Preprocedural 3D Transesophageal Echocardiography for the Prediction of Device Deformation Morphology and Peri-Device Leaks After Transcatheter Left Atrial Appendage Occlusion with the AmplatzerTM Device
Abstract
1. Introduction
2. Materials and Methods
2.1. TEE Protocol
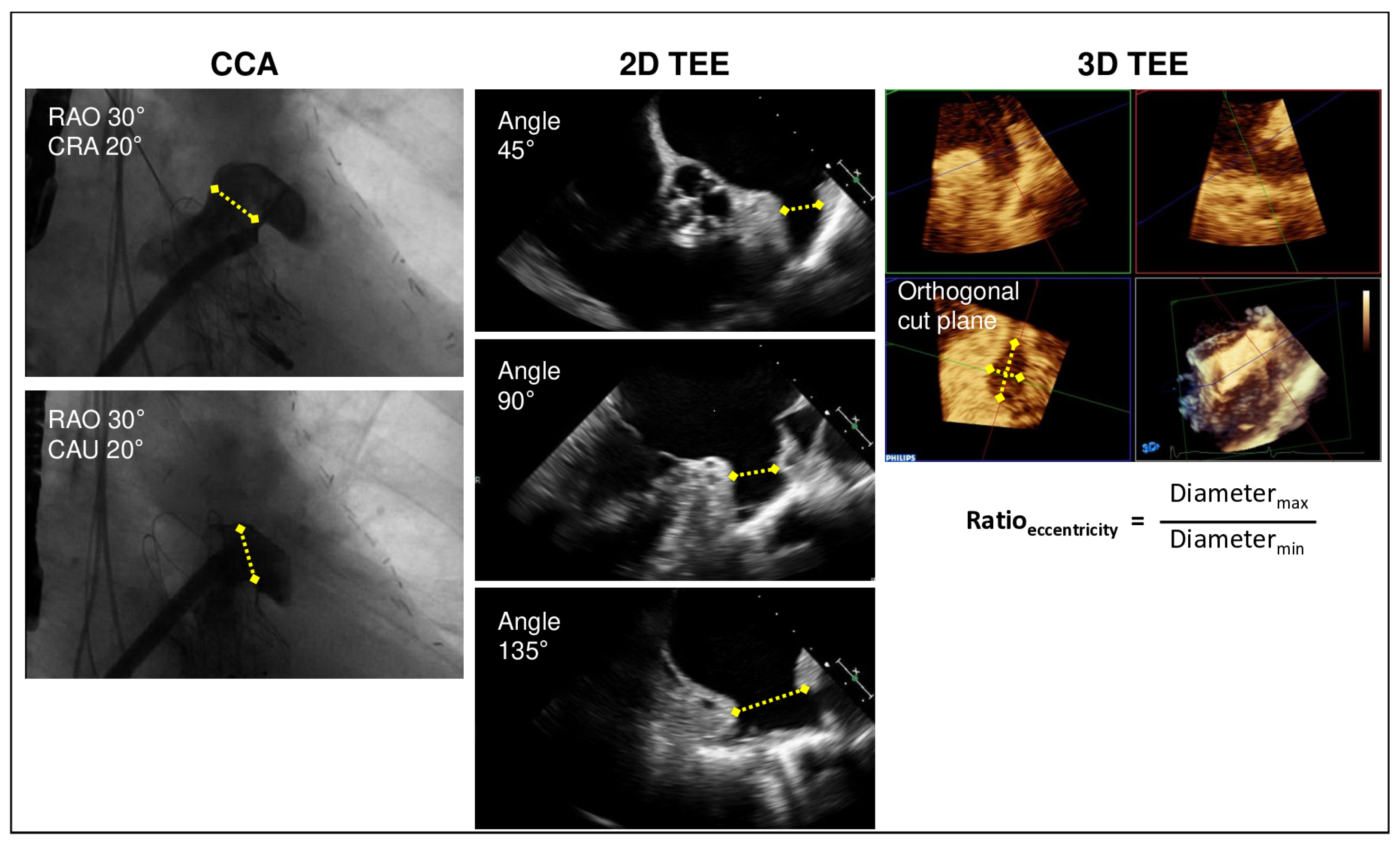
2.2. Imaging for LAA Sizing
2.2.1. Measurement by TEE
2.2.2. Measurement by Angiography
2.3. LAA Occluder Implantation
2.4. Statistical Analysis
3. Results
3.1. LAA Sizing
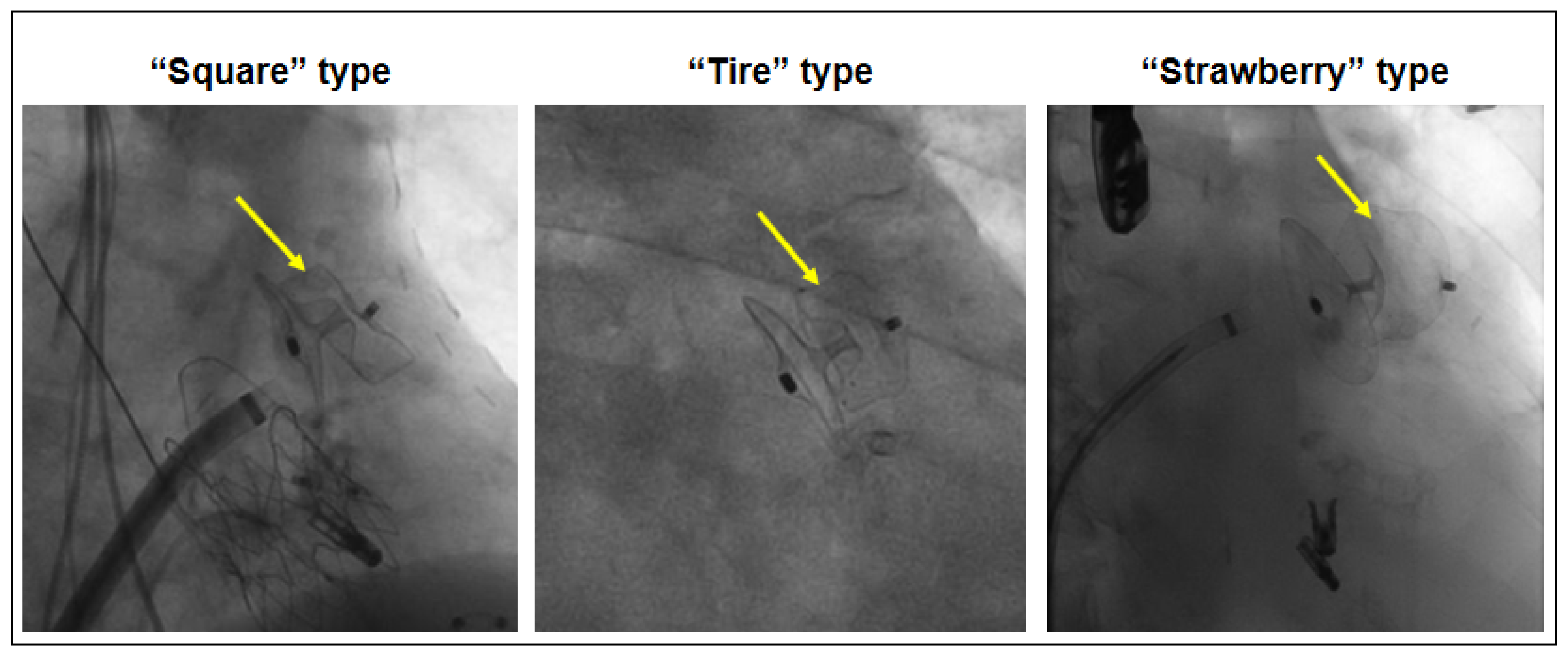
3.2. Device Morphology After Implantation and Peri-Device Leak
4. Discussion
4.1. LAA Measurements
4.2. Peri-Device Leak
4.3. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Benjamin, E.J.; Muntner, P.; Alonso, A.; Bittencourt, M.S.; Callaway, C.W.; Carson, A.P.; Chamberlain, A.M.; Chang, A.R.; Cheng, S.; Das, S.R.; et al. Heart Disease and Stroke Statistics—2019 Update: A Report From the American Heart Association. Circulation 2019, 139, e56–e528. Available online: https://pubmed.ncbi.nlm.nih.gov/30700139/ (accessed on 28 May 2025). [CrossRef]
- Van Gelder, I.C.; Rienstra, M.; Bunting, K.V.; Casado-Arroyo, R.; Caso, V.; Crijns, H.J.G.M.; De Potter, T.J.R.; Dwight, J.; Guasti, L.; Hanke, T.; et al. 2024 ESC Guidelines for the management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS). Eur. Heart J. 2024, 45, 3314–3414. Available online: https://pubmed.ncbi.nlm.nih.gov/39210723/ (accessed on 28 May 2025).
- Joglar, J.A.; Chung, M.K.; Armbruster, A.L.; Benjamin, E.J.; Chyou, J.Y.; Cronin, E.M.; Deswal, A.; Eckhardt, L.L.; Goldberger, Z.D.; Gopinathannair, R.; et al. 2023 ACC/AHA/ACCP/HRS Guideline for the Diagnosis and Management of Atrial Fibrillation: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2024, 149, E1–E156. Available online: https://pubmed.ncbi.nlm.nih.gov/38033089/ (accessed on 28 May 2025). [CrossRef] [PubMed]
- Lakkireddy, D.; Nielsen-Kudsk, J.E.; Windecker, S.; Thaler, D.; Price, M.J.; Gambhir, A.; Gupta, N.; Koulogiannis, K.; Marcoff, L.; Mediratta, A.; et al. Mechanisms, predictors, and evolution of severe peri-device leaks with two different left atrial appendage occluders. Europace 2023, 25, euad237. Available online: https://pubmed.ncbi.nlm.nih.gov/37584233 (accessed on 28 May 2025). [CrossRef] [PubMed]
- Kerut, E.K. Anatomy of the left atrial appendage. Echocardiography 2008, 25, 669–673. Available online: https://pubmed.ncbi.nlm.nih.gov/18279397/ (accessed on 28 May 2025). [CrossRef]
- Saw, J.; Holmes, D.R.; Cavalcante, J.L.; Freeman, J.V.; Goldsweig, A.M.; Kavinsky, C.J.; Moussa, I.D.; Munger, T.M.; Price, M.J.; Reisman, M.; et al. SCAI/HRS expert consensus statement on transcatheter left atrial appendage closure. Heart Rhythm 2023, 20, e1–e16. Available online: https://pubmed.ncbi.nlm.nih.gov/36990925/ (accessed on 28 May 2025). [CrossRef]
- Wang, D.D.; Eng, M.; Kupsky, D.; Myers, E.; Forbes, M.; Rahman, M.; Zaidan, M.; Parikh, S.; Wyman, J.; Pantelic, M.; et al. Application of 3-Dimensional Computed Tomographic Image Guidance to WATCHMAN Implantation and Impact on Early Operator Learning Curve: Single-Center Experience. JACC Cardiovasc. Interv. 2016, 9, 2329–2340. Available online: https://pubmed.ncbi.nlm.nih.gov/27884358/ (accessed on 28 May 2025). [CrossRef]
- Goitein, O.; Fink, N.; Hay, I.; Di Segni, E.; Guetta, V.; Goitein, D.; Brodov, Y.; Konen, E.; Glikson, M. Cardiac CT Angiography (CCTA) predicts left atrial appendage occluder device size and procedure outcome. Int. J. Cardiovasc. Imaging 2017, 33, 739–747. Available online: https://pubmed.ncbi.nlm.nih.gov/28070743/ (accessed on 28 May 2025). [CrossRef]
- Saw, J.; Fahmy, P.; Spencer, R.; Prakash, R.; Mclaughlin, P.; Nicolaou, S.; Tsang, M. Comparing Measurements of CT Angiography, TEE, and Fluoroscopy of the Left Atrial Appendage for Percutaneous Closure. J. Cardiovasc. Electrophysiol. 2016, 27, 414–422. Available online: https://pubmed.ncbi.nlm.nih.gov/26728988/ (accessed on 28 May 2025). [CrossRef]
- Rajwani, A.; Nelson, A.J.; Shirazi, M.G.; Disney, P.J.S.; Teo, K.S.L.; Wong, D.T.L.; Young, G.D.; Worthley, S.G. CT sizing for left atrial appendage closure is associated with favourable outcomes for procedural safety. Eur. Heart J. Cardiovasc. Imaging 2017, 18, 1361–1368. [Google Scholar] [CrossRef]
- Rajwani, A.; Shirazi, M.G.; Disney, P.J.; Wong, D.T.; Teo, K.S.; Delacroix, S.; Chokka, R.G.; Young, G.D.; Worthley, S.G. Left Atrial Appendage Eccentricity and Irregularity Are Associated with Residual Leaks After Percutaneous Closure. JACC Clin. Electrophysiol. 2015, 1, 478–485. Available online: https://pubmed.ncbi.nlm.nih.gov/29759401/ (accessed on 28 May 2025). [CrossRef] [PubMed]
- Nirmalan, J.G.; Kramer, A.; Korsholm, K.; Jensen, J.M.; Nielsen-Kudsk, J.E. Computed tomography–based device-sizing in Amplatzer Amulet left atrial appendage occlusion. J. Interv. Card. Electrophysiol. 2024, 67, 785–795. Available online: https://pubmed.ncbi.nlm.nih.gov/37882993/ (accessed on 28 May 2025). [CrossRef]
- Vainrib, A.F.; Harb, S.C.; Jaber, W.; Benenstein, R.J.; Aizer, A.; Chinitz, L.A.; Saric, M. Left Atrial Appendage Occlusion/Exclusion: Procedural Image Guidance with Transesophageal Echocardiography. J. Am. Soc. Echocardiogr. 2018, 31, 454–474. Available online: https://pubmed.ncbi.nlm.nih.gov/29158017/ (accessed on 28 May 2025). [CrossRef] [PubMed]
- Glikson, M.; Wolff, R.; Hindricks, G.; Mandrola, J.; Camm, A.J.; Lip, G.Y.H.; Fauchier, L.; Betts, T.R.; Lewalter, T.; Saw, J.; et al. EHRA/EAPCI expert consensus statement on catheter-based left atrial appendage occlusion—An update. Europace 2020, 22, 184. Available online: https://pubmed.ncbi.nlm.nih.gov/31504441/ (accessed on 28 May 2025). [CrossRef]
- Park, J.W.; Bethencourt, A.; Sievert, H.; Santoro, G.; Meier, B.; Walsh, K.; Lopez-Minquez, J.R.; Meerkin, D.; Valdés, M.; Ormerod, O.; et al. Left atrial appendage closure with Amplatzer cardiac plug in atrial fibrillation: Initial European experience. Catheter. Cardiovasc. Interv. 2011, 77, 700–706. Available online: https://pubmed.ncbi.nlm.nih.gov/20824765/ (accessed on 28 May 2025). [CrossRef]
- Clemente, A.; Avogliero, F.; Berti, S.; Paradossi, U.; Jamagidze, G.; Rezzaghi, M.; Della Latta, D.; Chiappino, D. Multimodality imaging in preoperative assessment of left atrial appendage transcatheter occlusion with the Amplatzer Cardiac Plug. Eur. Heart J. Cardiovasc. Imaging 2015, 16, 1276–1287. Available online: https://pubmed.ncbi.nlm.nih.gov/25916628/ (accessed on 28 May 2025). [CrossRef] [PubMed]
- Nucifora, G.; Faletra, F.F.; Regoli, F.; Pasotti, E.; Pedrazzini, G.; Moccetti, T.; Auricchio, A. Evaluation of the left atrial appendage with real-time 3-dimensional transesophageal echocardiography: Implications for catheter-based left atrial appendage closure. Circ. Cardiovasc. Imaging 2011, 4, 514–523. Available online: https://pubmed.ncbi.nlm.nih.gov/21737601/ (accessed on 28 May 2025). [CrossRef]
- Al-Kassou, B.; Tzikas, A.; Stock, F.; Neikes, F.; Völz, A.; Omran, H. A comparison of two-dimensional and real-time 3D transoesophageal echocardiography and angiography for assessing the left atrial appendage anatomy for sizing a left atrial appendage occlusion system: Impact of volume loading. EuroIntervention 2017, 12, 2083–2091. Available online: https://pubmed.ncbi.nlm.nih.gov/27973328/ (accessed on 28 May 2025). [CrossRef]
- Streb, W.; Mitręga, K.; Podolecki, T.; Szymała, M.; Leopold-Jadczyk, A.; Kukulski, T.; Kalarus, Z. Two-dimensional versus three-dimensional transesophageal echocardiography in percutaneous left atrial appendage occlusion. Cardiol. J. 2019, 26, 687–695. Available online: https://pubmed.ncbi.nlm.nih.gov/29512094/ (accessed on 28 May 2025). [CrossRef]
- Alkhouli, M.; Chaker, Z.; Clemetson, E.; Alqahtani, F.; Al Hajji, M.; Lobban, J.; Sengupta, P.P.; Raybuck, B. Incidence, Characteristics and Management of Persistent Peri-Device Flow after Percutaneous Left Atrial Appendage Occlusion. Struct. Heart 2019, 3, 491–498. [Google Scholar] [CrossRef]
- Price, M.J.; Ellis, C.R.; Nielsen-Kudsk, J.E.; Thaler, D.; Gupta, N.; Koulogiannis, K.; Anderson, J.A.; Gage, R.; Lakkireddy, D. Peridevice Leak After Transcatheter Left Atrial Appendage Occlusion: An Analysis of the Amulet IDE Trial. JACC Cardiovasc. Interv. 2022, 15, 2127–2138. Available online: https://pubmed.ncbi.nlm.nih.gov/36357016/ (accessed on 28 May 2025). [CrossRef] [PubMed]
- Alkhouli, M.; Du, C.; Killu, A.; Simard, T.; Noseworthy, P.A.; Friedman, P.A.; Curtis, J.P.; Freeman, J.V.; Holmes, D.R. Clinical Impact of Residual Leaks Following Left Atrial Appendage Occlusion: Insights From the NCDR LAAO Registry. JACC Clin. Electrophysiol. 2022, 8, 766–778. Available online: https://pubmed.ncbi.nlm.nih.gov/35387751/ (accessed on 28 May 2025). [CrossRef] [PubMed]
- Korsholm, K.; Jensen, J.M.; Nørgaard, B.L.; Samaras, A.; Saw, J.; Berti, S.; Tzikas, A.; Nielsen-Kudsk, J.E. Peridevice Leak Following Amplatzer Left Atrial Appendage Occlusion: Cardiac Computed Tomography Classification and Clinical Outcomes. JACC Cardiovasc. Interv. 2021, 14, 83–93. Available online: https://pubmed.ncbi.nlm.nih.gov/33413869/ (accessed on 28 May 2025). [CrossRef] [PubMed]
- Altiok, E.; Becker, M.M.; Moersen, W.; Mischke, K.; Schroeder, J.; Marx, N.; Reith, S. Additional value of preprocedural measurements by 3D transesophageal echocardiography for percutaneous left atrial appendage occlusion. In Proceedings of the 84th Annual Meeting of the German Society of Cardiology, Mannheim, Germany, 4–7 April 2018. [Google Scholar]
| Variables | n = 47 |
|---|---|
| Age, years | 76 ± 8 |
| Male gender, n (%) | 29 (62) |
| Type of atrial fibrillation | |
| Paroxysmal, n (%) | 21 (45) |
| Persistent, n (%) | 8 (17) |
| Permanent, n (%) | 18 (38) |
| CHA2DS2-VASc score | 4.3 ± 1.3 |
| HAS-BLED score | 3.6 ± 1.0 |
| NYHA class | |
| I, n (%) | 21 (45) |
| II, n (%) | 12 (26) |
| III, n (%) | 12 (26) |
| IV, n (%) | 2 (4) |
| Arterial hypertension, n (%) | 32 (68) |
| Chronic kidney disease, n (%) | 19 (40) |
| Congestive heart failure, n (%) | 21 (45) |
| Prior stroke, n (%) | 9 (19) |
| Prior bleeding, n (%) | 47 (100) |
| Indication for LAA occlusion | |
| Intracranial bleeding, n (%) | 11 (23) |
| Gastrointestinal bleeding, n (%) | 20 (43) |
| Urogenital bleeding, n (%) | 6 (13) |
| Other causes for bleeding, n (%) | 10 (21) |
| Medication on admission | |
| DOAC/Phenprocoumon/Enoxaparin, n (%) | 17 (36) |
| Variables | n = 47 |
|---|---|
| LAA occluder device | |
| AmplatzerTM ACP device, n (%) | 16 (34) |
| AmplatzerTM AmuletTM device, n (%) | 31 (66) |
| Adverse events | |
| Periprocedural pericardial effusion, n (%) | 3 (6) |
| Postprocedural device-related thrombus, n (%) | 3 (6) |
| Peri-device leak at 6-month follow-up, mm | 2.6 ± 0.7 |
| PDL > 5 mm, n (%) | 0 (0) |
| PDL 3–5 mm, n (%) | 4 (9) |
| PDL < 3 mm, n (%) | 7 (15) |
| Medication on discharge | |
| Phenprocoumon/Enoxaparin, n (%) | 2 (4) |
| Aspirin and Clopidogrel, n (%) | 45 (96) |
| CCA | 2D TEE | 3D TEE | |
|---|---|---|---|
| Diameter RAO 30° cranial 20° (mm) | 21.0 ± 3.6 | - | - |
| Diameter RAO 30° caudal 20° (mm) | 21.4 ± 4.2 | - | - |
| Diameter 45° (mm) | - | 17.0 ± 3.4 | - |
| Diameter 90° (mm) | - | 17.4 ± 3.7 | - |
| Diameter 135° (mm) | - | 18.8 ± 3.8 | - |
| Area by 3D assessment (mm2) | - | - | 293.0 ± 113.9 |
| Area-derived mean diameter by 3D assessment (mm) | - | - | 19.0 ± 3.6 |
| Maximal diameter (mm) | 22.2 ± 3.9 | 19.7 ± 3.7 * | 22.2 ± 4.1 # |
| Minimal diameter (mm) | - | - | 16.5 ± 3.5 |
| Eccentricity index (ratio of maximal and minimal diameter by 3D assessment) | - | - | 1.37 ± 0.26 |
| “Square” Type (n = 9) | “Tire” Type (n = 28) | “Strawberry” Type (n = 10) | |
|---|---|---|---|
| Peri-device leak after procedure | 5 (56%) | 3 (11%) * | 3 (30%) |
| Ratio max.-min. diameter by 3D TEE | 1.35 ± 0.28 | 1.45 ± 0.17 # | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kirschfink, A.; Puetz, A.; Frick, M.; Al-Khusein, R.; van Rijckeghem, P.; Alnaimi, A.; Kneizeh, K.; Vogt, F.; Marx, N.; Altiok, E.; et al. Preprocedural 3D Transesophageal Echocardiography for the Prediction of Device Deformation Morphology and Peri-Device Leaks After Transcatheter Left Atrial Appendage Occlusion with the AmplatzerTM Device. J. Clin. Med. 2025, 14, 4211. https://doi.org/10.3390/jcm14124211
Kirschfink A, Puetz A, Frick M, Al-Khusein R, van Rijckeghem P, Alnaimi A, Kneizeh K, Vogt F, Marx N, Altiok E, et al. Preprocedural 3D Transesophageal Echocardiography for the Prediction of Device Deformation Morphology and Peri-Device Leaks After Transcatheter Left Atrial Appendage Occlusion with the AmplatzerTM Device. Journal of Clinical Medicine. 2025; 14(12):4211. https://doi.org/10.3390/jcm14124211
Chicago/Turabian StyleKirschfink, Annemarie, Andreas Puetz, Michael Frick, Rami Al-Khusein, Pieterjan van Rijckeghem, Anas Alnaimi, Kinan Kneizeh, Felix Vogt, Nikolaus Marx, Ertunc Altiok, and et al. 2025. "Preprocedural 3D Transesophageal Echocardiography for the Prediction of Device Deformation Morphology and Peri-Device Leaks After Transcatheter Left Atrial Appendage Occlusion with the AmplatzerTM Device" Journal of Clinical Medicine 14, no. 12: 4211. https://doi.org/10.3390/jcm14124211
APA StyleKirschfink, A., Puetz, A., Frick, M., Al-Khusein, R., van Rijckeghem, P., Alnaimi, A., Kneizeh, K., Vogt, F., Marx, N., Altiok, E., & Schroeder, J. (2025). Preprocedural 3D Transesophageal Echocardiography for the Prediction of Device Deformation Morphology and Peri-Device Leaks After Transcatheter Left Atrial Appendage Occlusion with the AmplatzerTM Device. Journal of Clinical Medicine, 14(12), 4211. https://doi.org/10.3390/jcm14124211






