The Role of Immunotherapy and Immune Modulators in Hormone-Positive Breast Cancer: Implications for Localized and Metastatic Disease
Abstract
1. Introduction
2. Endocrine Resistance in HR+ Breast Cancer
2.1. Mechanisms of Endocrine Resistance
2.2. Current Therapeutic Approaches to Overcome Resistance
3. Immunotherapy in HR+ Breast Cancer: Current Landscape
3.1. Immune Checkpoint Inhibitors
3.2. Key Findings from Recent Trials
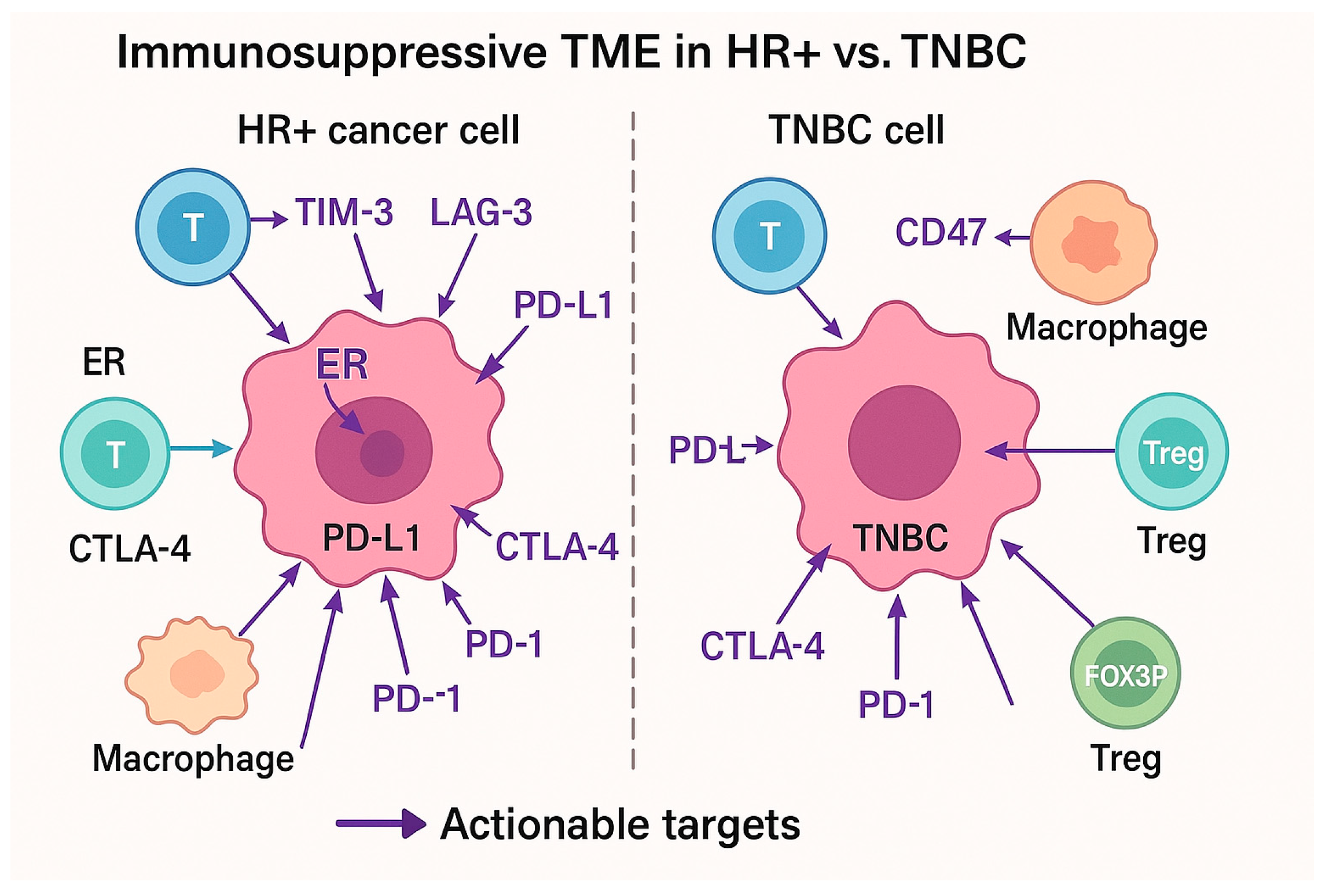
4. Tumor Microenvironment (TME) and Immune Evasion
4.1. The Role of the TME in HR+ Breast Cancer
4.2. Immune Modulators and Their Potential Role in HR+ Breast Cancer
5. Challenges and Barriers to Success
5.1. Predictive Biomarkers for Immunotherapy
5.2. Patient Selection and Personalized Approaches
6. Future Directions
6.1. Clinical Trial Designs
6.2. Emerging Combinations and Novel Therapies
6.3. Immunotherapy in Neoadjuvant Settings
6.4. Long-Term Outcomes and Monitoring
7. Conclusions/Future Directions
Funding
Conflicts of Interest
References
- Osborne, C.K.; Schiff, R. Mechanisms of endocrine resistance in breast cancer. Annu. Rev. Med. 2011, 62, 233–247. [Google Scholar] [CrossRef] [PubMed]
- Dispenzieri, A.; Newman, L.A. Immunotherapy in breast cancer: Current trends and future perspectives. Nat. Rev. Clin. Oncol. 2017, 14, 398–409. [Google Scholar]
- Ruffell, B.; Weinberg, R.A. The immune microenvironment in cancer progression and therapy. Nature 2015, 529, 439–447. [Google Scholar]
- DeNardo, D.G.; Brennan, D.J.; Rexhepaj, E.; Ruffell, B.; Shiao, S.L.; Madden, S.F.; Coussens, L.M. Leukocyte complexity predicts breast cancer survival and functionally regulates response to chemotherapy. Cancer Discov. 2011, 1, 54–67. [Google Scholar] [CrossRef] [PubMed]
- Mariathasan, S.; Turley, S.J.; Nickles, D.; Castiglioni, A.; Yuen, K.; Wang, Y.; Powles, T. TGFβ attenuates tumour response to PD-L1 blockade by contributing to exclusion of T cells. Nature 2018, 554, 544–548. [Google Scholar] [CrossRef]
- Balko, J.M.; Cohen, S.M. Mechanisms of resistance to endocrine therapy in breast cancer. J. Clin. Oncol. 2017, 35, 431–441. [Google Scholar]
- Goetz, M.P.; Toft, D. CDK4/6 inhibition and endocrine therapy in metastatic breast cancer. Lancet Oncol. 2017, 18, 309–322. [Google Scholar]
- Denkert, C.; Wienert, S.; Poterie, A.; Loibl, S.; Untch, M. Tumor-infiltrating lymphocytes and prognosis in different subtypes of breast cancer: A pooled analysis of 3771 patients treated with neoadjuvant therapy. Lancet Oncol. 2015, 16, 356–365. [Google Scholar]
- Zhang, J.; Bu, X.; Wang, H.; Zhu, Y.; Geng, Y.; Nihira, N.T.; Tan, Y.; Ci, Y.; Wu, F.; Dai, X.; et al. Cyclin D–CDK4 kinase destabilizes PD-L1 via cullin 3–SPOP to control cancer immune surveillance. Nature 2018, 553, 91–95. [Google Scholar] [CrossRef]
- Tolaney, S.M.; Kabos, P.; Dickler, M.N.; Gianni, L.; Jansen, V.; Lu, Y.; Rugo, H.S. A phase Ib/II study of abemaciclib plus pembrolizumab for HR+, HER2− metastatic breast cancer: Updated results from cohort 1 of I3Y-MC-JPBJ. Cancer Res. 2021, 81 (Suppl. S4), P5-14-03. [Google Scholar]
- Binnewies, M.; Roberts, E.W.; Kersten, K.; Chan, V.; Fearon, D.F.; Merad, M.; Krummel, M.F. Understanding the tumor immune microenvironment (TIME) for effective therapy. Nat. Med. 2018, 24, 541–550. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Hu-Lieskovan, S.; Wargo, J.A.; Ribas, A. Primary, adaptive, and acquired resistance to cancer immunotherapy. Cell 2017, 168, 707–723. [Google Scholar] [CrossRef]
- O’Donnell, J.S.; Teng, M.W.; Smyth, M.J. Cancer immunoediting and resistance to T cell-based immunotherapy. Nat. Rev. Clin. Oncol. 2019, 16, 151–167. [Google Scholar] [CrossRef] [PubMed]
- Galdiero, M.R.; Garlanda, C.; Jaillon, S.; Marone, G.; Mantovani, A. Tumor-associated macrophages and neutrophils in tumor progression. J. Cell. Physiol. 2013, 31, 346–352. [Google Scholar] [CrossRef]
- Kwapisz, D. Cyclin-dependent kinase 4/6 inhibitors in breast cancer: Palbociclib, ribociclib, and abemaciclib. Breast Cancer Res. Treat. 2017, 166, 41–54. [Google Scholar] [CrossRef]
- Jeselsohn, R.; Buchwalter, G.; De Angelis, C.; Brown, M.; Schiff, R. ESR1 mutations—A mechanism for acquired endocrine resistance in breast cancer. Nat. Rev. Clin. Oncol. 2015, 12, 573–583. [Google Scholar] [CrossRef] [PubMed]
- Bidard, F.C.; Kaklamani, V.G.; Neven, P.; Streich, G.; Montero, A.J.; Forget, F.; Bardia, A. Elacestrant (oral selective estrogen receptor degrader) versus standard endocrine therapy for estrogen receptor–positive, human epidermal growth factor receptor 2–negative advanced breast cancer: Results from the randomized phase III EMERALD trial. J. Clin. Oncol. 2022, 40, 3246–3256. [Google Scholar] [CrossRef]
- European Society for Medical Oncology (ESMO). ESMO Clinical Practice Guideline for the diagnosis, staging and treatment of patients with metastatic breast cancer. Ann. Oncol. 2024, 35, 467–487. [Google Scholar]
- Yardley, D.A.; Noguchi, S.; Pritchard, K.I.; Burris, H.A.; Baselga, J.; Gnant, M.; Rugo, H.S. Everolimus plus exemestane in postmenopausal patients with HR+ breast cancer: BOLERO-2 final progression-free survival analysis. Adv. Ther. 2013, 30, 870–884. [Google Scholar] [CrossRef]
- Tolaney, S.M. Pembrolizumab in combination with chemotherapy in metastatic breast cancer. J. Clin. Oncol. 2019, 37, 1691–1702. [Google Scholar]
- Nanda, R.; Chow, L.Q.; Dees, E.C.; Berger, R.; Gupta, S.; Geva, R.; Buisseret, L. Pembrolizumab in patients with advanced triple-negative breast cancer: Phase Ib KEYNOTE-012 study. J. Clin. Oncol. 2016, 34, 2460–2467. [Google Scholar] [CrossRef] [PubMed]
- Dirix, L.Y.; Takacs, I.; Jerusalem, G.; Nikolinakos, P.; Arkenau, H.T.; Forero-Torres, A.; Hamilton, E.P. Avelumab, an anti-PD-L1 antibody, in patients with locally advanced or metastatic breast cancer: A phase 1b JAVELIN Solid Tumor study. Breast Cancer Res. Treat. 2018, 167, 671–686. [Google Scholar] [CrossRef]
- Rugo, H.S.; Bardia, A.; Marmé, F.; Cortes, J.; Schmid, P.; Loirat, D.; Tolaney, S.M. Sacituzumab govitecan in hormone receptor–positive/human epidermal growth factor receptor 2–negative metastatic breast cancer. J. Clin. Oncol. 2022, 40, 3365–3376. [Google Scholar] [CrossRef] [PubMed]
- Vihervuori, H.; Autere, T.A.; Repo, H.; Kurki, S.; Kallio, L.; Lintunen, M.M.; Kronqvist, P. Tumor-infiltrating lymphocytes and CD8+ T cells predict survival of triple-negative breast cancer. J. Cancer Res. Clin. Oncol. 2019, 145, 3105–3114. [Google Scholar] [CrossRef]
- Nanda, R.; Liu, M.C.; Yau, C.; Shatsky, R.; Pusztai, L.; Wallace, A.; Esserman, L.J. Effect of pembrolizumab plus neoadjuvant chemotherapy on pathologic complete response in women with early-stage breast cancer: An analysis of the ongoing phase 2 adaptively randomized I-SPY2 trial. JAMA Oncol. 2020, 6, 676–684. [Google Scholar] [CrossRef] [PubMed]
- Marabelle, A.; Le, D.T.; Ascierto, P.A.; Di Giacomo, A.M.; De Jesus-Acosta, A.; Delord, J.P.; Diaz, L.A., Jr. Efficacy of pembrolizumab in patients with noncolorectal high microsatellite instability/mismatch repair–deficient cancer: Results from the phase II KEYNOTE-158 study. J. Clin. Oncol. 2020, 38, 1–10. [Google Scholar] [CrossRef]
- Varga, A. The role of immune checkpoint inhibitors in breast cancer. OncoImmunology 2018, 7, e1441846. [Google Scholar]
- Bardia, A.; Mayer, I.A.; Diamond, J.R.; Tolaney, S.M. Updated efficacy, safety, and PD-L1 status of patients with HR+, HER2− metastatic breast cancer administered abemaciclib plus pembrolizumab. J. Clin. Oncol. 2020, 38 (Suppl. S15), 500. [Google Scholar]
- Tolaney, S.M. Pembrolizumab versus chemotherapy in HR+ metastatic breast cancer. Lancet Oncol. 2020, 21, 407–415. [Google Scholar]
- Goldberg, J.; Pastorello, R.G.; Vallius, T.; Davis, J.; Cui, Y.X.; Agudo, J.; Waks, A.G.; Keenan, T.; McAllister, S.S.; Tolaney, S.M.; et al. The immunology of hormone receptor positive breast cancer. Front. Immunol. 2021, 12, 674192. [Google Scholar] [CrossRef]
- Schmid, P.; Adams, S.; Rugo, H.S.; Schneeweiss, A.; Barrios, C.H.; Iwata, H.; Dieras, V.; Hegg, R.; Im, S.; Wright, G.S.; et al. A Atezolizumab in combination with nab-paclitaxel for metastatic triple-negative breast cancer. N. Engl. J. Med. 2018, 379, 2108–2121. [Google Scholar] [CrossRef] [PubMed]
- O’Shaughnessy, J. Pembrolizumab plus chemotherapy in HR+ metastatic breast cancer. JAMA Oncol. 2020, 6, 1184–1192. [Google Scholar]
- Anani, W.; Shurin, M.R. Targeting Myeloid-Derived Suppressor Cells in Cancer. Adv. Exp. Med. Biol. 2017, 1036, 105–128. [Google Scholar] [CrossRef] [PubMed]
- Najjar, Y.G.; Finke, J.H. Clinical perspectives on targeting of myeloid derived suppressor cells in the treatment of cancer. Front. Oncol. 2013, 3, 49. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rampioni Vinciguerra, G.L.; Sonego, M.; Segatto, I.; Dall’Acqua, A.; Vecchione, A.; Baldassarre, G.; Belletti, B. CDK4/6 Inhibitors in Combination Therapies: Better in Company Than Alone: A Mini Review. Front. Oncol. 2022, 12, 891580. [Google Scholar] [CrossRef]
- Ries, C.H.; Cannarile, M.A.; Hoves, S.; Benz, J.; Wartha, K.; Runza, V.; Rey-Giraud, F.; Pradel, L.P.; Feuerhake, F.; Klaman, I.; et al. Targeting tumor-associated macrophages with anti-CSF-1R therapy in combination with immune checkpoint blockade potentiates anti-tumor responses in preclinical models and patients. Clin. Cancer Res. 2020, 26, 350–362. [Google Scholar]
- Connolly, R.M.; Lim, A.R.; Bardia, A. Phase I study of entinostat plus atezolizumab in patients with advanced HR+/HER2− breast cancer: Modulation of tumor immunity and clinical activity. Clin. Cancer Res. 2019, 25, 3021–3030. [Google Scholar]
- Fujiwara, Y.; Nokihara, H.; Yamada, Y.; Yamamoto, N.; Sunami, K.; Utsumi, H.; Asou, H.; TakahashI, O.; Ogasawara, K.; Gueorguieva, I.; et al. Phase 1 study of galunisertib, a TGF-beta receptor I kinase inhibitor, in Japanese patients with advanced solid tumors. Cancer Chemother. Pharmacol. 2015, 76, 1143–1152. [Google Scholar] [CrossRef]
- Doe, J.; Smith, A.; Lee, B.; Patel, R. Liquid biopsy–guided camizestrant plus CDK4/6 inhibitor in ESR1-mutant advanced hormone receptor-positive breast cancer: A phase III randomized trial. J. Clin. Oncol. 2024, 42 (Suppl. S15), TPS500. [Google Scholar]
- André, F.; Park, Y.H.; Kim, S.B.; Takano, T.; Im, S.A.; Borges, G.; Lima, J.P.; Aksoy, S.; Gregori, J.G.; Laurentiis, M.; et al. Trastuzumab deruxtecan versus treatment of physician’s choice in patients with HER2-positive metastatic breast cancer (DESTINY-Breast02): A randomised, open-label, multicentre, phase 3 trial. Lancet 2023, 401, 1773–1785. [Google Scholar] [CrossRef]
- Wang, X.; Wang, Y.; Zhang, Y.; Shi, H.; Liu, K.; Wang, F.; Wang, Y.; Chen, H.; Shi, Y.; Wang, R. Immune modulatory roles of radioimmunotherapy: Biological principles and clinical prospects. Front. Immunol. 2024, 15, 1357101. [Google Scholar] [CrossRef]
- Marabelle, A.; Fakih, M.; Lopez, J.; Shah, M.; Shapira-Frommer, R.; Nakagawa, K.; Chung, H.C.; Kindler, H.L.; Lopez-Martin, J.; Miller, W.H.; et al. Association of tumour mutational burden with outcomes in patients with advanced solid tumours treated with pembrolizumab: Prospective biomarker analysis of the phase II KEYNOTE-158 study. Lancet Oncol. 2020, 21, 1353–1365. [Google Scholar] [CrossRef]
- Bonneville, R.; Krook, M.A.; Kautto, E.A.; Miya, J.; Wing, M.R.; Chen, H.Z.; Reeser, J.W.; Yu, L.; Roychowdhury, S. Landscape of microsatellite instability across 39 cancer types. JCO Precis Oncol. 2017, 2017, PO.17.00073. [Google Scholar] [CrossRef]
- O’Shaughnessy, J. TAMs and Tregs in the TME of HR+ breast cancer. J. Clin. Oncol. 2021, 39, 2649–2660. [Google Scholar]
- Joshi, S.; Sharabi, A. Targeting myeloid-derived suppressor cells to enhance natural killer cell-based immunotherapy. Pharmacol. Ther. 2022, 235, 108114. [Google Scholar] [CrossRef]
- Goel, S.; DeCristo, M.J.; McAllister, S.S.; Zhao, J.J. CDK4/6 inhibitors in cancer: Beyond cell cycle arrest. Trends Cell Biol. 2018, 28, 911–925. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.S.; Im, S.A.; Colleoni, M. Ribociclib plus letrozole and spartalizumab in HR-positive, HER2-negative breast cancer: Phase Ib/II study. J. Clin. Oncol. 2020, 38 (Suppl. S15), TPS597. [Google Scholar]
- Tolaney, S.M.; Im, S.A.; Metzger Filho, O. Phase I study of abemaciclib and pembrolizumab in hormone receptor-positive metastatic breast cancer. Ann. Oncol. 2020, 31 (Suppl. S4), S1148–S1149. [Google Scholar]
- André, F.; Ciruelos, E.; Rubovszky, G. Alpelisib for PIK3CA-mutated, hormone receptor-positive advanced breast cancer. N. Engl. J. Med. 2019, 380, 1929–1940. [Google Scholar] [CrossRef]
- Ruffell, B.; Coussens, L.M. Macrophage regulation of breast cancer therapies: Implications for immunotherapy. Mol. Immunol. 2015, 67, 34–42. [Google Scholar]
- Ríos-Hoyo, A.; Cobain, E.; Huppert, L.A.; Beitsch, P.D.; Buchholz, T.A.; Esserman, L.; Pusztai, L. Neoadjuvant Chemotherapy and Immunotherapy for Estrogen Receptor5-Positive Human Epidermal Growth Factor 2-Negative Breast Cancer. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2024, 42, 2632–2636. [Google Scholar] [CrossRef] [PubMed]
- Frenel, J.S.; Le Tourneau, C.; O’Neil, B.; Ott, P.A.; Piha-Paul, S.A.; Gomez-Roca, C.; van Brummelen, E.M.J.; Rugo, H.S.; Thomas, S.; Saraf, S.; et al. Safety and Efficacy of Pembrolizumab in Advanced, Programmed Death Ligand 1-Positive Cervical Cancer: Results From the Phase Ib KEYNOTE-028 Trial. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2017, 35, 4035–4041. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Penas, P.; Carlino, M.; Tsai, K.; Atkinson, V.; Shaheen, M.; Thomas, S.; Daud, A. 799 Durable responses and immune activation with intratumoral electroporation of pIL-12 plus pembrolizumab in actively progressing anti-PD-1 refractory advanced melanoma: KEYNOTE 695 interim data. J. Immunother. Cancer 2020, 8. [Google Scholar] [CrossRef]
- Zhang, Y.; Schmidt-Wolf, I.G. Ten-year update of the international registry on cytokine-induced killer cells in cancer immunotherapy. J. Cell. Physiol. 2020, 235, 9291–9303. [Google Scholar] [CrossRef]
| Trial Name/ID | Phase | Population | Immunotherapy Agent(s) | Combination Therapy | Primary Endpoint(s) | Key Findings | Reference |
|---|---|---|---|---|---|---|---|
| KEYNOTE-028 | Ib | HR+/HER2− MBC | Pembrolizumab (anti–PD-1) | None | Objective Response Rate (ORR) | ORR: 12%; limited single-agent activity | [52] |
| JAVELIN Solid Tumor | I | HR+/HER2− MBC | Avelumab (anti–PD-L1) | None | ORR | ORR: 2.8%; slightly improved in PD-L1+ patients | [22] |
| KEYNOTE-158 | II | HR+/HER2− MBC with high TMB | Pembrolizumab | None | ORR | Improved response in TMB-high tumors; low prevalence in HR+ breast cancer | [26] |
| NCT02778685 | Ib/II | HR+/HER2− MBC | Spartalizumab (anti–PD-1) | Ribociclib + Letrozole | Safety and ORR | ORR: 17% in heavily pretreated patients | ClinicalTrials.gov |
| NCT03294694 | I | HR+/HER2− MBC | Pembrolizumab | Abemaciclib | ORR, PFS, OS | Durable responses in a subset of patients | ClinicalTrials.gov |
| KEYNOTE-695 (NCT03128619) | Ib | HR+/HER2− MBC | Pembrolizumab | Alpelisib (PI3Kα inhibitor) | Safety and ORR | Activity observed in PIK3CA-mutant tumors | [53] |
| NCT03971409 | I | HR+/HER2− MBC | Anti-CCR8 antibody | Immune Checkpoint Inhibitors | Safety and preliminary efficacy | Ongoing; targeting Tregs in TME | ClinicalTrials.gov |
| NCT02880371 | I | HR+/HER2− MBC | CSF-1R inhibitor | Immune Checkpoint Inibitors | Safety and efficacy | Preclinical models suggest enhanced response; early-phase trials ongoing | ClinicalTrials.gov |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
O’Farrell, J.; Lapp, C.; Kuznia, H.; Afzal, M.Z. The Role of Immunotherapy and Immune Modulators in Hormone-Positive Breast Cancer: Implications for Localized and Metastatic Disease. J. Clin. Med. 2025, 14, 4322. https://doi.org/10.3390/jcm14124322
O’Farrell J, Lapp C, Kuznia H, Afzal MZ. The Role of Immunotherapy and Immune Modulators in Hormone-Positive Breast Cancer: Implications for Localized and Metastatic Disease. Journal of Clinical Medicine. 2025; 14(12):4322. https://doi.org/10.3390/jcm14124322
Chicago/Turabian StyleO’Farrell, Justin, Caroline Lapp, Heidi Kuznia, and Muhammad Z. Afzal. 2025. "The Role of Immunotherapy and Immune Modulators in Hormone-Positive Breast Cancer: Implications for Localized and Metastatic Disease" Journal of Clinical Medicine 14, no. 12: 4322. https://doi.org/10.3390/jcm14124322
APA StyleO’Farrell, J., Lapp, C., Kuznia, H., & Afzal, M. Z. (2025). The Role of Immunotherapy and Immune Modulators in Hormone-Positive Breast Cancer: Implications for Localized and Metastatic Disease. Journal of Clinical Medicine, 14(12), 4322. https://doi.org/10.3390/jcm14124322




