Tr | Tramadol |
| VAS | Visual analogue scale |
References
- Liu, X.; Deng, C.; Deng, Y.; Luo, X.; Zhang, W. Molecule-rich solutions for achieving novel non-opioid analgesics. Drug. Discov. Today 2025, 30, 104329. [Google Scholar] [CrossRef]
- Matsuda, S.; Itoi, H.; Ryoke, T.; Yoshimura, H. How should clinicians assess acute dental pain? A review. Medicine 2022, 101, e31727. [Google Scholar] [CrossRef]
- Lökken, P.; Olsen, I.; Bruaset, I.; Norman-Pedersen, K. Bilateral surgical removal of impacted lower third molar teeth as a model for drug evaluation: A test with ibuprofen. Eur. J. Clin. Pharmacol. 1975, 8, 209–216. [Google Scholar] [CrossRef]
- Singla, N.K.; Desjardins, P.J.; Chang, P.D. A comparison of the clinical and experimental characteristics of four acute surgical pain models: Dental extraction, bunionectomy, joint replacement, and soft tissue surgery. Pain 2014, 155, 441–456. [Google Scholar] [CrossRef]
- Pergolizzi, J.V.; Magnusson, P.; LeQuan, J.A.; Gharibo, C.; Varrassi, G. The pharmacological management of dental pain. Expert. Opin. Pharmacother. 2020, 21, 591–601. [Google Scholar] [CrossRef]
- Esteve-Pérez, N.; Perez-Herrero, M.A.; Montes-Perez, A.; López-Alvarez, S. Management of acute postoperative pain: Conditions to guarantee the safety and effectiveness of analgesic treatments. Rev. Española Anestesiol. Reanim. 2024, 71, 304–316. [Google Scholar] [CrossRef]
- Hussien, E.; Aboelnile, D.; Aboelnile, A.; Kelly, L. Management of acute pain. Surgery 2025, 43, 240–248. [Google Scholar] [CrossRef]
- O’Neill, A.; Lirk, P. Multimodal Analgesia. Anesthesiol. Clin. 2022, 40, 455–468. [Google Scholar] [CrossRef]
- Zuqui-Ramírez, M.A.; Belalcazar-López, V.M.; Urenda-Quezada, A.; González-Rebatu, Y.; González, A.; Sander-Padilla, J.G.; Lugo-Sánchez, L.A.; Rodríguez-Vázquez, I.C.; Rios-Brito, K.F.; Arguedas-Núñez, M.M.; et al. Multimodal analgesia approach in acute low back pain management: A phase III study of a novel analgesic combination of etoricoxib/tramadol. Pain Ther. 2024, 13, 1511–1528. [Google Scholar] [CrossRef]
- Ambatkar, M.P.; Rarokar, N.R.; Khedekar, P.B. Clinical Use of COX-2 Inhibitors Containing Quinoline Heterocycle as a Selective Therapeutic Agents for Complementary Medicine. Clin. Compl. Med. Pharmacol. 2023, 3, 100102. [Google Scholar] [CrossRef]
- Takemoto, J.K.; Reynolds, J.K.; Remsberg, C.M.; Vega-Villa, K.R.; Davies, N.M. Clinical pharmacokinetic and pharmacodynamic profile of etoricoxib. Clin. Pharmacokinet. 2008, 47, 703–720. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Urizar, J.; Martínez-Rider, R.; Torres-Roque, I.; Garrocho-Rangel, A.P.-G.A. Analgesic efficacy of lysine clonixinate plus tramadol versus tramadol in multiple doses following impacted third molar surgery. Int. J. Oral Maxillofac. Surg. 2014, 21, 348–354. [Google Scholar] [CrossRef]
- Rosas-Peralta, M.; Santos-Martínez, L.E.; Magaña-Serrano, J.A.; Valencia-Sánchez, J.S.; Garrido-Garduño, M.; Pérez-Rodríguez, G. Methodology for superiority versus equivalence and non- inferior clinical studies. A practical review. Rev. Med. Inst. Mex. Seguro Soc. 2016, 54, 344–353. [Google Scholar]
- Martins, L.D.; Rezende, M.; Loguercio, A.D.; Bortoluzzi, M.C.; Reis, A. Analgesic efficacy of Ketorolac associated with a tramadol/acetaminophen combination after third molar surgery—A randomized, triple-blind clinical trial. Med. Oral Patol. Oral Cir. Bucal 2019, 24, e96–e102. [Google Scholar] [CrossRef]
- Pell, G.J.; Gregory, B.T. Impacted mandibular third molars: Classification and Impacted mandibular third molars: Classification and modified technique for removal. Dent. Digest. 1933, 39, 330–338. [Google Scholar]
- Tubach, F.; Ravaud, P.; Baron, G.; Falissard, B.; Logeart, I.; Bellamy, N.; Bombardier, C.; Felson, D.; Hochberg, M.; van der Heijde, D.; et al. Evaluation of clinically relevant changes in patient reported outcomes in knee and hip osteoarthritis: The minimal clinically important improvement. Ann. Rheum. Dis. 2005, 64, 29–33. [Google Scholar] [CrossRef] [PubMed]
- Trivedi, P.; Malini, D.M. A Study of Efficacy of a Single Dose of a Transdermal Diclofenac Patch a Study of Efficacy of a Single Dose of a Transdermal Diclofenac Patch and Intramuscular Diclofenac-As Pre-Emptive Postoperative Analgesia in Patients Undergoing Abdominal Hysterectomy. Int. J. Res. Med. 2015, 4, 194–197. [Google Scholar] [CrossRef]
- Julious, S.A. Sample sizes for clinical trials with Normal data. Stat. Med. 2004, 23, 1921–1986. [Google Scholar] [CrossRef]
- Falci, S.G.M.; Fernandes, I.A.; Guimarães, M.T.B.Á.; Galvão, E.L.; de Souza, G.M.; Al-Moraissi, E.A. Complementary and alternative therapies for managing postoperative pain after lower third molar surgery: A systematic review and network meta-analysis. Clin. Oral Investig. 2024, 28, 231. [Google Scholar] [CrossRef]
- Apfelbaum, J.L.; Chen, C.; Mehta, S.S.; Gan, T.J. Postoperative pain experience: Results from a national survey suggest postoperative pain continues to be undermanaged. Anesth. Analg. 2003, 97, 534–540. [Google Scholar] [CrossRef]
- Moore, P.A.; Ziegler, K.M.; Lipman, R.D.; Aminoshariae, A.; Carrasco-Labra, A.; Mariotti, A. Benefits and Harms Associated with Analgesic Medications Used in the Management of Acute Dental Pain: An overview of systematic reviews. J. Am. Dental Assoc. 2018, 149, 256–268. [Google Scholar] [CrossRef] [PubMed]
- Gandhi, K.; Heitz, J.W.; Viscusi, E.R. Challenges in acute pain management. Anesthesiol. Clin. 2011, 29, 291–309. [Google Scholar] [CrossRef] [PubMed]
- Langford, R.; Morte, A.; Sust, M.; Cebrecos, J.; Vaqué, A.; Ortiz, E.; Fettiplace, J.; Adeyemi, S.; Raba, G.; But-Husaim, L.; et al. Efficacy and safety of co-crystal of tramadol-celecoxib (CTC) in acute moderate-to-severe pain after abdominal hysterectomy: A randomized, double-blind, phase 3 trial (STARDOM2). Eur. J. Pain. 2022, 26, 2083–2096. [Google Scholar] [CrossRef] [PubMed]
- López-Cedrún, J.; Videla, S.; Burgueño, M.; Juárez, I.; Aboul-Hosn, S.; Martín-Granizo, R.; Grau, J.; Puche, M.; Gil-Diez, J.L.; Hueto, J.A.; et al. Co-crystal of Tramadol-Celecoxib in Patients with Moderate to Severe Acute Post-surgical Oral Pain: A Dose-Finding, Randomised, Double-Blind, Placebo- and Active-Controlled, Multicentre, Phase II Trial. Drugs R D 2018, 18, 137–148. [Google Scholar] [CrossRef]
- Gupta, D.; Pandey, M.; Maiti, A.; Pujari, N.M. Bilayer tablet technology: A concept ff immediate and controlled drug delivery. J. Pharm. Neg. Res. 2023, 14, 503–512. [Google Scholar] [CrossRef]
| Total | Nap-Tr | Eto-Tr | |
|---|---|---|---|
| Population distribution, n (%) | 57 (100) | 28 (49.10) | 29 (50.90) |
| Age Years, M (IQR) | 26 (6) | 26.50 (6) | 26 (7) |
| Sex Female, n (%) | 34 (59.60) | 17 (60.70) | 17 (58.60) |
| Weight kg, (σ) | 68.97 (14.56) | 67.27 (12.85) | 70.60 (16.09) |
| Temperature °C, M (IQR) | 36.20 (0.45) | 36.15 (0.58) | 36.20 (0.40) |
| Heart Rate bpm, (σ) | 74.39 (7.89) | 73.61 (7.47) | 75.14 (8.34) |
| Respiratory Rate bpm, M (IQR) | 18 (4) | 18 (3) | 18 (4) |
| SBP mmHg, M (IQR) | 110 (14) | 110 (19) | 110 (10) |
| DBP mmHg, M (IQR) | 70 (19) | 70 (13) | 70 (15) |
| Difficulty | |||
| I, n (%) | 0 (0.00) | 0 (0.00) | 0 (0.00) |
| II, n (%) | 4 (7.00) | 2 (7.10) | 2 (6.90) |
| III, n (%) | 11 (19.30) | 7 (25.00) | 4 (13.80) |
| IV, n (%) | 42 (73.70) | 19 (67.90) | 23 (79.30) |
| Procedure time min, M (IQR) | 37(13) | 38 (21) | 37 (10) |
| Basal pain (VAS) mm, M (IQR) | 5 (23) | 5 (15) | 10 (30) |
| Basal interincisal distance mm, M (IQR) | 32.80 (9.10) | 35.50 (14.55) | 30.40 (21.90) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sibaja, T.A.; Espinoza, G.A.; Dávila, Y.I.; Salinas, E.M.; Venegas, J.J.; Batista, D.; Delgado-Roche, L. Efficacy and Safety of a Fixed-Dose Combination of Etoricoxib–Tramadol Biphasic Tablet in Moderate-to-Severe Acute Pain: A Randomized, Double-Blind, Parallel-Group, Active-Controlled Trial. J. Clin. Med. 2025, 14, 4327. https://doi.org/10.3390/jcm14124327
Sibaja TA, Espinoza GA, Dávila YI, Salinas EM, Venegas JJ, Batista D, Delgado-Roche L. Efficacy and Safety of a Fixed-Dose Combination of Etoricoxib–Tramadol Biphasic Tablet in Moderate-to-Severe Acute Pain: A Randomized, Double-Blind, Parallel-Group, Active-Controlled Trial. Journal of Clinical Medicine. 2025; 14(12):4327. https://doi.org/10.3390/jcm14124327
Chicago/Turabian StyleSibaja, Tania A., Guadalupe A. Espinoza, Yazmin I. Dávila, Erick M. Salinas, Juan J. Venegas, Dany Batista, and Livan Delgado-Roche. 2025. "Efficacy and Safety of a Fixed-Dose Combination of Etoricoxib–Tramadol Biphasic Tablet in Moderate-to-Severe Acute Pain: A Randomized, Double-Blind, Parallel-Group, Active-Controlled Trial" Journal of Clinical Medicine 14, no. 12: 4327. https://doi.org/10.3390/jcm14124327
APA StyleSibaja, T. A., Espinoza, G. A., Dávila, Y. I., Salinas, E. M., Venegas, J. J., Batista, D., & Delgado-Roche, L. (2025). Efficacy and Safety of a Fixed-Dose Combination of Etoricoxib–Tramadol Biphasic Tablet in Moderate-to-Severe Acute Pain: A Randomized, Double-Blind, Parallel-Group, Active-Controlled Trial. Journal of Clinical Medicine, 14(12), 4327. https://doi.org/10.3390/jcm14124327






