Acute Myocardial Infarction and Diffuse Coronary Artery Disease in a Patient with Multiple Sclerosis: A Case Report and Literature Review
Abstract
1. Introduction
1.1. The Role of Inflammation
1.2. Multiple Sclerosis and MI—Two Similar Pathologies
1.3. MS Treatment and Cardiovascular Side Effects
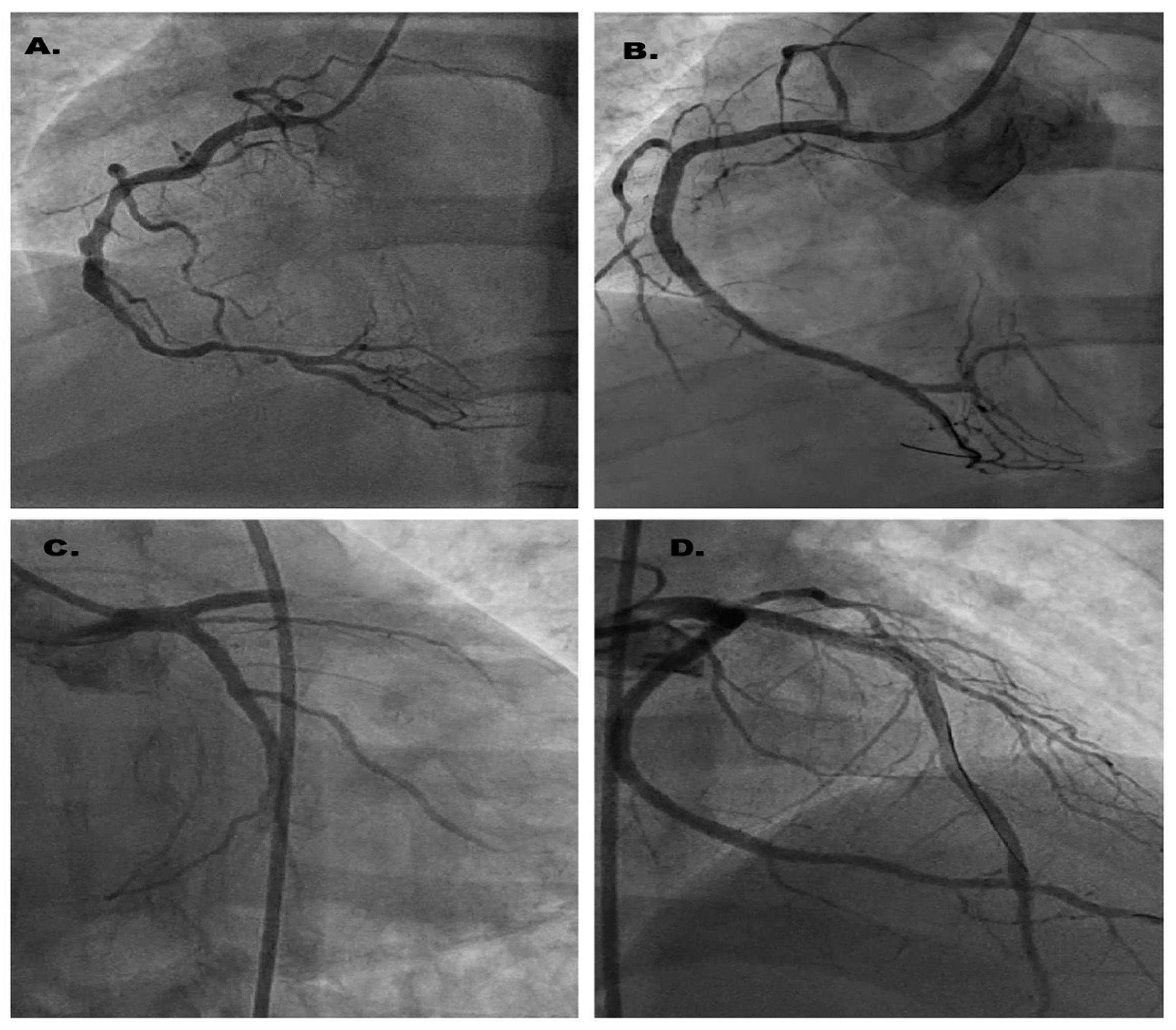
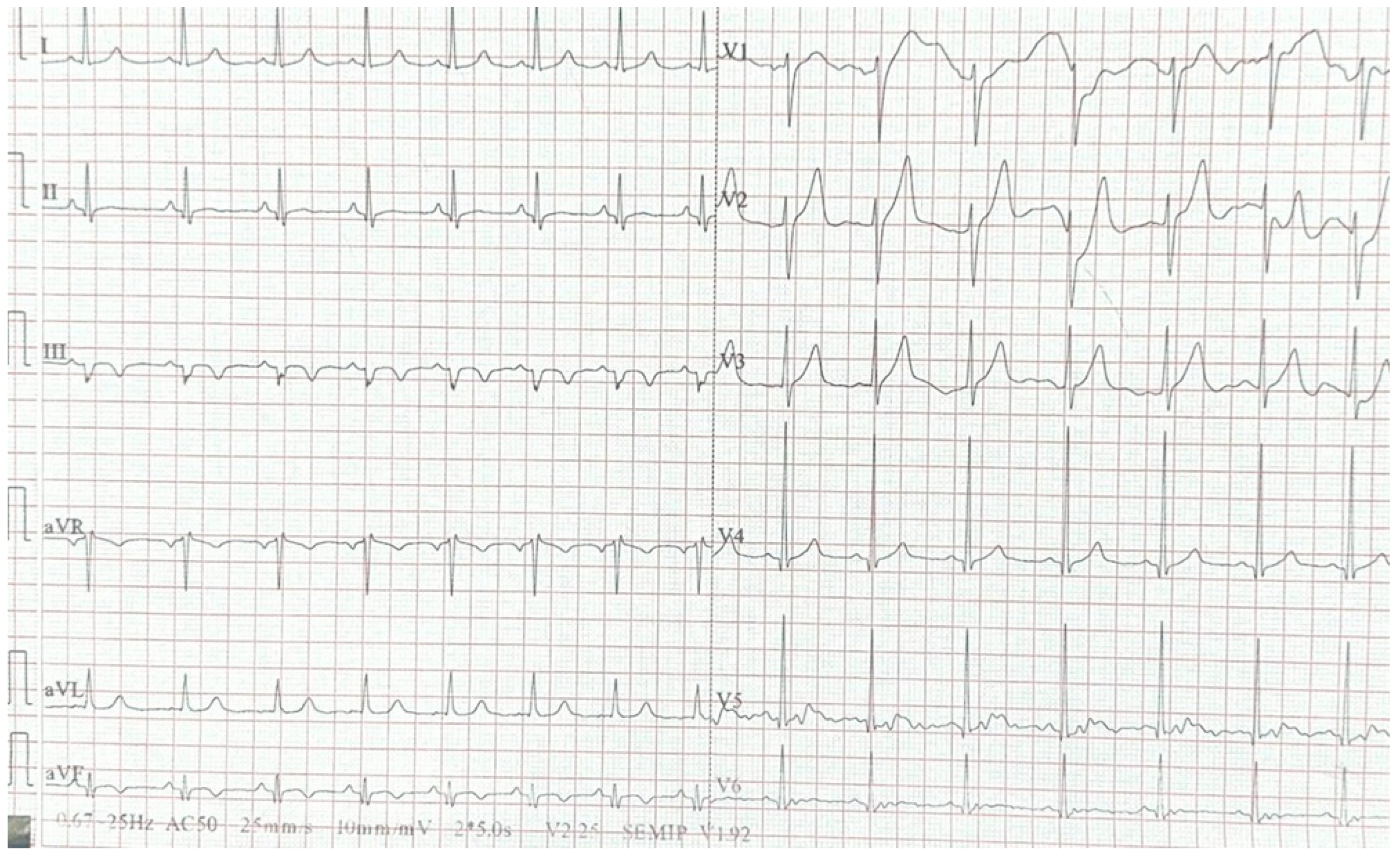
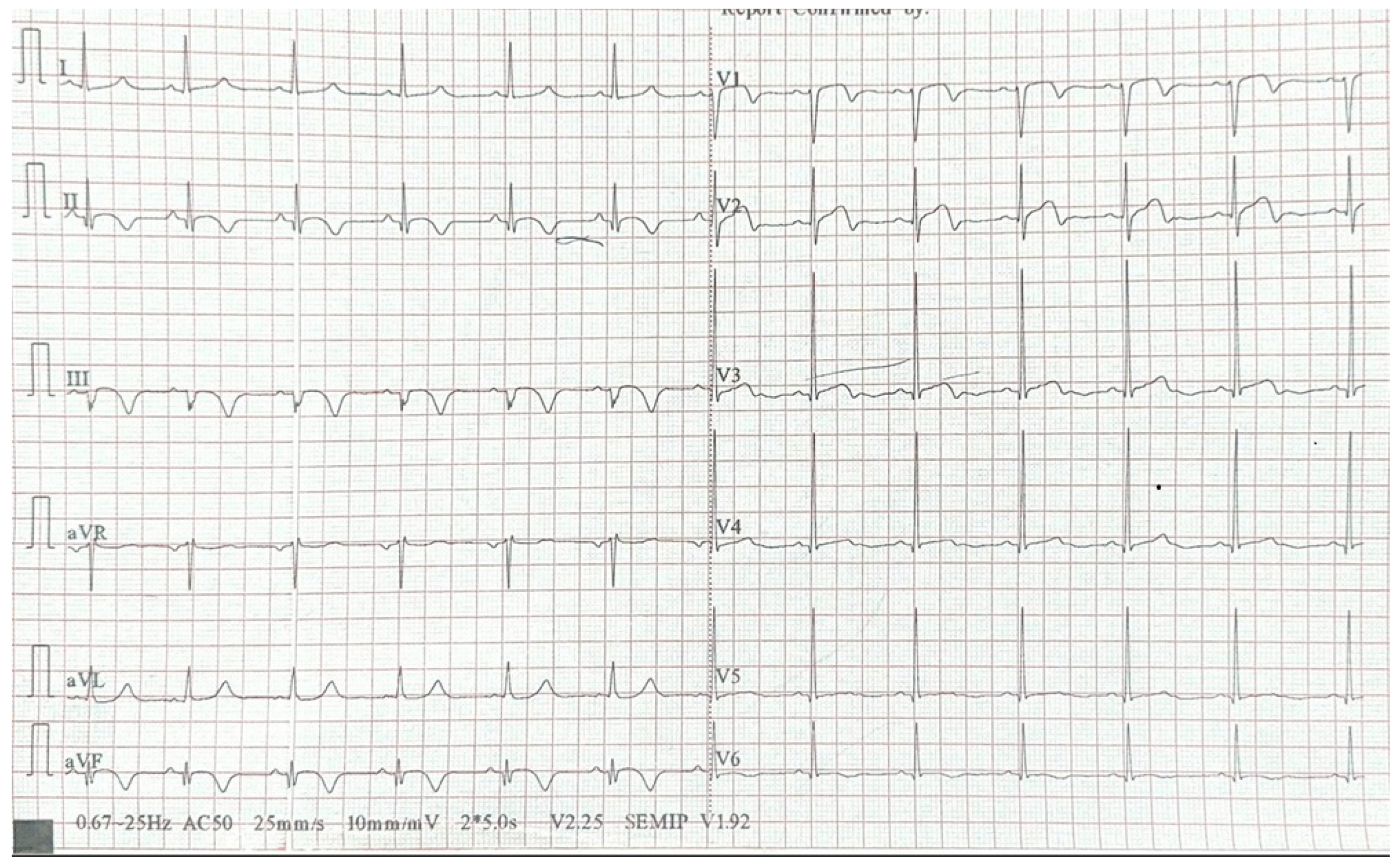
2. Case Report
3. Discussion
4. Conclusions
5. Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ECG | Electrocardiogram |
| LAD | Anterior descending artery |
| LCX | Left circumflex artery |
| RCD | Right coronary artery |
| MS | Multiple sclerosis |
| CVD | Cardiovascular disease |
| CAD | Coronary artery disease |
| MI | Myocardial infarction |
References
- Lennon, R.P.; Claussen, K.A.; Kuersteiner, K.A. State of the Heart: An Overview of the Disease Burden of Cardiovascular Disease from an Epidemiologic Perspective. Prim. Care 2018, 45, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Davia, J.E.; Hallal, F.J.; Cheitlin, M.D.; Gregoratos, G.; McCarty, R.; Foote, W. Coronary artery disease in young patients: Arteriographic and clinical review of 40 cases aged 35 and under. Am. Heart J. 1974, 87, 689–696. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Biery, D.W.; Singh, A.; Divakaran, S.; DeFilippis, E.M.; Wu, W.Y.; Klein, J.; Hainer, J.; Ramsis, M.; Natarajan, P.; et al. Risk Factors and Outcomes of Very Young Adults Who Experience Myocardial Infarction: The Partners YOUNG-MI Registry. Am. J. Med. 2020, 133, 605–612.e1. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Shi, R.-J.; Zhang, Y.-M.; Cheng, Y.-H.; Yang, B.-S.; Zhang, Y.-K.; Huang, B.-T.; Chen, M. Risk factors, clinical features, and outcomes of premature acute myocardial infarction. Front. Cardiovasc. Med. 2022, 9, 1012095. [Google Scholar] [CrossRef]
- Trimarchi, G.; Pizzino, F.; Lilli, A.; De Caterina, A.R.; Esposito, A.; Dalmiani, S.; Mazzone, A.; Di Bella, G.; Berti, S.; Paradossi, U. Advanced Lung Cancer Inflammation Index as Predictor of All-Cause Mortality in ST-Elevation Myocardial Infarction Patients Undergoing Primary Percutaneous Coronary Intervention. J. Clin. Med. 2024, 13, 6059. [Google Scholar] [CrossRef]
- Zasada, W.; Bobrowska, B.; Plens, K.; Dziewierz, A.; Siudak, Z.; Surdacki, A.; Dudek, D.; Bartuś, S. Acute myocardial infarction in young patients. Kardiol. Pol. 2021, 79, 1093–1098. [Google Scholar] [CrossRef]
- Arora, S.; Stouffer, G.A.; Kucharska-Newton, A.M.; Qamar, A.; Vaduganathan, M.; Pandey, A.; Porterfield, D.; Blankstein, R.; Rosamond, W.D.; Bhatt, D.L.; et al. Twenty Year Trends and Sex Differences in Young Adults Hospitalized With Acute Myocardial Infarction. Circulation 2019, 139, 1047–1056. [Google Scholar] [CrossRef]
- Paradossi, U.; De Caterina, A.R.; Trimarchi, G.; Pizzino, F.; Bastiani, L.; Dossi, F.; Raccis, M.; Bianchi, G.; Palmieri, C.; de Gregorio, C.; et al. The enigma of the “smoker’s paradox”: Results from a single-center registry of patients with STEMI undergoing primary percutaneous coronary intervention. Cardiovasc. Revasc. Med. 2024, 69, 42–49. [Google Scholar] [CrossRef]
- Weinberger, I.; Rotenberg, Z.; Fuchs, J.; Sagy, A.; Friedmann, J.; Agmon, J. Myocardial infarction in young adults under 30 years: Risk factors and clinical course. Clin. Cardiol. 1987, 10, 9–15. [Google Scholar] [CrossRef]
- Tibblin, G.; Wilhelmsen, L.; Werkö, L. Risk factors for myocardial infarction and death due to ischemic heart disease and other causes. Am. J. Cardiol. 1975, 35, 514–522. [Google Scholar] [CrossRef]
- Kannel, W.B.; Sorlie, P.; McNamara, P.M. Prognosis after initial myocardial infarction: The Framingham study. Am. J. Cardiol. 1979, 44, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Roskamm, H.; Gohlke, H.; Stürzenhofecker, P.; Samek, L.; Betz, P. Myocardial infarction at a young age (under 40 years). Int. J. Sports Med. 1984, 5, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Magyari, M.; Sorensen, P.S. Comorbidity in Multiple Sclerosis. Front. Neurol. 2020, 11, 851. [Google Scholar] [CrossRef] [PubMed]
- Reich, D.S.; Lucchinetti, C.F.; Calabresi, P.A. Multiple Sclerosis. N. Engl. J. Med. 2018, 378, 169–180. [Google Scholar] [CrossRef]
- Christiansen, C.F. Risk of vascular disease in patients with multiple sclerosis: A review. Neurol. Res. 2012, 34, 746–753. [Google Scholar] [CrossRef]
- Wei, L.; MacDonald, T.M.; Walker, B.R. Taking glucocorticoids by prescription is associated with subsequent cardiovascular disease. Ann. Intern. Med. 2004, 141, 764–770. [Google Scholar] [CrossRef]
- Marrie, R.A.; Horwitz, R.I.; Cutter, G.; Tyry, T.; Vollmer, T. Smokers with multiple sclerosis are more likely to report comorbid autoimmune diseases. Neuroepidemiology 2011, 36, 85–90. [Google Scholar] [CrossRef]
- Geissmann, F.; Manz, M.G.; Jung, S.; Sieweke, M.H.; Merad, M.; Ley, K. Development of monocytes, macrophages, and dendritic cells. Science 2010, 327, 656–661. [Google Scholar] [CrossRef]
- Ajoolabady, A.; Pratico, D.; Lin, L.; Mantzoros, C.S.; Bahijri, S.; Tuomilehto, J.; Ren, J. Inflammation in atherosclerosis: Pathophysiology and mechanisms. Cell Death Dis. 2024, 15, 817. [Google Scholar] [CrossRef]
- Iwaszko, M.; Biały, S.; Bogunia-Kubik, K. Significance of Interleukin (IL)-4 and IL-13 in Inflammatory Arthritis. Cells 2021, 10, 3000. [Google Scholar] [CrossRef]
- Freemerman, A.J.; Johnson, A.R.; Sacks, G.N.; Milner, J.J.; Kirk, E.L.; Troester, M.A.; Macintyre, A.N.; Goraksha-Hicks, P.; Rathmell, J.C.; Makowski, L. Metabolic reprogramming of macrophages: Glucose transporter 1 (GLUT1)-mediated glucose metabolism drives a proinflammatory phenotype. J. Biol. Chem. 2014, 289, 7884–7896. [Google Scholar] [CrossRef] [PubMed]
- Palsson-McDermott, E.M.; Curtis, A.M.; Goel, G.; Lauterbach, M.A.R.; Sheedy, F.J.; Gleeson, L.E.; van den Bosch, M.W.M.; Quinn, S.R.; Domingo-Fernandez, R.; Johnston, D.G.W.; et al. Pyruvate kinase M2 regulates Hif-1α activity and IL-1β induction and is a critical determinant of the warburg effect in LPS-activated macrophages. Cell Metab. 2015, 21, 65–80. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, T.; Natoli, G. Transcriptional regulation of macrophage polarization: Enabling diversity with identity. Nat. Rev. Immunol. 2011, 11, 750–761. [Google Scholar] [CrossRef] [PubMed]
- Soehnlein, O.; Swirski, F.K. Hypercholesterolemia links hematopoiesis with atherosclerosis. Trends Endocrinol. Metab. 2013, 24, 129–136. [Google Scholar] [CrossRef]
- Combadière, C.; Potteaux, S.; Rodero, M.; Simon, T.; Pezard, A.; Esposito, B.; Merval, R.; Proudfoot, A.; Tedgui, A.; Mallat, Z. Combined inhibition of CCL2, CX3CR1, and CCR5 abrogates Ly6C(hi) and Ly6C(lo) monocytosis and almost abolishes atherosclerosis in hypercholesterolemic mice. Circulation 2008, 117, 1649–1657. [Google Scholar] [CrossRef]
- Engberink, R.D.O.; van der Pol, S.M.A.; Walczak, P.; van der Toorn, A.; Viergever, M.A.; Dijkstra, C.D.; Bulte, J.W.M.; de Vries, H.E.; Blezer, E.L.A. Magnetic resonance imaging of monocytes labeled with ultrasmall superparamagnetic particles of iron oxide using magnetoelectroporation in an animal model of multiple sclerosis. Mol. Imaging 2010, 9, 268–277. [Google Scholar] [CrossRef]
- DePaula-Silva, A.B. The Contribution of Microglia and Brain-Infiltrating Macrophages to the Pathogenesis of Neuroinflammatory and Neurodegenerative Diseases during TMEV Infection of the Central Nervous System. Viruses 2024, 16, 119. [Google Scholar] [CrossRef]
- Liu, T.; Chen, Y.; Hou, L.; Yu, Y.; Ma, D.; Jiang, T.; Zhao, G. Immune cell-mediated features of atherosclerosis. Front. Cardiovasc. Med. 2024, 11, 1450737. [Google Scholar] [CrossRef]
- Swerdlow, D.I.; Holmes, M.V.; Kuchenbaecker, K.B.; Engmann, J.E.L.; Shah, T.; Sofat, R.; Guo, Y.; Chung, C.; Peasey, A.; Pfister, R.; et al. The interleukin-6 receptor as a target for prevention of coronary heart disease: A mendelian randomisation analysis. Lancet 2012, 379, 1214–1224. [Google Scholar] [CrossRef]
- Abbate, A.; Toldo, S.; Marchetti, C.; Kron, J.; Van Tassell, B.W.; Dinarello, C.A. Interleukin-1 and the Inflammasome as Therapeutic Targets in Cardiovascular Disease. Circ. Res. 2020, 126, 1260–1280. [Google Scholar] [CrossRef]
- Ajala, O.N.; Everett, B.M. Targeting Inflammation to Reduce Residual Cardiovascular Risk. Curr. Atheroscler. Rep. 2020, 22, 66. [Google Scholar] [CrossRef] [PubMed]
- Dendrou, C.A.; Fugger, L.; Friese, M.A. Immunopathology of multiple sclerosis. Nat. Rev. Immunol. 2015, 15, 545–558. [Google Scholar] [CrossRef] [PubMed]
- Kutzelnigg, A.; Lucchinetti, C.F.; Stadelmann, C.; Brück, W.; Rauschka, H.; Bergmann, M.; Schmidbauer, M.; Parisi, J.E.; Lassmann, H. Cortical demyelination and diffuse white matter injury in multiple sclerosis. Brain 2005, 128, 2705–2712. [Google Scholar] [CrossRef] [PubMed]
- Roifman, I.; Beck, P.L.; Anderson, T.J.; Eisenberg, M.J.; Genest, J. Chronic inflammatory diseases and cardiovascular risk: A systematic review. Can. J. Cardiol. 2011, 27, 174–182. [Google Scholar] [CrossRef]
- Lassmann, H.; van Horssen, J.; Mahad, D. Progressive multiple sclerosis: Pathology and pathogenesis. Nat. Rev. Neurol. 2012, 8, 647–656. [Google Scholar] [CrossRef]
- Tschöpe, C.; Ammirati, E.; Bozkurt, B.; Caforio, A.L.P.; Cooper, L.T.; Felix, S.B.; Hare, J.M.; Heidecker, B.; Heymans, S.; Hübner, N.; et al. Myocarditis and inflammatory cardiomyopathy: Current evidence and future directions. Nat. Rev. Cardiol. 2021, 18, 169–193. [Google Scholar] [CrossRef]
- Hauser, S.L.; Bar-Or, A.; Comi, G.; Giovannoni, G.; Hartung, H.-P.; Hemmer, B.; Lublin, F.; Montalban, X.; Rammohan, K.W.; Selmaj, K.; et al. Ocrelizumab versus Interferon Beta-1a in Relapsing Multiple Sclerosis. N. Engl. J. Med. 2017, 376, 221–234. [Google Scholar] [CrossRef]
- Cappannoli, L.; Scacciavillani, R.; Iannaccone, G.; Anastasia, G.; Di Giusto, F.; Loria, V.; Aspromonte, N. 2019 novel-coronavirus: Cardiovascular insights about risk factors, myocardial injury, therapy and clinical implications. Chronic Dis. Transl. Med. 2020, 6, 246–250. [Google Scholar] [CrossRef]
- Kaufmann, C.C.; Villatore, A.; Heugl, M.; Kvakan, H.; Zweiker, D.; Sala, S.; Mazzone, P.; Huber, K.; Peretto, G. Cardiac inflammation associated with COVID-19 mRNA vaccination in patients with and without previous myocarditis. Minerva Cardiol. Angiol. 2023, 71, 242–248. [Google Scholar] [CrossRef]
- Toljan, K.; Amin, M.; Kunchok, A.; Ontaneda, D. New diagnosis of multiple sclerosis in the setting of mRNA COVID-19 vaccine exposure. J. Neuroimmunol. 2022, 362, 577785. [Google Scholar] [CrossRef]
- Marshall, N.A.; Vickers, M.A.; Barker, R.N. Regulatory T cells secreting IL-10 dominate the immune response to EBV latent membrane protein 1. J. Immunol. 2003, 170, 6183–6189. [Google Scholar] [CrossRef] [PubMed]
- Dominguez-Villar, M.; Baecher-Allan, C.M.; Hafler, D.A. Identification of T helper type 1-like, Foxp3+ regulatory T cells in human autoimmune disease. Nat. Med. 2011, 17, 673–675. [Google Scholar] [CrossRef] [PubMed]
- Waickman, A.T.; Keller, H.R.; Kim, T.-H.; Luckey, M.A.; Tai, X.; Hong, C.; Molina-París, C.; Walsh, S.T.R.; Park, J.-H. The Cytokine Receptor IL-7Rα Impairs IL-2 Receptor Signaling and Constrains the In Vitro Differentiation of Foxp3+ Treg Cells. iScience 2020, 23, 101421. [Google Scholar] [CrossRef]
- Hartmann, F.J.; Khademi, M.; Aram, J.; Ammann, S.; Kockum, I.; Constantinescu, C.; Gran, B.; Piehl, F.; Olsson, T.; Codarri, L.; et al. Multiple sclerosis-associated IL2RA polymorphism controls GM-CSF production in human TH cells. Nat. Commun. 2014, 5, 5056. [Google Scholar] [CrossRef] [PubMed]
- Kreft, K.L.; Verbraak, E.; Wierenga-Wolf, A.F.; van Meurs, M.; Oostra, B.A.; Laman, J.D.; Hintzen, R.Q. Decreased systemic IL-7 and soluble IL-7Rα in multiple sclerosis patients. Genes Immun. 2012, 13, 587–592. [Google Scholar] [CrossRef]
- Weber, F.; Fontaine, B.; Cournu-Rebeix, I.; Kroner, A.; Knop, M.; Lutz, S.; Müller-Sarnowski, F.; Uhr, M.; Bettecken, T.; Kohli, M.; et al. IL2RA and IL7RA genes confer susceptibility for multiple sclerosis in two independent European populations. Genes Immun. 2008, 9, 259–263. [Google Scholar] [CrossRef]
- van Langelaar, J.; Rijvers, L.; Smolders, J.; van Luijn, M.M. B and T Cells Driving Multiple Sclerosis: Identity, Mechanisms and Potential Triggers. Front. Immunol. 2020, 11, 760. [Google Scholar] [CrossRef]
- Bjornevik, K.; Cortese, M.; Healy, B.C.; Kuhle, J.; Mina, M.J.; Leng, Y.; Elledge, S.J.; Niebuhr, D.W.; Scher, A.I.; Munger, K.L.; et al. Longitudinal analysis reveals high prevalence of Epstein-Barr virus associated with multiple sclerosis. Science 2022, 375, 296–301. [Google Scholar] [CrossRef]
- Ghenea, A.E.; Ungureanu, A.M.; Turculeanu, A.; Popescu, M.; Carsote, M.; Ţieranu, M.L.; Ţieranu, E.N.; Vasile, C.M.; Cioboată, R.; Udriştoiu, A.L.; et al. Predictors of early and sustained virological response of viral hepatitis C. Rom. J. Morphol. Embryol. = Rev. Roum. Morphol. Embryol. 2020, 61, 1185–1192. [Google Scholar] [CrossRef]
- Ascherio, A.; Munger, K.L. Environmental risk factors for multiple sclerosis. Part I: The role of infection. Ann. Neurol. 2007, 61, 288–299. [Google Scholar] [CrossRef]
- Aubert-Broche, B.; Fonov, V.; Narayanan, S.; Arnold, D.L.; Araujo, D.; Fetco, D.; Till, C.; Sled, J.G.; Banwell, B.; Collins, D.L. Onset of multiple sclerosis before adulthood leads to failure of age-expected brain growth. Neurology 2014, 83, 2140–2146. [Google Scholar] [CrossRef] [PubMed]
- Dobson, R.; Giovannoni, G. Multiple sclerosis—A review. Eur. J. Neurol. 2019, 26, 27–40. [Google Scholar] [CrossRef] [PubMed]
- Multiple sclerosis genomic map implicates peripheral immune cells and microglia in susceptibility. Science 2019, 365, aav7188. [CrossRef] [PubMed]
- Zavarella, M.; Villatore, A.; Rocca, M.A.; Peretto, G.; Filippi, M. The Heart-Brain Interplay in Multiple Sclerosis from Pathophysiology to Clinical Practice: A Narrative Review. J. Cardiovasc. Dev. Dis. 2023, 10, 153. [Google Scholar] [CrossRef]
- Tseng, C.-H.; Huang, W.-S.; Lin, C.-L.; Chang, Y.-J. Increased risk of ischaemic stroke among patients with multiple sclerosis. Eur. J. Neurol. 2015, 22, 500–506. [Google Scholar] [CrossRef]
- Roshanisefat, H.; Bahmanyar, S.; Hillert, J.; Olsson, T.; Montgomery, S. Multiple sclerosis clinical course and cardiovascular disease risk—Swedish cohort study. Eur. J. Neurol. 2014, 21, 1353-e88. [Google Scholar] [CrossRef]
- Yang, F.; Hu, T.; He, K.; Ying, J.; Cui, H. Multiple Sclerosis and the Risk of Cardiovascular Diseases: A Mendelian Randomization Study. Front. Immunol. 2022, 13, 861885. [Google Scholar] [CrossRef]
- Swanberg, M.; Lidman, O.; Padyukov, L.; Eriksson, P.; Akesson, E.; Jagodic, M.; Lobell, A.; Khademi, M.; Börjesson, O.; Lindgren, C.M.; et al. MHC2TA is associated with differential MHC molecule expression and susceptibility to rheumatoid arthritis, multiple sclerosis and myocardial infarction. Nat. Genet. 2005, 37, 486–494. [Google Scholar] [CrossRef]
- Jain, S. Role of interleukin-17 signaling pathway in the interaction between multiple sclerosis and acute myocardial infarction. Mult. Scler. Relat. Disord. 2022, 58, 103515. [Google Scholar] [CrossRef]
- Enzinger, C.; Fazekas, F.; Matthews, P.M.; Ropele, S.; Schmidt, H.; Smith, S.; Schmidt, R. Risk factors for progression of brain atrophy in aging: Six-year follow-up of normal subjects. Neurology 2005, 64, 1704–1711. [Google Scholar] [CrossRef]
- Jefferson, A.L.; Massaro, J.M.; Wolf, P.A.; Seshadri, S.; Au, R.; Vasan, R.S.; Larson, M.G.; Meigs, J.B.; Keaney, J.F.J.; Lipinska, I.; et al. Inflammatory biomarkers are associated with total brain volume: The Framingham Heart Study. Neurology 2007, 68, 1032–1038. [Google Scholar] [CrossRef] [PubMed]
- Minagar, A.; Jy, W.; Jimenez, J.J.; Alexander, J.S. Multiple sclerosis as a vascular disease. Neurol. Res. 2006, 28, 230–235. [Google Scholar] [CrossRef] [PubMed]
- Hauser, S.L.; Cree, B.A.C. Treatment of Multiple Sclerosis: A Review. Am. J. Med. 2020, 133, 1380–1390.e2. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, T.B.; Berkowitz, A.L.; Samuels, M.A. Cardiovascular Dysfunction in Multiple Sclerosis. Neurologist 2015, 20, 108–114. [Google Scholar] [CrossRef]
- Vanoli, E.; Montano, N.; De Angelis, G.; Badilini, F.; Mirabella, M.; Bonavita, S.; Patti, F.; Bianco, A.; Sparaco, M.; Chisari, C.; et al. Cardiovascular autonomic individual profile of relapsing-remitting multiple sclerosis patients and risk of extending cardiac monitoring after first dose fingolimod. J. Neurol. Sci. 2019, 405, 116423. [Google Scholar] [CrossRef]
- Aimo, A.; Tavoni, A.; Buda, G.; Emdin, M. Rituximab as a novel treatment for heart failure: Evidence from a case series. Eur. Heart J. Case Rep. 2019, 3, 1–2. [Google Scholar] [CrossRef]
- Kuang, Y.; Zhang, Y.; Xiao, Z.; Xu, L.; Wang, P.; Ma, Q. Protective effect of dimethyl fumarate on oxidative damage and signaling in cardiomyocytes. Mol. Med. Rep. 2020, 22, 2783–2790. [Google Scholar] [CrossRef]
- Cîrstea, I.; Mîndrilă, B.; Țieranu, E.; Țieranu, L.M.; Istratoaie, O.; Militaru, C.; Donoiu, I. Overview of non-vitamin K oral anticoagulants. Farmacia 2020, 68, 206. [Google Scholar] [CrossRef]
- Poznyak, A.V.; Sadykhov, N.K.; Kartuesov, A.G.; Borisov, E.E.; Melnichenko, A.A.; Grechko, A.V.; Orekhov, A.N. Hypertension as a risk factor for atherosclerosis: Cardiovascular risk assessment. Front. Cardiovasc. Med. 2022, 9, 959285. [Google Scholar] [CrossRef]
- Bays, H.E.; Kulkarni, A.; German, C.; Satish, P.; Iluyomade, A.; Dudum, R.; Thakkar, A.; Al Rifai, M.; Mehta, A.; Thobani, A.; et al. Ten things to know about ten cardiovascular disease risk factors—2022. Am. J. Prev. Cardiol. 2022, 10, 100342. [Google Scholar] [CrossRef]
- Mohammed, E.M.A. Understanding Multiple Sclerosis Pathophysiology and Current Disease-Modifying Therapies: A Review of Unaddressed Aspects. FBL 2024, 29, 386. [Google Scholar] [CrossRef] [PubMed]
- Prado, C.; Herrada, A.A.; Hevia, D.; Goiry, L.G.; Escobedo, N. Role of innate immune cells in multiple sclerosis. Front. Immunol. 2025, 16, 1540263. [Google Scholar] [CrossRef] [PubMed]
- Findling, O.; Hauer, L.; Pezawas, T.; Rommer, P.S.; Struhal, W.; Sellner, J. Cardiac Autonomic Dysfunction in Multiple Sclerosis: A Systematic Review of Current Knowledge and Impact of Immunotherapies. J. Clin. Med. 2020, 9, 335. [Google Scholar] [CrossRef] [PubMed]
- Jaramillo-Torres, M.J.; Limpert, R.H.; Butak, W.J.; Cohen, K.E.; Whitaker-Hilbig, A.A.; SenthilKumar, G.; Durand, M.J.; Freed, J.K. Promoting Resiliency to Stress in the Vascular Endothelium. Basic Clin. Pharmacol. Toxicol. 2025, 136, e70001. [Google Scholar] [CrossRef]
- Liu, T.; Yang, L.; Lv, X.; Zuo, C.; Jia, C.; Yang, Z.; Fan, C.; Chen, H. Cumulative evidence for associations between genetic variants in interleukin 17 family gene and risk of human diseases. Front. Immunol. 2022, 13, 1008184. [Google Scholar] [CrossRef]
- Banait, T.; Wanjari, A.; Danade, V.; Banait, S.; Jain, J. Role of High-Sensitivity C-reactive Protein (Hs-CRP) in Non-communicable Diseases: A Review. Cureus 2022, 14, e30225. [Google Scholar] [CrossRef]
- Casas-Deza, D.; Martínez-Sapiña, A.; Espina, S.; Garcia-Rodriguez, B.; Fernandez-Bonilla, E.M.; Sanz-Paris, A.; Gonzalez-Irazabal, Y.; Bernal-Monterde, V.; Arbones-Mainar, J.M. Evaluation of Cardiovascular Risk Factors after Hepatitis C Virus Eradication with Direct-Acting Antivirals in a Cohort of Treatment-Naïve Patients without History of Cardiovascular Disease. J. Clin. Med. 2022, 11, 4049. [Google Scholar] [CrossRef]
- Sacchetti, S.; Puricelli, C.; Mennuni, M.; Zanotti, V.; Giacomini, L.; Giordano, M.; Dianzani, U.; Patti, G.; Rolla, R. Research into New Molecular Mechanisms in Thrombotic Diseases Paves the Way for Innovative Therapeutic Approaches. Int. J. Mol. Sci. 2024, 25, 2523. [Google Scholar] [CrossRef]
- Coerver, E.M.E.; Fung, W.H.; de Beukelaar, J.; Bouvy, W.H.; Canta, L.R.; Gerlach, O.H.H.; Hoitsma, E.; Hoogervorst, E.L.J.; de Jong, B.A.; Kalkers, N.F.; et al. Discontinuation of First-Line Disease-Modifying Therapy in Patients With Stable Multiple Sclerosis: The DOT-MS Randomized Clinical Trial. JAMA Neurol. 2025, 82, 123–131. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Țieranu, E.N.; Cureraru, S.I.; Târtea, G.C.; Vladuțu, V.-C.; Cojocaru, P.A.; Piorescu, M.T.L.; Țieranu, L.M. Acute Myocardial Infarction and Diffuse Coronary Artery Disease in a Patient with Multiple Sclerosis: A Case Report and Literature Review. J. Clin. Med. 2025, 14, 4304. https://doi.org/10.3390/jcm14124304
Țieranu EN, Cureraru SI, Târtea GC, Vladuțu V-C, Cojocaru PA, Piorescu MTL, Țieranu LM. Acute Myocardial Infarction and Diffuse Coronary Artery Disease in a Patient with Multiple Sclerosis: A Case Report and Literature Review. Journal of Clinical Medicine. 2025; 14(12):4304. https://doi.org/10.3390/jcm14124304
Chicago/Turabian StyleȚieranu, Eugen Nicolae, Silvana Isabella Cureraru, Georgică Costinel Târtea, Viorel-Cristian Vladuțu, Petre Alexandru Cojocaru, Mina Teodora Luminița Piorescu, and Loredana Maria Țieranu. 2025. "Acute Myocardial Infarction and Diffuse Coronary Artery Disease in a Patient with Multiple Sclerosis: A Case Report and Literature Review" Journal of Clinical Medicine 14, no. 12: 4304. https://doi.org/10.3390/jcm14124304
APA StyleȚieranu, E. N., Cureraru, S. I., Târtea, G. C., Vladuțu, V.-C., Cojocaru, P. A., Piorescu, M. T. L., & Țieranu, L. M. (2025). Acute Myocardial Infarction and Diffuse Coronary Artery Disease in a Patient with Multiple Sclerosis: A Case Report and Literature Review. Journal of Clinical Medicine, 14(12), 4304. https://doi.org/10.3390/jcm14124304





