Questioning the Role of Psoas Measurements: Limited Predictive Value for Outcomes After Aortic Repair
Abstract
1. Introduction
Sarcopenia and Imaging-Based Assessment
2. Materials and Methods
2.1. Study Design and Population
2.2. Data Collection
2.3. Imaging Analysis
2.4. Statistical Analysis
3. Results
3.1. Patient Characteristics
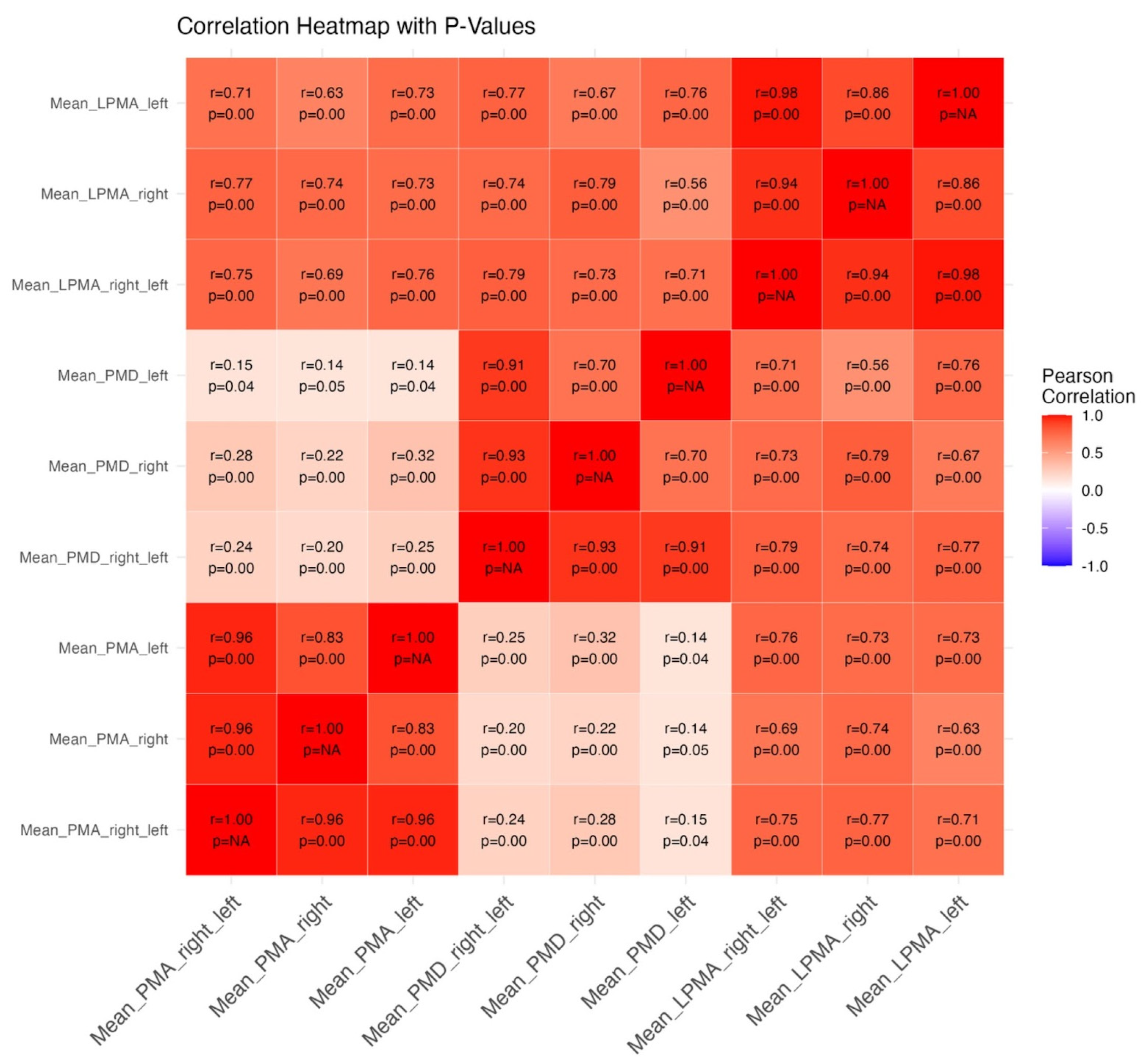
3.2. Psoas Muscle Measurements
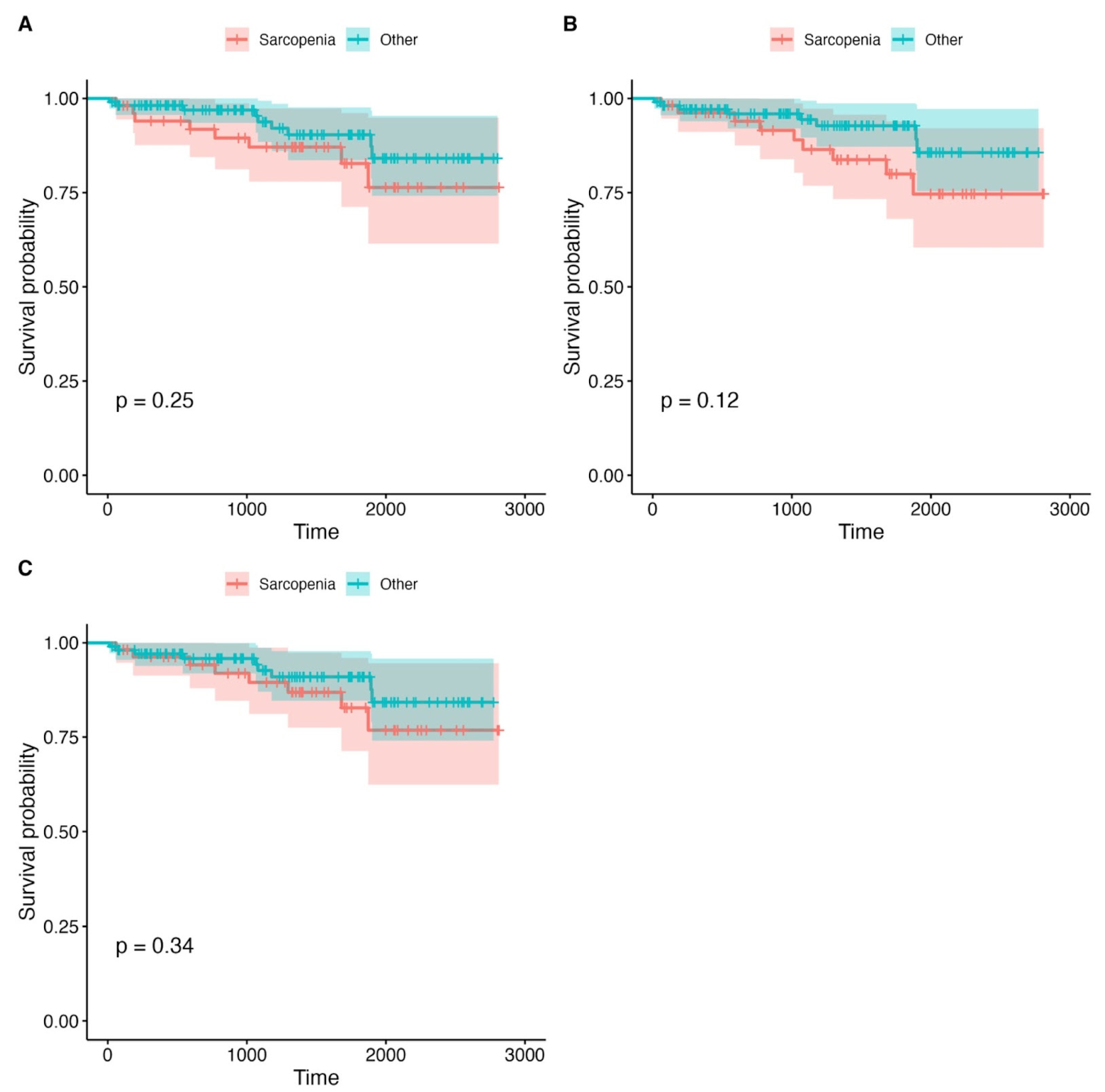
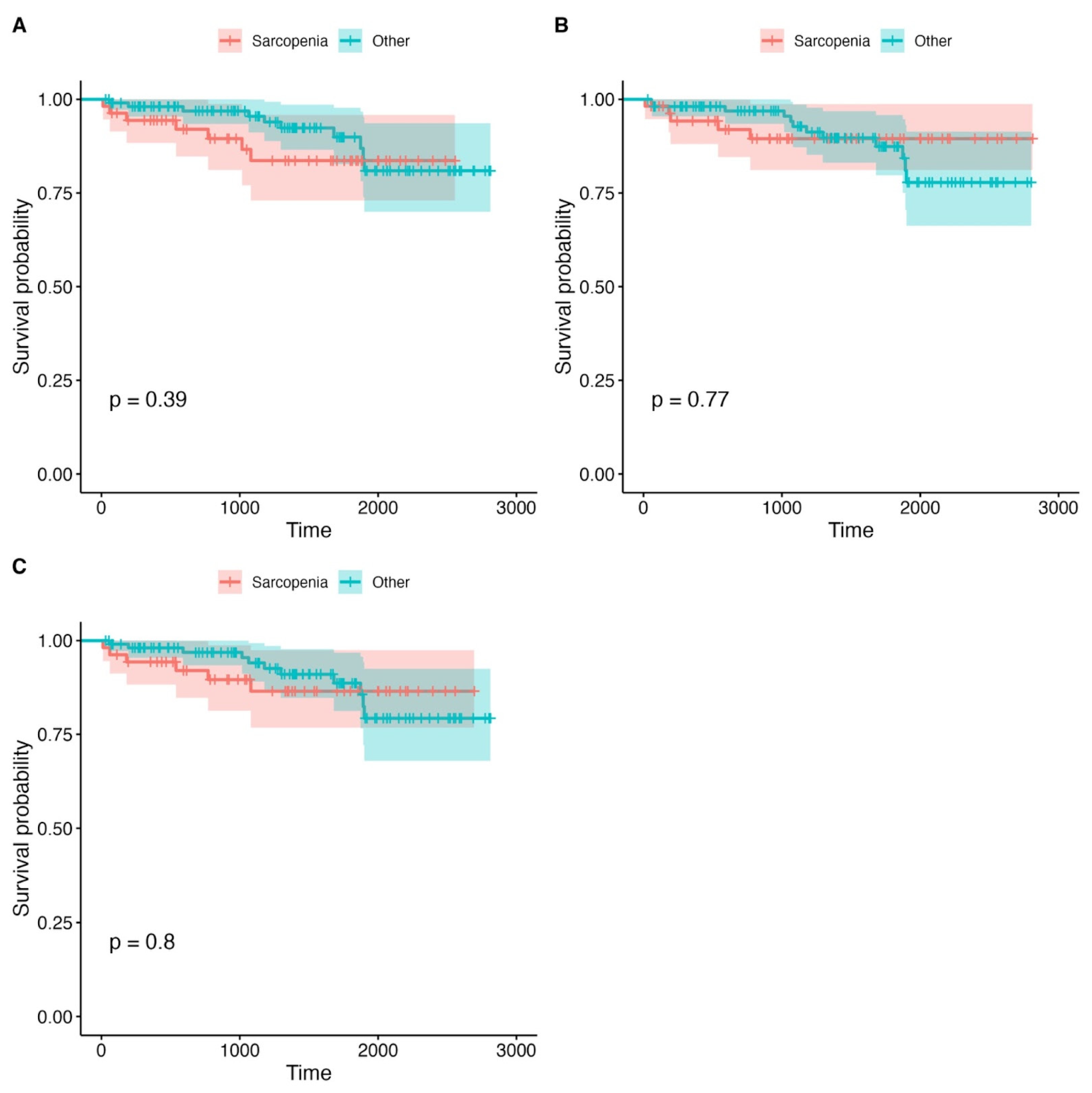
3.3. Survival Analysis
3.4. Complication Rates
3.5. Logistic Regression
4. Discussion
5. Conclusions
6. Limitations
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Abbreviation | Full Term |
| AAA | Abdominal Aortic Aneurysm |
| CTA | Computed Tomography Angiography |
| PMA | Psoas Muscle Area |
| PMD | Psoas Muscle Density |
| LPMA | Lean Psoas Muscle Area |
| EVAR | Endovascular Aneurysm Repair |
| OAR | Open Aneurysm Repair |
| FDR | False Discovery Rate |
| CT | Computed Tomography |
| MRI | Magnetic Resonance Imaging |
| DEXA | Dual-Energy X-ray Absorptiometry |
| BMI | Body Mass Index |
| Q1 | First Quartile |
| Q3 | Third Quartile |
References
- Cruz-Jentoft, A.J.; Baeyens, J.P.; Bauer, J.M.; Boirie, Y.; Cederholm, T.; Landi, F.; Martin, F.C.; Michel, J.P.; Rolland, Y.; Schneider, S.M.; et al. Sarcopenia: European Consensus on Definition and Diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age Ageing 2010, 39, 412–423. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Newton, D.H.; Kim, C.; Lee, N.; Wolfe, L.; Pfeifer, J.; Amendola, M. Sarcopenia predicts poor long-term survival in patients undergoing endovascular aortic aneurysm repair. J. Vasc. Surg. 2018, 67, 453–459. [Google Scholar] [CrossRef] [PubMed]
- Antoniou, G.A.; Rojoa, D.; Antoniou, S.A.; Alfahad, A.; Torella, F.; Juszczak, M.T. Effect of Low Skeletal Muscle Mass on Post-Operative Survival of Patients with Abdominal Aortic Aneurysm: A Prognostic Factor Review and Meta-Analysis of Time-to-Event Data. Eur. J. Vasc. Endovasc. Surg. 2019, 58, 190–198. [Google Scholar] [CrossRef] [PubMed]
- Minawala, R.; Faye, A.S. Sarcopenia as a Preoperative Risk Stratification Tool Among Older Adults with Inflammatory Bowel Disease. Adv. Geriatr. Med. Res. 2024, 6, e240003. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zannoni, S.; Albano, D.; Jannone, M.L.; Messina, C.; Sconfienza, L.M. Correlation between muscle mass and quality around the hip and of psoas muscles at L3 level using unenhanced CT scans. Skelet. Radiol. 2020, 49, 1649–1655. [Google Scholar] [CrossRef] [PubMed]
- Al Shakarchi, J.; Fairhead, J.; Rajagopalan, S.; Pherwani, A.; Jaipersad, A. Impact of frailty on outcomes in patients undergoing open abdominal aortic aneurysm repair. Ann. Vasc. Surg. 2020, 67, 100–104. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, V.C.; Oliveira, P.; Moreira, M.; Correia, M.; Lima, P.; Silva, J.C.; Pereira, R.V.; Fonseca, M. Impact of Total Psoas Area and Lean Muscular Area on Mortality After Endovascular Aortic Aneurysm Repair. Ann. Vasc. Surg. 2021, 72, 479–487. [Google Scholar] [CrossRef] [PubMed]
- Kays, J.K.; Liang, T.W.; Zimmers, T.A.; Milgrom, D.P.; Abduljabar, H.; Young, A.; Kim, B.J.; Bell, T.M.; Fajardo, A.; Murphy, M.P.; et al. Sarcopenia is a Significant Predictor of Mortality After Abdominal Aortic Aneurysm Repair. JCSM Clin. Rep. 2018, 3, e00053. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hawkins, R.B.; Mehaffey, J.H.; Charles, E.J.; Kern, J.A.; Lim, D.S.; Teman, N.R.; Ailawadi, G. Psoas Muscle Size Predicts Risk-Adjusted Outcomes After Surgical Aortic Valve Replacement. Ann. Thorac. Surg. 2018, 106, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Drudi, L.M.; Phung, K.; Ades, M.; Zuckerman, J.; Mullie, L.; Steinmetz, O.K.; Obrand, D.I.; Afilalo, J. Psoas Muscle Area Predicts All-Cause Mortality After Endovascular and Open Aortic Aneurysm Repair. Eur. J. Vasc. Endovasc. Surg. 2016, 52, 764–769. [Google Scholar] [CrossRef] [PubMed]
- Huber, T.C.; Keefe, N.; Patrie, J.; Tracci, M.C.; Sheeran, D.; Angle, J.F.; Wilkins, L.R. Predictors of All-Cause Mortality After Endovascular Aneurysm Repair: Assessing the Role of Psoas Muscle Cross-Sectional Area. J. Vasc. Interv. Radiol. 2019, 30, 1972–1979. [Google Scholar] [CrossRef] [PubMed]
- Lindström, I.; Khan, N.; Vänttinen, T.; Peltokangas, M.; Sillanpää, N.; Oksala, N. Psoas Muscle Area and Quality are Independent Predictors of Survival in Patients Treated for Abdominal Aortic Aneurysms. Ann. Vasc. Surg. 2019, 56, 183–193.e3. [Google Scholar] [CrossRef] [PubMed]
- Kleczynski, P.; Tokarek, T.; Dziewierz, A.; Sorysz, D.; Bagienski, M.; Rzeszutko, L.; Dudek, D. Usefulness of Psoas Muscle Area and Volume and Frailty Scoring to Predict Outcomes After Transcatheter Aortic Valve Implantation. Am. J. Cardiol. 2018, 122, 135–140. [Google Scholar] [CrossRef] [PubMed]
- Margadant, C.C.; Bruns, E.R.; Sloothaak, D.A.; van Duijvendijk, P.; van Raamt, A.F.; van der Zaag, H.J.; Buskens, C.J.; van Munster, B.C.; van der Zaag, E.S. Lower muscle density is associated with major postoperative complications in older patients after surgery for colorectal cancer. Eur. J. Surg. Oncol. 2016, 42, 1654–1659. [Google Scholar] [CrossRef] [PubMed]
- Vázquez Pérez, R.; Álvarez Marcos, F.; Tello Díaz, C.; Bellmunt Montoya, S.; Fernández-Samos Gutiérrez, R.; Gil Sala, D. Psoas muscle analysis as a surrogate marker of sarcopenia and frailty: A multicenter analysis of predictive capacities over short- and long-term outcomes after abdominal aortic aneurysm repair. Vascular 2024, 32, 973–982. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.Y.; Kim, K.I.; Choi, Y.; Ahn, S.H.; Kang, E.; Oh, H.K.; Kim, D.W.; Kim, E.K.; Yoon, Y.S.; Kang, S.B.; et al. Comparison of multidimensional frailty score, grip strength, and gait speed in older surgical patients. J. Cachexia Sarcopenia Muscle 2020, 11, 432–440. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]

| Type of Procedure | Number |
|---|---|
| OAR | 160 |
| EVAR | 39 |
| Basic characteristics | |
| Number of patients | n = 199 |
| Age | 72.3 ± 6.7 (years) |
| Male | 159 (71.9%) |
| Female | 40 (20.1%) |
| Comorbidity | Prevalence |
| Coronary disease | 70 (35.2%) |
| History of myocardial infarction | 53 (26.6%) |
| Previous coronary intervention | 45 (22.6%) |
| Hypertension | 179 (89.9%) |
| Diabetes | 46 (23.1%) |
| Hyperlipidemia | 25 (12.6%) |
| Chronic heart failure | 24 (12.1%) |
| COPD | 20 (10.1%) |
| PAD | 25 (12.6%) |
| CKD | 21 (10.6%) |
| Dialysis | 2 (1%) |
| History of neurologic incidents | 25(12.6%) |
| Thyroid diseases | 9 (4.5%) |
| Liver failure | 1 (0.5%) |
| Nicotinism | 46 (23.1%) |
| Complication | Number, n (%) |
|---|---|
| MI | 4 (2%) |
| Major bleeding | 2 (1%) |
| AKI | 2 (1%) |
| Reintervention | 3 (1.52%) |
| Other | 7 (4.24%) |
| Death | 5 (2.5%) |
| Complication | Number, n (%) |
|---|---|
| MI | 7 (4.24%) |
| Stroke | 2 (1.21%) |
| Hernia | 25 (15%) |
| Wound infection | 3 (1.52%) |
| Late reintervention | 10 (6.1%) |
| Death | 18 (9.05%) |
| Overall complication rate | 83 (41.7%) |
| Overall mortality rate | 23 (12%) |
| Length of hospital stay | 9.7 ± 5.7 (days) |
| Grouping Variable | Total Patients (n) | Sarcopenia Definition | Deaths in Sarcopenic Group (n) | Deaths in Non-Sarcopenic Group (n) | p-Value |
|---|---|---|---|---|---|
| PMA | 199 | <11.1 | 9 (17.3%) | 14 (12.2%) | 0.467 |
| PMD | 199 | <32 (HU) | 8 (15.1%) | 15 (13.2%) | 0.810 |
| LPMA | 199 | <326.3 | 12 (21.2%) | 11 (11.3%) | 0.503 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Halman, J.; Szydłowska, K.; Znaniecki, Ł.; Wojciechowski, J. Questioning the Role of Psoas Measurements: Limited Predictive Value for Outcomes After Aortic Repair. J. Clin. Med. 2025, 14, 4227. https://doi.org/10.3390/jcm14124227
Halman J, Szydłowska K, Znaniecki Ł, Wojciechowski J. Questioning the Role of Psoas Measurements: Limited Predictive Value for Outcomes After Aortic Repair. Journal of Clinical Medicine. 2025; 14(12):4227. https://doi.org/10.3390/jcm14124227
Chicago/Turabian StyleHalman, Joanna, Klaudia Szydłowska, Łukasz Znaniecki, and Jacek Wojciechowski. 2025. "Questioning the Role of Psoas Measurements: Limited Predictive Value for Outcomes After Aortic Repair" Journal of Clinical Medicine 14, no. 12: 4227. https://doi.org/10.3390/jcm14124227
APA StyleHalman, J., Szydłowska, K., Znaniecki, Ł., & Wojciechowski, J. (2025). Questioning the Role of Psoas Measurements: Limited Predictive Value for Outcomes After Aortic Repair. Journal of Clinical Medicine, 14(12), 4227. https://doi.org/10.3390/jcm14124227








