Diagnostic Challenges and Risk Stratification of Periprosthetic Joint Infection in Patients with Inflammatory Arthritis
Abstract
1. Background
2. Methods
3. Results
3.1. Assessment of Bias and Level of Evidence of the Enrolled Studies
3.2. Demography Data and Risk Factors of Patients with Inflammatory Arthritis with PJI Diagnosis
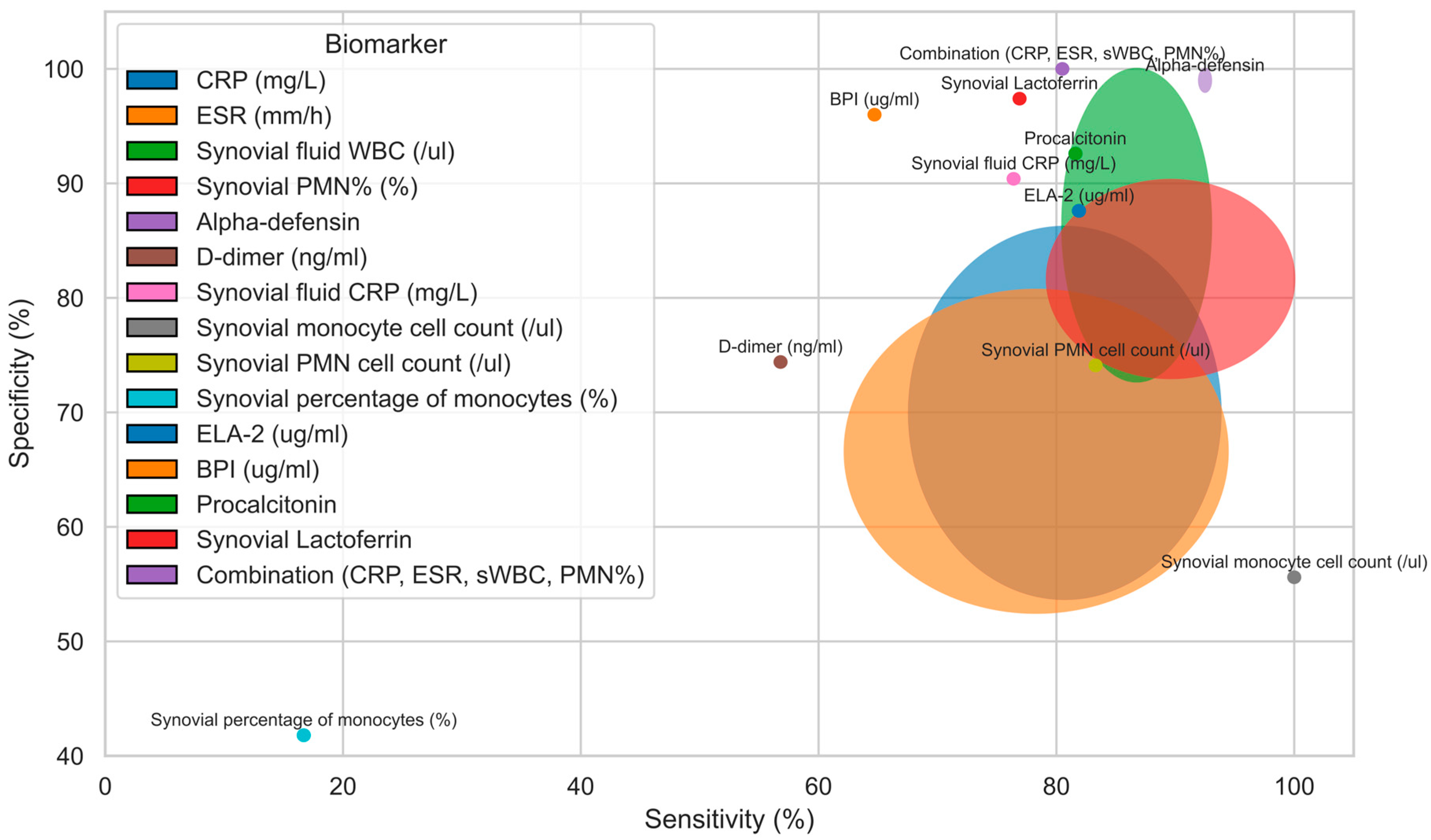
3.3. Efficacy and Diagnostic Value of Preoperative and Intraoperative Criteria for PJI Diagnosis in Patients with Inflammatory Arthritis
4. Discussion
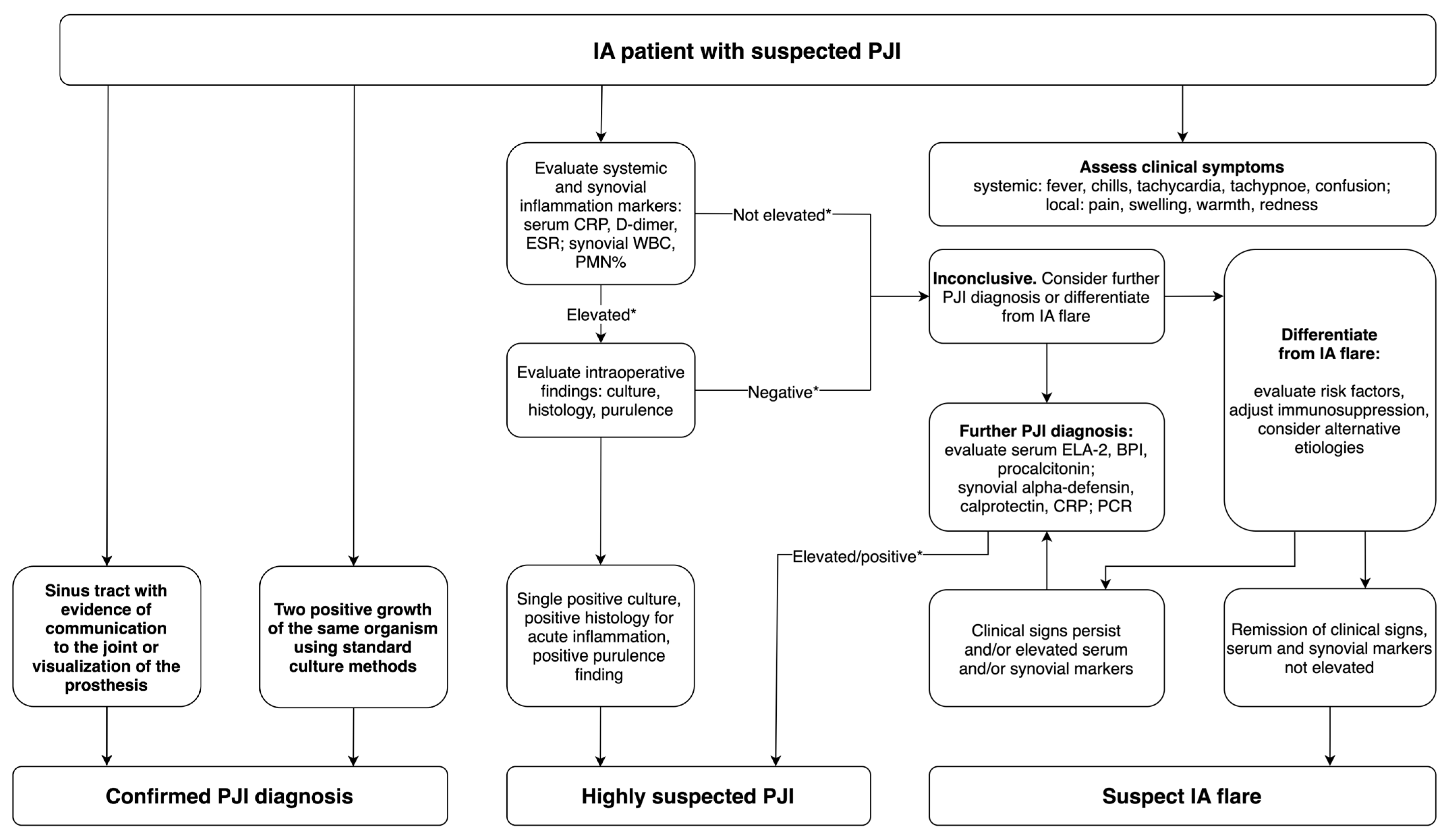
4.1. Diagnostic Challenges
4.2. Perioperative Risk Factors and Medication Management
4.3. Guidelines and Clinical Implications
4.4. Limitations
5. Conclusions
5.1. Risk Factors for PJI in Patients with Inflammatory Arthritis
- Patients with IA undergoing alloplasty have an increased risk of infection due to disease activity and IA treatment.
- Key contributors to increased PJI risk include elevated systemic inflammation, extended disease duration, corticosteroid use, and the uninterrupted administration of biologic agents during the perioperative phase.
- Future studies should determine a more accurate optimal timing of withholding IA therapy and consider the role of comorbidities such as metabolic syndrome or cardiovascular disease in the pathogenesis of PJI in patients with inflammatory arthritis.
5.2. Suggestions for PJI Diagnosis in Patients with Inflammatory Arthritis
- In patients with inflammatory arthritis, diagnostic efforts should primarily rely on fulfilling major MSIS criteria, such as dual positive cultures or the presence of a sinus tract.
- The application of the scoring system recommended in the updated MSIS criteria for cases that do not meet the main criteria is limited in patients with autoimmune inflammation due to the low efficacy of the available diagnostic tests. Adhering to recommended cut-off points may lead to false-positive interpretations.
- Synovial fluid markers such as sWBC, PMN%, and alpha-defensin are relatively the most reliable and may be most helpful in diagnosing uncertain infection cases. Serum ESR and CRP can be applied in combination with synovial markers.
- Additional markers not included in the MSIS criteria, such as serum ELA–2, BPI, procalcitonin, synovial CRP, calprotectin, and molecular techniques like PCR present promising diagnostic values for the diagnosis of PJI, but more studies are needed to confirm their efficacy for patients with IA.
- To advance diagnostic accuracy in IA-related PJI, additional clinical studies are necessary to formulate and validate a tailored diagnostic algorithm or scoring model.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Learmonth, I.D.; Young, C.; Rorabeck, C. The operation of the century: Total hip replacement. Lancet 2007, 370, 1508–1519. [Google Scholar] [CrossRef] [PubMed]
- Natsuhara, K.M.; Shelton, T.J.; Meehan, J.P.; Lum, Z.C. Mortality During Total Hip Periprosthetic Joint Infection. J. Arthroplast. 2019, 34 (Suppl. 7), S337–S342. [Google Scholar] [CrossRef] [PubMed]
- Parvizi, J.; Tan, T.L.; Goswami, K.; Higuera, C.; Della Valle, C.; Chen, A.F.; Shohat, N. The 2018 Definition of Periprosthetic Hip and Knee Infection: An Evidence-Based and Validated Criteria. J. Arthroplast. 2018, 33, 1309–1314.e2. [Google Scholar] [CrossRef] [PubMed]
- Shohat, N.; Bauer, T.; Buttaro, M.; Budhiparama, N.; Cashman, J.; Della Valle, C.J.; Drago, L.; Gehrke, T.; Gomes, L.S.M.; Goswami, K.; et al. Hip and Knee Section, What is the Definition of a Periprosthetic Joint Infection (PJI) of the Knee and the Hip? Can the Same Criteria be Used for Both Joints?: Proceedings of International Consensus on Orthopedic Infections. J. Arthroplast. 2019, 34, S325–S327. [Google Scholar] [CrossRef]
- Goodman, S.M.; Figgie, M.A. Arthroplasty in patients with established rheumatoid arthritis (RA): Mitigating risks and optimizing outcomes. Best. Pract. Res. Clin. Rheumatol. 2015, 29, 628–642. [Google Scholar] [CrossRef]
- Morrison, T.A.; Figgie, M.; Miller, A.O.; Goodman, S.M. Periprosthetic Joint Infection in Patients with Inflammatory Joint Disease: A Review of Risk Factors and Current Approaches to Diagnosis and Management. HSS J. 2013, 9, 183–194. [Google Scholar] [CrossRef]
- Silman, A.J.; Pearson, J.E. Epidemiology and genetics of rheumatoid arthritis. Arthritis Res. 2002, 4 (Suppl. S3), S265–S272. [Google Scholar] [CrossRef]
- Smolen, J.S.; Aletaha, D.; McInnes, I.B. Rheumatoid arthritis. Lancet 2016, 388, 2023–2038. [Google Scholar] [CrossRef]
- Yeganeh, M.H.; Kheir, M.M.; Shahi, A.; Parvizi, J. Rheumatoid Arthritis, Disease Modifying Agents, and Periprosthetic Joint Infection: What Does a Joint Surgeon Need to Know? J. Arthroplast. 2018, 33, 1258–1264. [Google Scholar] [CrossRef]
- Doran, M.F.; Crowson, C.S.; Pond, G.R.; O’FAllon, W.M.; Gabriel, S.E. Frequency of infection in patients with rheumatoid arthritis compared with controls: A population-based study. Arthritis Rheum. 2002, 46, 2287–2293. [Google Scholar] [CrossRef]
- Bernatsky, S.; Hudson, M.; Suissa, S. Anti-rheumatic drug use and risk of serious infections in rheumatoid arthritis. Rheumatology 2007, 46, 1157–1160. [Google Scholar] [CrossRef] [PubMed]
- Shohat, N.; Goswami, K.; Fillingham, Y.; Tan, T.L.; Calkins, T.; Della Valle, C.J.; George, J.; Higuera, C.; Parvizi, J. Diagnosing Periprosthetic Joint Infection in Inflammatory Arthritis: Assumption Is the Enemy of True Understanding. J. Arthroplast. 2018, 33, 3561–3566. [Google Scholar] [CrossRef] [PubMed]
- Sculco, P.; Kapadia, M.; Moezinia, C.J.; Mannstadt, I.; Miller, A.O.; Donlin, L.; Henry, M.; Russell, L.; Figgie, M.; Nocon, A.; et al. Clinical and Histological Features of Prosthetic Joint Infections May Differ in Patients With Inflammatory Arthritis and Osteoarthritis. HSS J. 2023, 19, 146–153. [Google Scholar] [CrossRef]
- Xu, H.; Xie, J.; Wan, X.; Liu, L.; Wang, D.; Zhou, Z. Combination of C-reactive protein and fibrinogen is useful for diagnosing periprosthetic joint infection in patients with inflammatory diseases. Chin. Med. J. 2022, 135, 1986–1992. [Google Scholar] [CrossRef]
- Wang, Y.; Li, G.; Ji, B.; Xu, B.; Zhang, X.; Maimaitiyiming, A.; Cao, L. Diagnosis of periprosthetic joint infections in patients who have rheumatoid arthritis: Application of routine serological and synovial fluid indexes. Bone Jt. Res. 2023, 12, 559–570. [Google Scholar] [CrossRef]
- Miyamae, Y.; George, J.; Klika, A.K.; Barsoum, W.K.; Higuera, C.A. Diagnostic Accuracy of the Alpha-Defensin Test for Periprosthetic Joint Infection in Patients With Inflammatory Diseases. J. Arthroplast. 2019, 34, 1767–1771. [Google Scholar] [CrossRef]
- Tahta, M.; Simsek, M.E.; Isik, C.; Akkaya, M.; Gursoy, S.; Bozkurt, M. Does inflammatory joint diseases affect the accuracy of infection biomarkers in patients with periprosthetic joint infections? A prospective comparative reliability study. J. Orthop. Sci. 2018, 24, 286–289. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Li, L.; Wang, H.-Y.; Ding, L.; Wang, Y.; Liu, X.; Tian, S.; Wang, Y. Efficacy analysis of clinical serological indicators in the diagnosis of postoperative periprosthetic joint infection in patients with rheumatoid arthritis or osteoarthritis. Int. Orthop. 2024, 48, 1945–1952. [Google Scholar] [CrossRef]
- de Araujo, L.C.T.; Westerholt, A.; Sandiford, A.N.; Gursche, A.; Kendoff, D. Periprosthetic joint infections in patients with rheumatoid arthritis are associated with higher complication and mortality rates. Arch. Orthop. Trauma Surg. 2024, 144, 5101–5109. [Google Scholar] [CrossRef]
- Momohara, S.; Kawakami, K.; Iwamoto, T.; Yano, K.; Sakuma, Y.; Hiroshima, R.; Imamura, H.; Masuda, I.; Tokita, A.; Ikari, K. Prosthetic joint infection after total hip or knee arthroplasty in rheumatoid arthritis patients treated with nonbiologic and biologic disease-modifying antirheumatic drugs. Mod. Rheumatol. 2011, 21, 469–475. [Google Scholar] [CrossRef]
- Cipriano, C.A.; Brown, N.M.; Michael, A.M.; Moric, M.; Sporer, S.M.; Della Valle, C.J. Serum and Synovial Fluid Analysis for Diagnosing Chronic Periprosthetic Infection in Patients with Inflammatory Arthritis. J. Bone Jt. Surg. 2012, 94, 594–600. [Google Scholar] [CrossRef] [PubMed]
- Carlson, V.R.; Anderson, L.A.; Lu, C.-C.; Sauer, B.C.; Blackburn, B.E.; Gililland, J.M. Perioperative Continuation of Biologic Medications Increases Odds of Periprosthetic Joint Infection in Patients With Inflammatory Arthropathy. J. Arthroplast. 2021, 36, 2546–2550. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.; Biedermann, L.; Gwinner, C.; Perka, C.; Kienzle, A. Serum and Synovial Markers in Patients with Rheumatoid Arthritis and Periprosthetic Joint Infection. J. Pers. Med. 2022, 12, 810. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Xu, H.; Wang, X.; Jia, Z.; Liao, C.; Huang, Q.; Zhou, Z.; Pei, F. More complications and higher transfusion rate in patients with rheumatoid arthritis than osteoarthritis undergoing total hip arthroplasty. Int. Orthop. 2023, 47, 1189–1196. [Google Scholar] [CrossRef]
- Lai, Y.; Tang, H.; Ding, Z.; Huang, C.; Cai, Y.; Luo, Z.; Zhou, Z. Association between disease activity of rheumatoid arthritis and risk of complications following total hip arthroplasty: A retrospective cohort study. J. Orthop. Surg. Res. 2024, 19, 455. [Google Scholar] [CrossRef]
- Zhang, J.; Gui, B.; Cheng, F.; Rong, G.; Tang, Z.; Shen, C. Influence of inflammatory arthritis on leukocyte esterase strip results in the diagnosis of periprosthetic joint infection. J. Orthop. Surg. Res. 2020, 15, 10. [Google Scholar] [CrossRef]
- Alkadhem, M.F.; Jutte, P.C.; Wouthuyzen-Bakker, M.; Kobold, A.C.M. Analytical and clinical considerations of synovial fluid calprotectin in diagnosing periprosthetic joint infections. Crit. Rev. Clin. Lab. Sci. 2025, 62, 228–239. [Google Scholar] [CrossRef]
- Hantouly, A.T.; Salameh, M.; Toubasi, A.A.; Salman, L.A.; Alzobi, O.; Ahmed, A.F.; Hameed, S.; Zikria, B.; Ahmed, G. Synovial fluid calprotectin in diagnosing periprosthetic joint infection: A meta-analysis. Int. Orthop. 2022, 46, 971–981. [Google Scholar] [CrossRef]
- Xing, J.; Li, J.; Yan, Z.; Li, Y.; Liu, X.; He, L.; Xu, T.; Wang, C.; Zhao, L.; Jie, K. Diagnostic accuracy of calprotectin in periprosthetic joint infection: A diagnostic meta-analysis. J. Orthop. Surg. Res. 2022, 17, 11. [Google Scholar] [CrossRef]
- Abildtrup, M.; Kingsley, G.H.; Scott, D.L. Calprotectin as a Biomarker for Rheumatoid Arthritis: A Systematic Review. J. Rheumatol. 2015, 42, 760–770. [Google Scholar] [CrossRef]
- Suren, C.; Lazic, I.; Haller, B.; Pohlig, F.; von Eisenhart-Rothe, R.; Prodinger, P. The synovial fluid calprotectin lateral flow test for the diagnosis of chronic prosthetic joint infection in failed primary and revision total hip and knee arthroplasty. Int. Orthop. 2023, 47, 929–944. [Google Scholar] [CrossRef]
- Mazzella, F.M.; Zhang, Y.; Bauer, T.W. Update on the role of pathology and laboratory medicine in diagnosing periprosthetic infection. Hum. Pathol. 2024, 147, 5–14. [Google Scholar] [CrossRef] [PubMed]
- Fenollar, F.; Roux, V.; Stein, A.; Drancourt, M.; Raoult, D. Analysis of 525 Samples To Determine the Usefulness of PCR Amplification and Sequencing of the 16S rRNA Gene for Diagnosis of Bone and Joint Infections. J. Clin. Microbiol. 2006, 44, 1018–1028. [Google Scholar] [CrossRef]
- Torchia, M.T.; Amakiri, I.; Werth, P.; Moschetti, W. Characterization of native knee microorganisms using next-generation sequencing in patients undergoing primary total knee arthroplasty. Knee 2020, 27, 1113–1119. [Google Scholar] [CrossRef]
- Kildow, B.J.; Ryan, S.P.; Danilkowicz, R.; Lazarides, A.L.; Penrose, C.; Bolognesi, M.P.; Jiranek, W.; Seyler, T.M. Next-generation sequencing not superior to culture in periprosthetic joint infection diagnosis. Bone Jt. J. 2021, 103-B, 26–31. [Google Scholar] [CrossRef] [PubMed]
- Flurin, L.; Hemenway, J.J.; Fisher, C.R.; Vaillant, J.J.; Azad, M.; Wolf, M.J.; Greenwood-Quaintance, K.E.; Abdel, M.P.; Patel, R.; Frank, K.M.; et al. Clinical Use of a 16S Ribosomal RNA Gene-Based Sanger and/or Next Generation Sequencing Assay to Test Preoperative Synovial Fluid for Periprosthetic Joint Infection Diagnosis. mBio 2022, 13, e0132222. [Google Scholar] [CrossRef]
- Ahmad, S.S.; Shaker, A.; Saffarini, M.; Chen, A.F.; Hirschmann, M.T.; Kohl, S. Accuracy of diagnostic tests for prosthetic joint infection: A systematic review. Knee Surg. Sports Traumatol. Arthrosc. 2016, 24, 3064–3074. [Google Scholar] [CrossRef]
- Goodman, S.M.; Mannstadt, I.B.; Tam, K.; Mehta, B.M.; Kochen, A.B.; Shakib, L.B.; Sculco, P.; Carli, A.; Batter, S.B.; Rodriguez, J.; et al. Periprosthetic Joint Infection in Patients With Inflammatory Arthritis: Optimal Tests to Differentiate From Flares. J. Clin. Rheumatol. 2024, 30, 309–314. [Google Scholar] [CrossRef] [PubMed]
- Goodman, S.M.; Springer, B.D.; Chen, A.F.; Davis, M.; Fernandez, D.R.; Figgie, M.; Finlayson, H.; George, M.D.; Giles, J.T.; Gilliland, J.; et al. 2022 American College of Rheumatology/American Association of Hip and Knee Surgeons Guideline for the Perioperative Management of Antirheumatic Medication in Patients With Rheumatic Diseases Undergoing Elective Total Hip or Total Knee Arthroplasty. Arthritis Care Res. 2022, 74, 1399–1408. [Google Scholar] [CrossRef]
- Wouthuyzen-Bakker, M.; Ploegmakers, J.J.; Ottink, K.; Kampinga, G.A.; Wagenmakers-Huizenga, L.; Jutte, P.C.; Kobold, A.C. Synovial Calprotectin: An Inexpensive Biomarker to Exclude a Chronic Prosthetic Joint Infection. J. Arthroplast. 2018, 33, 1149–1153. [Google Scholar] [CrossRef]
- Bongartz, T.; Halligan, C.S.; Osmon, D.R.; Reinalda, M.S.; Bamlet, W.R.; Crowson, C.S.; Hanssen, A.D.; Matteson, E.L. Incidence and risk factors of prosthetic joint infection after total hip or knee replacement in patients with rheumatoid arthritis. Arthritis Care Res. 2008, 59, 1713–1720. [Google Scholar] [CrossRef]
- Stone, W.Z.; Gray, C.F.; Parvataneni, H.K.; Al-Rashid, M.; Vlasak, R.G.; Horodyski, M.; Prieto, H.A. Clinical Evaluation of Synovial Alpha Defensin and Synovial C-Reactive Protein in the Diagnosis of Periprosthetic Joint Infection. J. Bone Jt. Surg. 2018, 100, 1184–1190. [Google Scholar] [CrossRef] [PubMed]
- Plate, A.; Stadler, L.; Sutter, R.; Anagnostopoulos, A.; Frustaci, D.; Zbinden, R.; Fucentese, S.; Zinkernagel, A.; Zingg, P.; Achermann, Y. Inflammatory disorders mimicking periprosthetic joint infections may result in false-positive α-defensin. Clin. Microbiol. Infect. 2018, 24, 1212.e1–1212.e6. [Google Scholar] [CrossRef] [PubMed]
- Deirmengian, C.; Kardos, K.; Kilmartin, P.; Cameron, A.; Schiller, K.; Parvizi, J. Diagnosing Periprosthetic Joint Infection: Has the Era of the Biomarker Arrived? Clin. Orthop. Relat. Res. 2014, 472, 3254–3262. [Google Scholar] [CrossRef] [PubMed]
- Salt, E.; Wiggins, A.T.; Rayens, M.K.; Morris, B.J.; Mannino, D.; Hoellein, A.; Donegan, R.P.; Crofford, L.J. Moderating effects of immunosuppressive medications and risk factors for post-operative joint infection following total joint arthroplasty in patients with rheumatoid arthritis or osteoarthritis. Semin. Arthritis Rheum. 2017, 46, 423–429. [Google Scholar] [CrossRef]
- Cordtz, R.L.; Zobbe, K.; Højgaard, P.; Kristensen, L.E.; Overgaard, S.; Odgaard, A.; Lindegaard, H.; Dreyer, L. Predictors of revision, prosthetic joint infection and mortality following total hip or total knee arthroplasty in patients with rheumatoid arthritis: A nationwide cohort study using Danish healthcare registers. Ann. Rheum. Dis. 2018, 77, 281–288. [Google Scholar] [CrossRef]
- Albrecht, K.; Poddubnyy, D.; Leipe, J.; Sewerin, P.; Iking-Konert, C.; Scholz, R.; Krüger, K. Perioperative management of patients with inflammatory rheumatic diseases: Updated recommendations of the German Society for Rheumatology. Z. Rheumatol. 2022, 82, 1–11. [Google Scholar] [CrossRef]
- Cordtz, R.; Odgaard, A.; Kristensen, L.E.; Overgaard, S.; Dreyer, L. Risk of medical complications following total hip or knee arthroplasty in patients with rheumatoid arthritis: A register-based cohort study from Denmark. Semin. Arthritis Rheum. 2020, 50, 30–35. [Google Scholar] [CrossRef]
- Lin, Z.-H.; Chang, H.-C.; Wu, Y.-L.; Gau, S.-Y. Increased Risk of New-Onset Rheumatoid Arthritis Among Osteoarthritis Patients Received Total Knee Arthroplasty: A global federated health network analysis. Int. J. Med. Sci. 2024, 21, 994–1002. [Google Scholar] [CrossRef]
- Goodman, S.M.; Springer, B.; Guyatt, G.; Abdel, M.P.; Dasa, V.; George, M.; Gewurz-Singer, O.; Giles, J.T.; Johnson, B.; Lee, S.; et al. 2017 American College of Rheumatology/American Association of Hip and Knee Surgeons Guideline for the Perioperative Management of Antirheumatic Medication in Patients With Rheumatic Diseases Undergoing Elective Total Hip or Total Knee Arthroplasty. Arthritis Care Res. 2017, 69, 1111–1124. [Google Scholar] [CrossRef]
| Study | Study Type | Sample Size of IA PJI | Sample Size of nIA PJI | Sample Size of IA nPJI | Sample Size of nIA nPJI | Type of PJI | Definition of an Acute PJI |
|---|---|---|---|---|---|---|---|
| Shohat et al. [12] | Retrospective | 55 | 512 | 61 | 592 | Chronic | Less than 3 months from surgery |
| Sculco et al. [13] | Retrospective | 36 | 771 | N/A | N/A | Not specified | N/A |
| Xu et al. [14] | Retrospective | 30 | N/A | 32 | N/A | Acute and chronic * | Less than 4–6 weeks from surgery |
| Wang et al. [15] | Retrospective | 60 | 104 | 80 | 104 | Chronic | Less than 3 months from surgery |
| Miyamae et al. [16] | Retrospective | 41 | N/A | N/A | N/A | Chronic | Less than 4–6 weeks from surgery |
| Tahta et al. [17] | Prospective | 17 | N/A | 21 | N/A | Not specified | N/A |
| Zhao et al. [18] | Retrospective | 40 | 102 | 538 | 15,022 | Chronic | Less than 3 months from surgery |
| De Araujo et al. [19] | Retrospective | 53 | N/A | N/A | N/A | Acute and chronic | Definition not provided |
| Momohara et al. [20] | Retrospective | 3 | N/A | 417 | N/A | Not specified | N/A |
| Cipriano et al. [21] | Prospective | 19 | 146 | 42 | 664 | Chronic | Less than 3 months from surgery |
| Carlson et al., [22] | Retrospective | 26 | N/A | 58 | N/A | Not specified | N/A |
| Ren et al. [23] | Retrospective | 17 | 121 | N/A | N/A | Acute and chronic | Less than 4–6 weeks from surgery |
| Jiang et al. [24] | Retrospective | 1 | 1 | 219 | 260 | Not specified | N/A |
| Lai et al. [25] | Retrospective | 4 | 1 | 333 | 336 | Not specified | N/A |
| Predictive Cutoff (IA) | Predictive Cutoff (MSIS) ⊕ | |
|---|---|---|
| CRP (mg/L) | 10.0–29.05 ∗ | 10.00 |
| D-dimer (ng/mL) | 796.50 • | 860 |
| ESR (mm/h) | 30–39 ° | 30 |
| Synovial fluid WBC (/ul) | 1948–3654 + | 3000 |
| Synovial PMN% (%) | 65.9–85.3 # | 70 |
| Alpha-defensin | Positive ⊥ | Positive |
| Authors | Predictive Cutoff | Sensitivity (%) | Specificity (%) | Positive Predictive Value (%) | Negative Predictive Value (%) | AUC | |
|---|---|---|---|---|---|---|---|
| CRP (mg/L) | [12,15,16,17,18,21,23] | 10.0–29.05 | 67.6–93.8 | 53.7–86.2 | 18.8–86.2 | 73.4–96.0 | 0.676–0.920 |
| ESR (mm/h) | [12,15,16,17,18,21] | 30–39 | 62.2–94.4 | 52.5–80.7 | 53.0–85.8 | 66.4–96.0 | 0.613–0.890 |
| Synovial fluid WBC (/ul) | [12,15,16,17,21,23] | 1948–3654 | 80.5–93.0 | 72.7–100.0 | 25.0–100.0 | 68.0–97.6 | 0.780–0.938 |
| Synovial PMN% (%) | [12,15,17,21,23] | 65.9–85.3 | 79.2–100.0 | 73.0–90.3 | 18.5–89.7 | 70.0–100.0 | 0.710–0.936 |
| Alpha- defensin | [16,17] | P | 92.0–93.0 | 98.0–100.0 | 100 | 96 | 0.960–0.970 |
| D-dimer (ng/mL) | [18] | 796.50 | 56.8 | 74.4 | 79.8 | 66.4 | 0.657 |
| Synovial fluid CRP (mg/L) | [17] | 11.7 | 76.4 | 90.4 | ND | ND | 0.920 |
| Synovial monocyte cell count (/ul) | [23] | 830 | 100 | 55.6 | 20.0 | 100.0 | 0.750 |
| Synovial PMN cell count (/ul) | [23] | 1618 | 83.3 | 74.1 | 26.3 | 97.6 | 0.800 |
| Synovial percentage of monocytes (%) | [23] | 14.7 | 16.7 | 41.8 | 3.00 | 82.1 | 0.69 |
| ELA-2 (ug/mL) | [17] | 1.9 | 81.9 | 87.6 | ND | ND | 0.950 |
| BPI (ug/mL) | [17] | 3.47 | 64.7 | 96.0 | ND | ND | 0.920 |
| Procalcitonin | [17] | 0.1 | 81.6 | 92.6 | ND | ND | 0.930 |
| Synovial Lactoferrin | [17] | 9.1 | 76.9 | 97.4 | ND | ND | 0.900 |
| Combination (CRP, ESR, sWBC, PMN%) | [15] | N/A | 80.5 | 100.0 | 100.0 | 69.2 | 0.944 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kasprzak, P.; Skała, W.; Gniadek, M.; Kobiernik, A.; Pulik, Ł.; Łęgosz, P. Diagnostic Challenges and Risk Stratification of Periprosthetic Joint Infection in Patients with Inflammatory Arthritis. J. Clin. Med. 2025, 14, 4302. https://doi.org/10.3390/jcm14124302
Kasprzak P, Skała W, Gniadek M, Kobiernik A, Pulik Ł, Łęgosz P. Diagnostic Challenges and Risk Stratification of Periprosthetic Joint Infection in Patients with Inflammatory Arthritis. Journal of Clinical Medicine. 2025; 14(12):4302. https://doi.org/10.3390/jcm14124302
Chicago/Turabian StyleKasprzak, Paweł, Wiktoria Skała, Mariusz Gniadek, Adam Kobiernik, Łukasz Pulik, and Paweł Łęgosz. 2025. "Diagnostic Challenges and Risk Stratification of Periprosthetic Joint Infection in Patients with Inflammatory Arthritis" Journal of Clinical Medicine 14, no. 12: 4302. https://doi.org/10.3390/jcm14124302
APA StyleKasprzak, P., Skała, W., Gniadek, M., Kobiernik, A., Pulik, Ł., & Łęgosz, P. (2025). Diagnostic Challenges and Risk Stratification of Periprosthetic Joint Infection in Patients with Inflammatory Arthritis. Journal of Clinical Medicine, 14(12), 4302. https://doi.org/10.3390/jcm14124302








