Possibilities of Using Tensiomyography to Assess Early Changes in Muscle Function in Patients with Multiple Sclerosis—Pilot Study
Abstract
1. Introduction
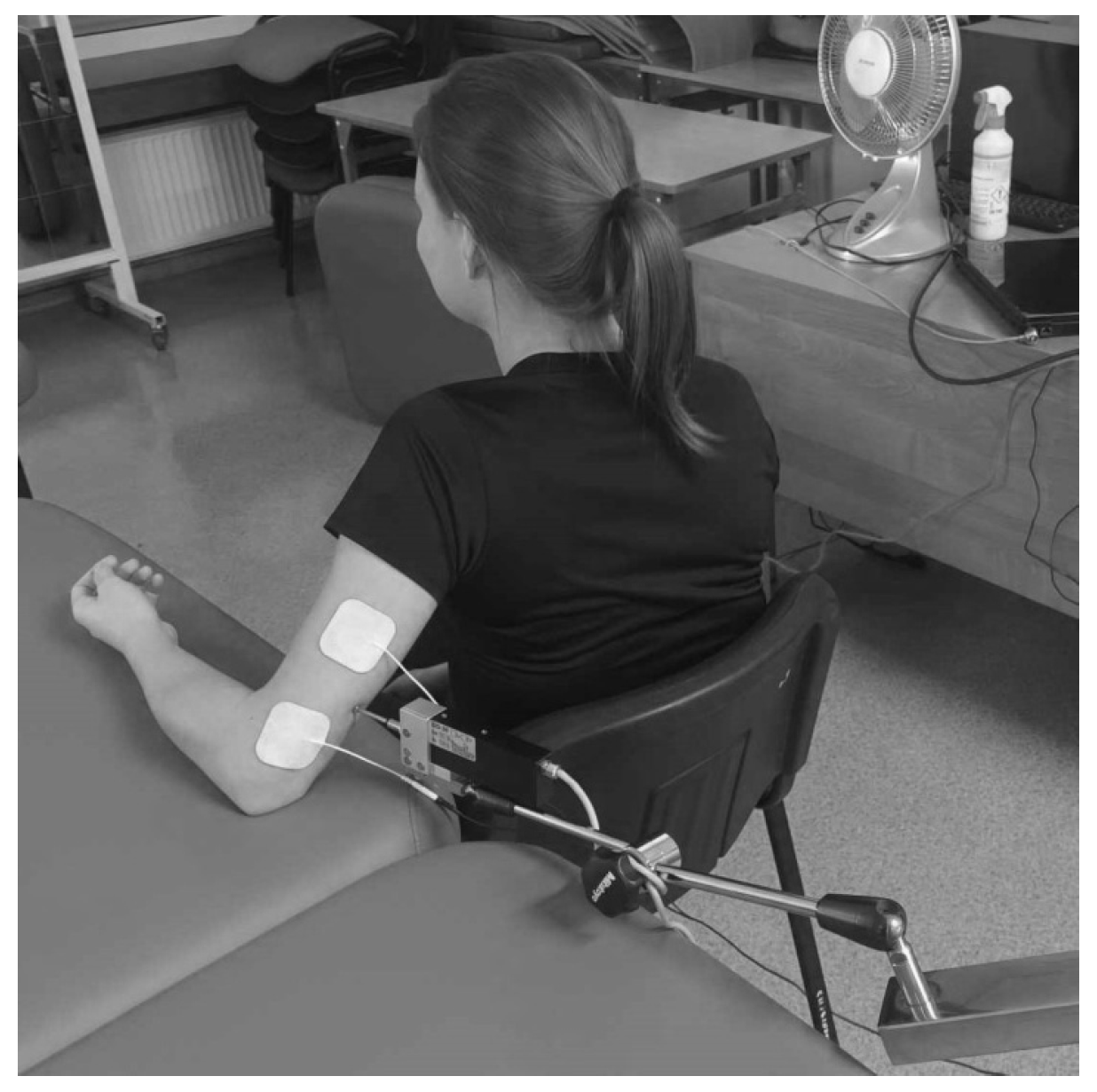
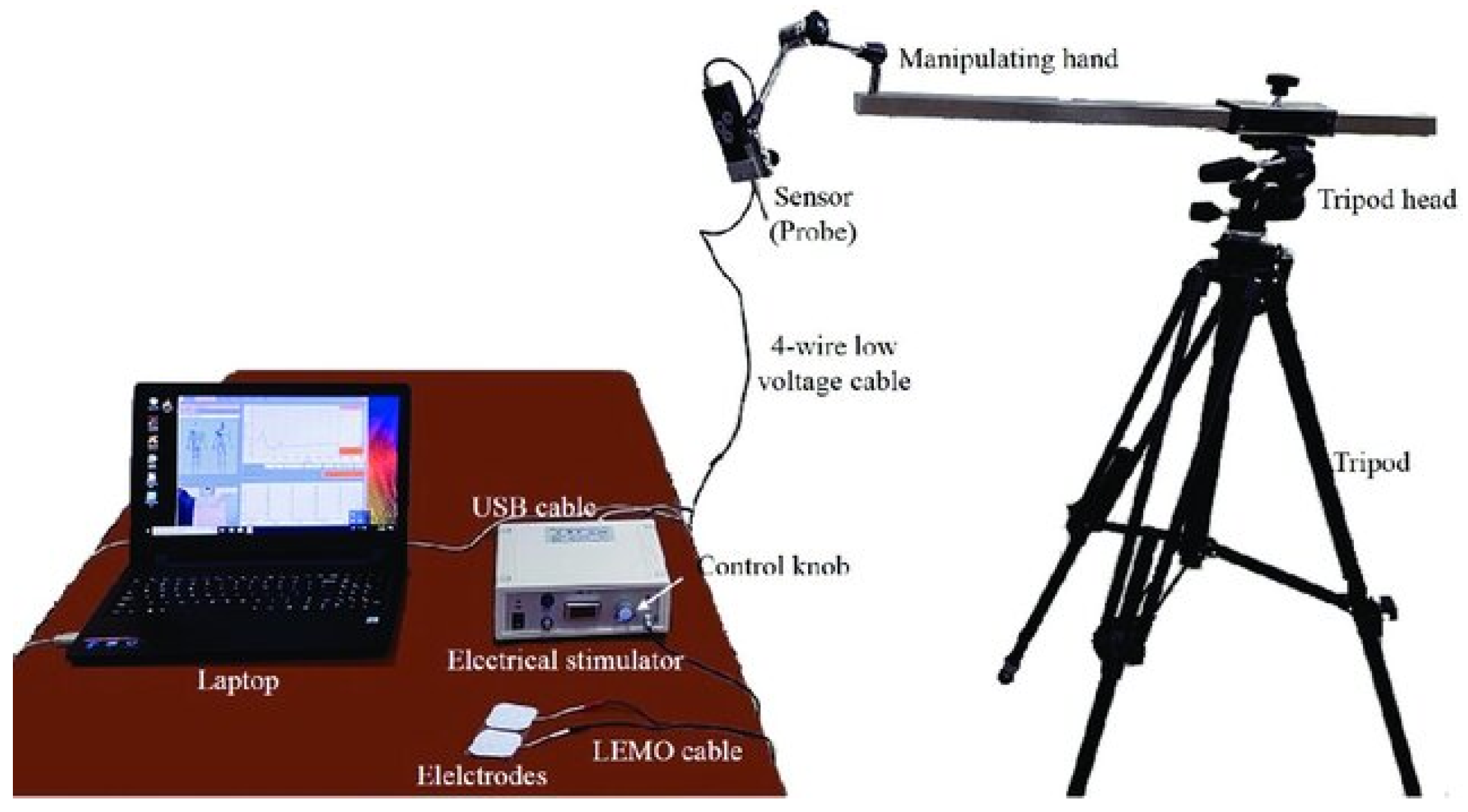
2. Materials and Methods
2.1. Study Design
2.2. Setting
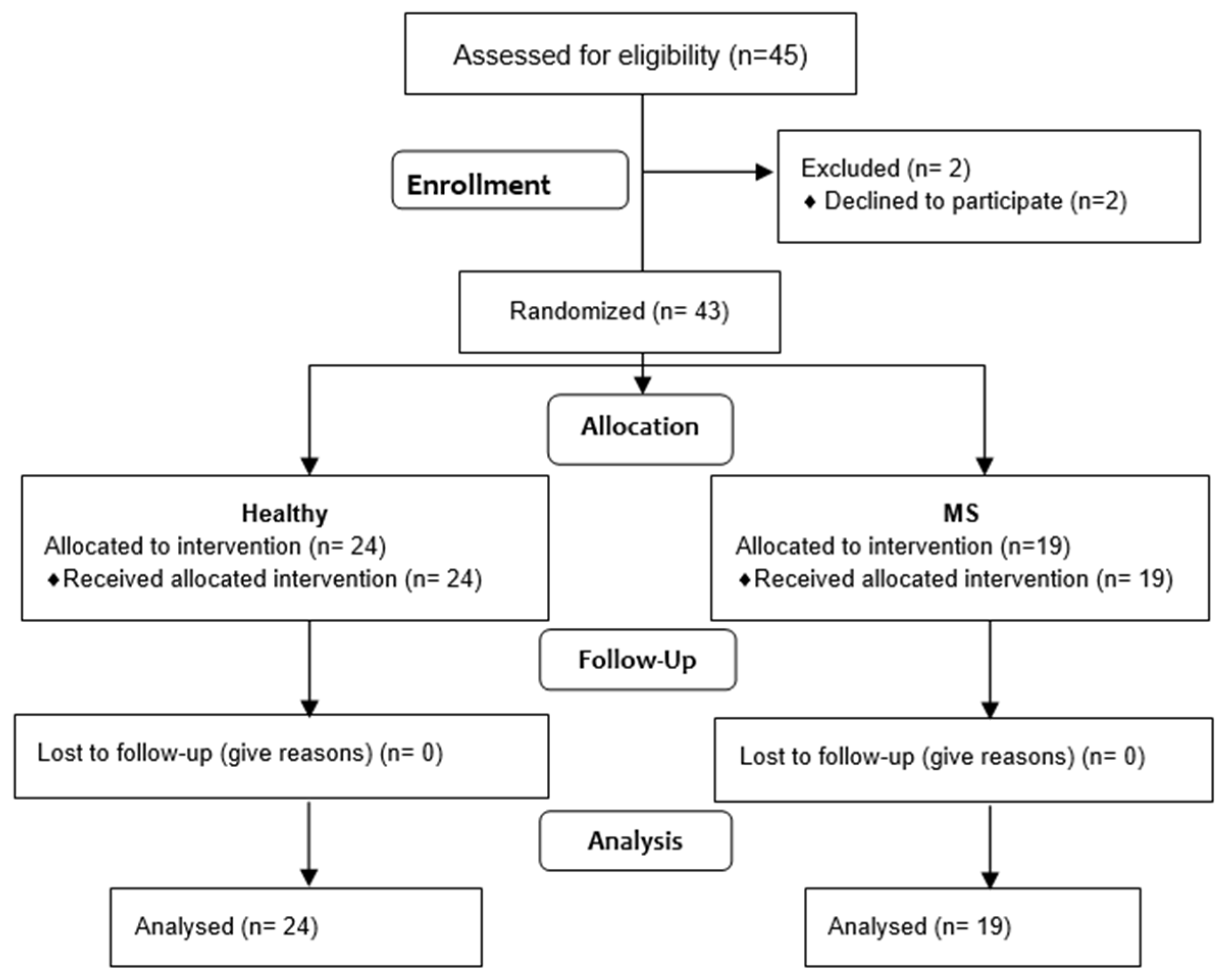
2.3. Participants
- The diagnosis of MS (MS) was based on a neurological examination, which was conducted by a neurologist, according to the McDonald criteria.
- The patients were deemed to be clinically stable MS patients, characterised by no worsening or exacerbation of the Expanded Disability Status Score (EDSS) in the six months preceding this study.
- The patients had no other diseases or injuries to the dominant upper limb.
- The patients did not participate in any form of rehabilitation in the month prior to the measurements.
- Written consent to participate in this study was obtained.
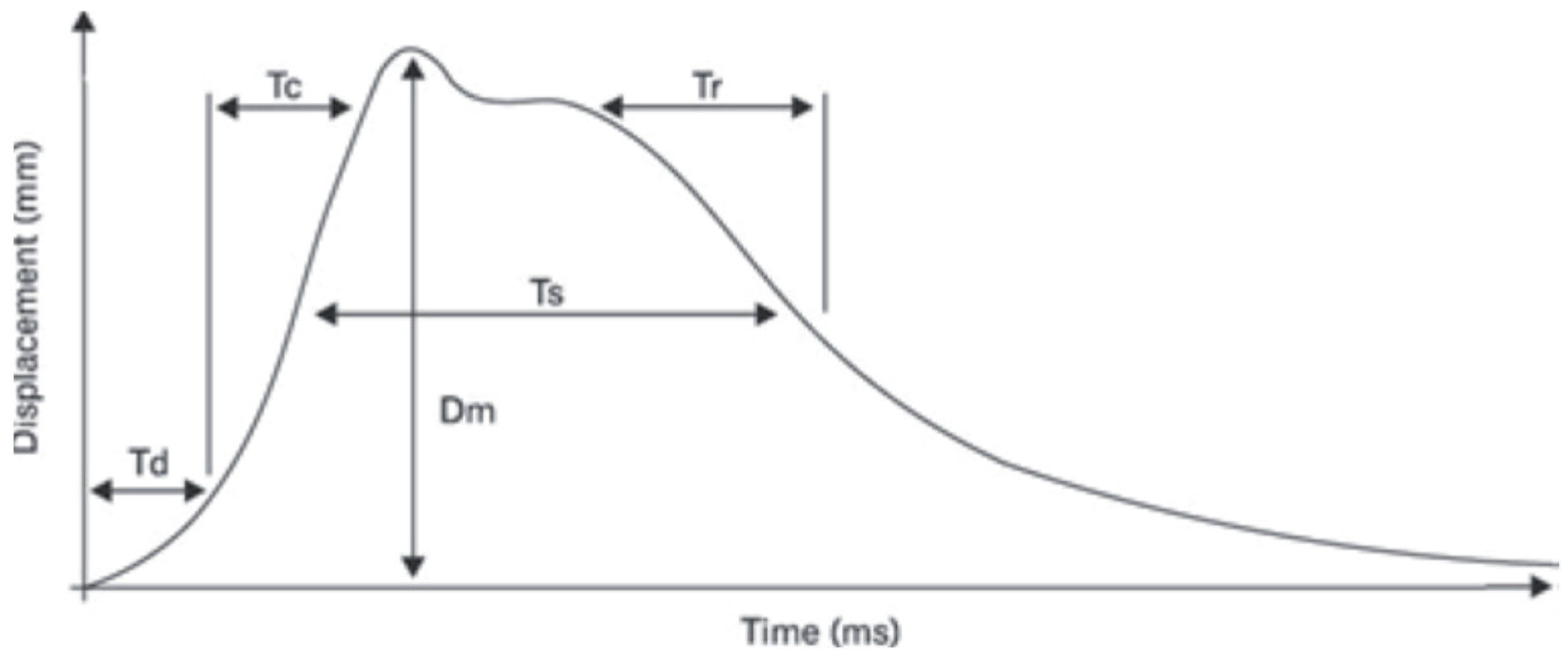
- Tc (ms)—the contraction time, defined as the time interval between the onset of 10% of muscle contraction and the subsequent attainment of 90% of the maximum level;
- Td (ms)—the delay time, which is measured from the moment of stimulation to the point at which 10% of muscle contraction is achieved;
- Dm (mm)—muscle displacement, a variable that is associated with Tc and contingent on the elasticity of the muscle tissue. The magnitude of the force exerted by the muscles during explosive movement is found to increase in proportion to the degree to which muscle tone is elevated. Conversely, the force is observed to decrease in proportion to the degree of muscle tone elevation.
2.4. Outcome Measures
2.5. Intervention
2.6. Statistical Analysis
3. Results
4. Discussion
Study Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Baecher-Allan, C.; Kaskow, B.J.; Weiner, H.L. Multiple Sclerosis: Mechanisms and Immunotherapy. Neuron 2018, 97, 742–768. [Google Scholar] [CrossRef] [PubMed]
- International Multiple Sclerosis Genetics Consortium (IMSGC). The Multiple Sclerosis Genomic Map: Role of peripheral immune cells and resident microglia in susceptibility. Science 2019, 365, eaav7188. [Google Scholar] [CrossRef] [PubMed]
- Hemmer, B.; Kerschensteiner, M.; Korn, T. Role of the innate and adaptive immune responses in the course of multiple sclerosis. Lancet Neurol. 2015, 14, 406–419. [Google Scholar] [CrossRef]
- Walton, C.; King, R.; Rechtman, L.; Kaye, W.; Leray, E.; Marrie, R.A.; Robertson, N.; La Rocca, N.; Uitdehaag, B.; van Der Mei, I.; et al. Rising prevalence of multiple sclerosis worldwide: Insights from the Atlas of MS, third edition. Mult. Scler. 2020, 26, 1816–1821. [Google Scholar] [CrossRef] [PubMed]
- Thompson, A.J.; Banwell, B.L.; Barkhof, F.; Carroll, W.M.; Coetzee, T.; Comi, G.; Correale, J.; Fazekas, F.; Filippi, M.; Freedman, M.S.; et al. Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol. 2018, 17, 162–173. [Google Scholar] [CrossRef]
- Lublin, F.D.; Reingold, S.C.; Cohen, J.A.; Cutter, G.R.; Sørensen, P.S.; Thompson, A.J.; Wolinsky, J.S.; Balcer, L.J.; Banwell, B.; Barkhof, F.; et al. Defining the clinical course of multiple sclerosis: The 2013 revisions. Neurology 2014, 83, 278–286. [Google Scholar] [CrossRef]
- Alentorn-Geli, E.; Alvarez-Diaz, P.; Ramon, S.; Marin, M.; Steinbacher, G.; Rius, M.; Seijas, R.; Ares, O.; Cugat, R. Assessment of gastrocnemius tensiomyographic neuromuscular characteristics as risk factors for anterior cruciate ligament injury in male soccer players. Knee Surg. Sports Traumatol. Arthrosc. 2015, 23, 2502–2507. [Google Scholar] [CrossRef]
- Alentorn-Geli, E.; Alvarez-Diaz, P.; Ramon, S.; Marin, M.; Steinbacher, G.; Boffa, J.J.; Cuscó, X.; Ballester, J.; Cugat, R. Assessment of neuromuscular risk factors for anterior cruciate ligament injury through tensiomyography in male soccer players. Knee Surg. Sports Traumatol. Arthrosc. 2015, 23, 2508–2513. [Google Scholar] [CrossRef]
- Achiron, A.; Barak, Y. Multiple sclerosis—From probable to definite diagnosis: A 7-year prospective study. Arch. Neurol. 2000, 57, 974–979. [Google Scholar] [CrossRef][Green Version]
- Dahmane, R.; Valenčič, V.; Knez, N.; Eržen, I. Evaluation of the ability to make non-invasive estimation of muscle contractile properties on the basis of the muscle belly response. Med. Biol. Eng. Comput. 2001, 39, 51–55. [Google Scholar] [CrossRef]
- Ingram, L.A.; Butler, A.A.; Brodie, M.A.; Hoang, P.; Gandevia, S.C.; Lord, S.R. Quantifying upper-limb motor impairment in people with multiple sclerosis: A physiological profiling approach. Ann. Phys. Rehabil. Med. 2022, 65, 101625. [Google Scholar] [CrossRef] [PubMed]
- Rotstein, D.; Solomon, J.M.; Sormani, M.P.; Montalban, X.; Ye, X.Y.; Dababneh, D.; Muccilli, A.; Shah, P. Association of no evidence of disease activity with no long-term disability progression in multiple sclerosis: A systematic review and meta-analysis. Neurology 2022, 99, e209–e220. [Google Scholar] [CrossRef] [PubMed]
- Scalfari, A.; Romualdi, C.; Nicholas, R.S.; Mattoscio, M.; Magliozzi, R.; Morra, A.; Monaco, S.; Muraro, P.A.; Calabrese, M. The cortical damage, early relapses, and onset of the progressive phase in multiple sclerosis. Neurology 2018, 90, e2107–e2118. [Google Scholar] [CrossRef] [PubMed]
- Rotstein, D.; Montalban, X. Reaching an evidence-based prognosis for personalized treatment of multiple sclerosis. Nat. Rev. Neurol. 2019, 15, 287–300. [Google Scholar] [CrossRef]
- Saguil, A. Evaluation of the patient with muscle weakness. Am. Fam. Physician 2005, 71, 1327–1336. [Google Scholar]
- Šimunič, B. Model of Longitudinal Contractions and Transverse Deformations in Skeletal Muscles. Ph.D. Thesis, University of Ljubljana Faculty of Electrical Engineering, Ljubljana, Slovenia, 2003. [Google Scholar]
- Dahmane, R.; Djordjevic, S.; Smerdu, V. Adaptive potential of human biceps femoris muscle demonstrated by histochemical, immunohistochemical and mechanomyographical methods. Med. Biol. Eng. Comput. 2006, 11, 999–1006. [Google Scholar] [CrossRef]
- Martín-Rodríguez, S.; Alentorn-Geli, E.; Tous-Fajardo, J.; Samuelsson, K.; Marín, M.; Álvarez-Díaz, P.; Cugat, R. Is tensiomyography a useful assessment tool in sports medicine? Knee Surg. Sports Traumatol. Arthrosc. 2017, 25, 3980–3981. [Google Scholar] [CrossRef]
- Paravlic, A.H. Establishing reference values for tensiomyography-derived parameters in soccer players: Insights from a systematic review, meta-analysis and meta-regression. Biol. Sport 2025, 42, 171–192. [Google Scholar] [CrossRef]
- Pus, K.; Paravlic, A.H.; Šimunič, B. The use of tensiomyography in older adults: A systematic review. Front. Physiol. 2023, 14, 1213993. [Google Scholar] [CrossRef]
- Park, S. Theory and usage of tensiomyography and the analysis method for the patient with low back pain. J. Exerc. Rehabil. 2020, 16, 325–331. [Google Scholar] [CrossRef]
- Neamtu, M.C.; Neamtu, O.M.; Rusu, M.R.; Marin, M.I.; Rusu, L. Functional muscle balance assessment in multiple sclerosis. J. Back. Musculoskelet. Rehabil. 2020, 33, 607–612. [Google Scholar] [CrossRef] [PubMed]
- Bibrowicz, K.; Szurmik, T.; Ogrodzka-Ciechanowicz, K.; Hudakova, Z.; Gąsienica-Walczak, B.; Kurzeja, P. Asymmetry of the pelvis in Polish young adults. Front. Psychol. 2023, 14, 1148239. [Google Scholar] [CrossRef] [PubMed]
- von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P. Initiative STROBE. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: Guidelines for reporting observational studies. J. Clin. Epidemiol. 2008, 61, 344–349. [Google Scholar] [CrossRef] [PubMed]
- Pakosz, P.; Konieczny, M.; Gnoiński, M. Change in the neuromuscular profile of the lower limbs after a 15-minute warm-up, assessed by TMG, in young females group. In A Man In Health And Disease Health Promotion, Care And Rehabilitation; University of Applied Sciences in Tarnow: Tarnow, Poland, 2018; pp. 376–384. [Google Scholar]
- Šimunič, B.; Koren, K.; Rittweger, J.; Lazzer, S.; Reggiani, C.; Rejc, E.; Pišot, R.; Narici, M.; Degens, H. Tensiomyography detects early hallmarks of bed-rest-induced atrophy before changes in muscle architecture. J. Appl. Physiol. 2019, 126, 815–822. [Google Scholar] [CrossRef]
- Wilson, M.T.; Ryan, A.M.F.; Vallance, S.R.; Dias-Dougan, A.; Dugdale, J.H.; Hunter, A.M.; Hamilton, L.; Macgregor, L.J. Tensiomyography Derived Parameters Reflect Skeletal Muscle Architectural Adaptations Following 6-Weeks of Lower Body Resistance Training. Front. Physiol. 2019, 10, 1493. [Google Scholar] [CrossRef]
- Martín-Rodríguez, S.; Loturco, I.; Hunter, A.M.; Rodríguez-Ruiz, D.; Munguia-Izquierdo, D. Reliability and Measurement Error of Tensiomyography to Assess Mechanical Muscle Function: A Systematic Review. J. Strength. Cond. Res. 2017, 31, 3524–3536. [Google Scholar] [CrossRef]
- Available online: https://www.tmg-bodyevolution.com/research/tmg-research-measuring-device/ (accessed on 2 March 2025).
- Lohr, C.; Schmidt, T.; Medina-Porqueres, I.; Braumann, K.M.; Reer, R.; Porthun, J. Diagnostic accuracy, validity, and reliability of Tensiomyography to assess muscle function and exercise-induced fatigue in healthy participants. A systematic review with meta-analysis. J. Electromyogr. Kinesiol. 2019, 47, 65–87. [Google Scholar] [CrossRef]
- Mamoei, S.; Hvid, L.G.; Boye Jensen, H. Neurophysiological impairments in multiple sclerosis—Central and peripheral motor pathways. Acta Neurol. Scand. 2020, 142, 401–417. [Google Scholar] [CrossRef]
- Rusu, L.D.; Cosma, G.G.; Cernaianu, S.M.; Marin, M.N.; Rusu, P.A.; Ciocănescu, D.P.; Neferu, F.N. Tensiomyography method used for neuromuscular assessment of muscle training. J. Neuroeng. Rehabil. 2013, 10, 67. [Google Scholar] [CrossRef]
- Haff, G.G.; Whitley, A.; Potteiger, J.A. A brief review: Explosive exercises and sports performance. Strength Cond. J. 2001, 23, 13. [Google Scholar] [CrossRef]
- García-Manso, J.M.; Rodríguez-Ruiz, D.; Rodríguez-Matoso, D.; De Saa, Y.; Sarmiento, S.; Quiroga, M. Assessment of muscle fatigue after an ultra-endurance triathlon using tensiomyography (TMG). J. Sports Sci. 2011, 29, 619–625. [Google Scholar] [CrossRef] [PubMed]
- Loturco, I.; Gil, S.; de Souza Laurino, C.F.; Roschel, H.; Kobal, R.; Abad, C.C.C.; Nakamura, F.Y. Differences in muscle mechanical properties between elite power and endurance athletes: A comparative study. J. Strength Cond. Res. 2015, 29, 1723–1728. [Google Scholar] [CrossRef] [PubMed]
- De Paula Simola, R.Á.; Raeder, C.; Wiewelhove, T.; Kellmann, M.; Meyer, T.; Pfeiffer, M.; Ferrauti, A. Muscle mechanical properties of strength and endurance athletes and changes after one week of intensive training. J. Electromyogr. Kinesiol. 2016, 30, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Šimunič, B.; Pišot, R.; Rittweger, J.; Degens, H. Age-related slowing of contractile properties differs between power, endurance, and nonathletes: A tensiomyographic assessment. J. Gerontol. Biol. Sci. Med. Sci. 2018, 73, 1602–1608. [Google Scholar] [CrossRef]
- Compston, A.; Lassmann, H.; Wekerle, H.; Smith, K.J.; Confavreux, C.; Miller, D.H.; Noseworthy, J.; McDonald, I.R. Chapter 9—Multiple sclerosis in the individual and in groups: A conspectus. In McAlpine’s Multiple Sclerosis; Churchill Livingstone: London, UK, 2006; pp. 439–446. [Google Scholar]
- Hoang, P.D.; Gandevia, S.C.; Herbert, R.D. Prevalence of joint contractures and muscle weakness in people with multiple sclerosis. Disabil. Rehabil. 2014, 36, 1588–1593. [Google Scholar] [CrossRef]
- Chung, L.H.; Remelius, J.G.; Van Emmerik, R.E.A.; Kent-Braun, J.A. Leg power asymmetry and postural control in women with multiple sclerosis. Med. Sci. Sports Exerc. 2008, 40, 1717–1724. [Google Scholar] [CrossRef]
- Thoumie, P.; Lamotte, D.; Cantalloube, S.; Faucher, M.; Amarenco, G. Motor determinants of gait in 100 ambulatory patients with multiple sclerosis. Mult. Scler. 2005, 11, 485–491. [Google Scholar] [CrossRef]
- Lassmann, H. Classification of demyelinating diseases at the interface between etiology and pathogenesis. Curr. Opin. Neurol. 2001, 14, 253–258. [Google Scholar] [CrossRef]
- Kent-Braun, J.A.; Ng, A.V.; Castro, M.; Weiner, M.W.; Gelinas, D.; Dudley, G.A.; Miller, R.G. Strength, skeletal muscle composition, and enzyme activity in multiple sclerosis. J. Appl. Physiol. 1997, 83, 1998–2004. [Google Scholar] [CrossRef]
- Ng, A.V.; Miller, R.G.; Gelinas, D.; Kent-Braun, J.A. Functional relationships of central and peripheral muscle alterations in multiple sclerosis. Muscle Nerve 2004, 29, 843–852. [Google Scholar] [CrossRef]
- Jorgensen, L.M.; Nielsen, J.E.; Ravnborg, M. MEP recruitment curves in multiple sclerosis and hereditary spastic paraplegia. J. Neurol. Sci. 2005, 237, 25–29. [Google Scholar] [CrossRef]
- Neamţu, M.C.; Rusu, L.; Neamţu, O.M.; Enescu Bieru, D.; Marin, M.I.; Croitoru, I.C.; Tarniţă, D.N. Analysis of neuromuscular parameters in patients with multiple sclerosis and gait disorders. Rom. J. Morphol. Embryol. 2014, 55, 1423–1428. [Google Scholar]
| Variable | SM n = 19 | Healthy n = 24 |
|---|---|---|
| Mean ± Std. | Mean ± Std. | |
| Weight (kg) | 79.4 ± 12.23 | 81.3 ± 10.11 |
| Height (cm) | 174.5 ± 8.45 | 179.4 ± 8.21 |
| Age (years) | 31 ± 1.15 | 22 ± 2.31 |
| Sex | 10 F/9 M | 14 F/10 M |
| Displacement—Dm (mm) | ||||||
|---|---|---|---|---|---|---|
| Group—Side/Muscle | Left Side | Right Side | ||||
| MS X ± SD | Healthy X ± SD | Difference Test t p-Value | MS X ± SD | Healthy Mean ± SD | Difference Test t p-Value | |
| Biceps brachii | 7.0 ± 4.05 | 13.4 ± 3.52 | t = −5.54 p < 0.0001 * | 6.1 ± 3.51 | 10.8 ± 3.18 | t = −4.45 p < 0.0001 * |
| Triceps brachii | 5.4 ± 3.91 | 7.1 ± 3.91 | t = −1.44 p = 1.55 | 5.4 ± 3.68 | 6.7 ± 3.88 | t = −1.17 p = 0.24 |
| Contraction Time—Tc (ms) | ||||||
|---|---|---|---|---|---|---|
| Group—Side/Muscle | Left Side | Right Side | ||||
| MS X ± SD | Healthy X ± SD | Difference Test t p-Value | MS X ± SD | Healthy Mean ± SD | Difference Test t p-Value | |
| Biceps brachii | 23.4 ± 4.08 | 24.4 ± 3.58 | t = −0.28 p = 0.77 | 22.3 ± 3.08 | 24.4 ± 6.58 | t = −4.45 p = 0.20 |
| Triceps brachii | 27.6 ± 11.91 | 22.9 ± 9.29 | t = −1.44 p = 1.49 | 22.1 ± 9.19 | 33.7 ± 20.87 | t = 2.45 p = 0.01 * |
| Delay Time—Td (ms) | ||||||
|---|---|---|---|---|---|---|
| Group—Side/Muscle | Left Side | Right Side | ||||
| MS X ± SD | Healthy X ± SD | Difference Test t p-Value | MS X ± SD | Healthy Mean ± SD | Difference Test t p-Value | |
| Biceps brachii | 26.7 ± 3.73 | 31.2 ± 6.36 | t = 2.73 p = 0.01 * | 26.1 ± 2.86 | 30.8 ± 7.95 | t = 2.43 p = 0.01 * |
| Triceps brachii | 23.5 ± 3.19 | 25.6 ± 8.27 | t = −1.06 p = 0.29 | 25.6 ± 6.33 | 22.8 ± 5.11 | t = 1.58 p = 0.12 |
| Bilateral Asymmetry Index (%) | |||
|---|---|---|---|
| Group—Side/Muscle | Left/Right Side | ||
| MS X ± SD | Healthy X ± SD | Difference Test t p-Value | |
| Biceps brachii | 84.4 ± 8.34 | 82.0 ± 9.53 | t = −0.85 p = 0.39 |
| Triceps brachii | 69.7 ± 15.41 | 78.7 ± 11.24 | t = −2.17 p = 0.03 * |
| Functional Asymmetry Index (%) | |||
|---|---|---|---|
| Group—Side/Muscle | Biceps/Triceps | ||
| MS X ± SD | Healthy X ± SD | Difference Test t p-Value | |
| Left side | 74.2 ± 12.22 | 73.3 ± 11.74 | t = 0.21 p = 0.83 |
| Right side | 68.7 ± 12.89 | 67.9 ± 12.89 | t = 0.15 p = 0.88 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kurzeja, P.; Ogrodzka-Ciechanowicz, K.; Szurmik, T.; Daszkiewicz, E.; Madarász, Š.; Hudakova, Z.; Rożek, K.; Bibrowicz, K. Possibilities of Using Tensiomyography to Assess Early Changes in Muscle Function in Patients with Multiple Sclerosis—Pilot Study. J. Clin. Med. 2025, 14, 4212. https://doi.org/10.3390/jcm14124212
Kurzeja P, Ogrodzka-Ciechanowicz K, Szurmik T, Daszkiewicz E, Madarász Š, Hudakova Z, Rożek K, Bibrowicz K. Possibilities of Using Tensiomyography to Assess Early Changes in Muscle Function in Patients with Multiple Sclerosis—Pilot Study. Journal of Clinical Medicine. 2025; 14(12):4212. https://doi.org/10.3390/jcm14124212
Chicago/Turabian StyleKurzeja, Piotr, Katarzyna Ogrodzka-Ciechanowicz, Tomasz Szurmik, Edyta Daszkiewicz, Štefan Madarász, Zuzana Hudakova, Karina Rożek, and Karol Bibrowicz. 2025. "Possibilities of Using Tensiomyography to Assess Early Changes in Muscle Function in Patients with Multiple Sclerosis—Pilot Study" Journal of Clinical Medicine 14, no. 12: 4212. https://doi.org/10.3390/jcm14124212
APA StyleKurzeja, P., Ogrodzka-Ciechanowicz, K., Szurmik, T., Daszkiewicz, E., Madarász, Š., Hudakova, Z., Rożek, K., & Bibrowicz, K. (2025). Possibilities of Using Tensiomyography to Assess Early Changes in Muscle Function in Patients with Multiple Sclerosis—Pilot Study. Journal of Clinical Medicine, 14(12), 4212. https://doi.org/10.3390/jcm14124212






