Transvenous Lead Extraction in Patients with Congenital Heart Disease
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Population
2.2. Primary Hypothesis and Study Design
2.3. Data Collection
2.4. Consent and Ethics
2.5. Definitions
2.6. Extraction Procedures
2.7. Statistical Analysis
3. Results
3.1. Study Population
3.2. Device Type and Lead Characteristics
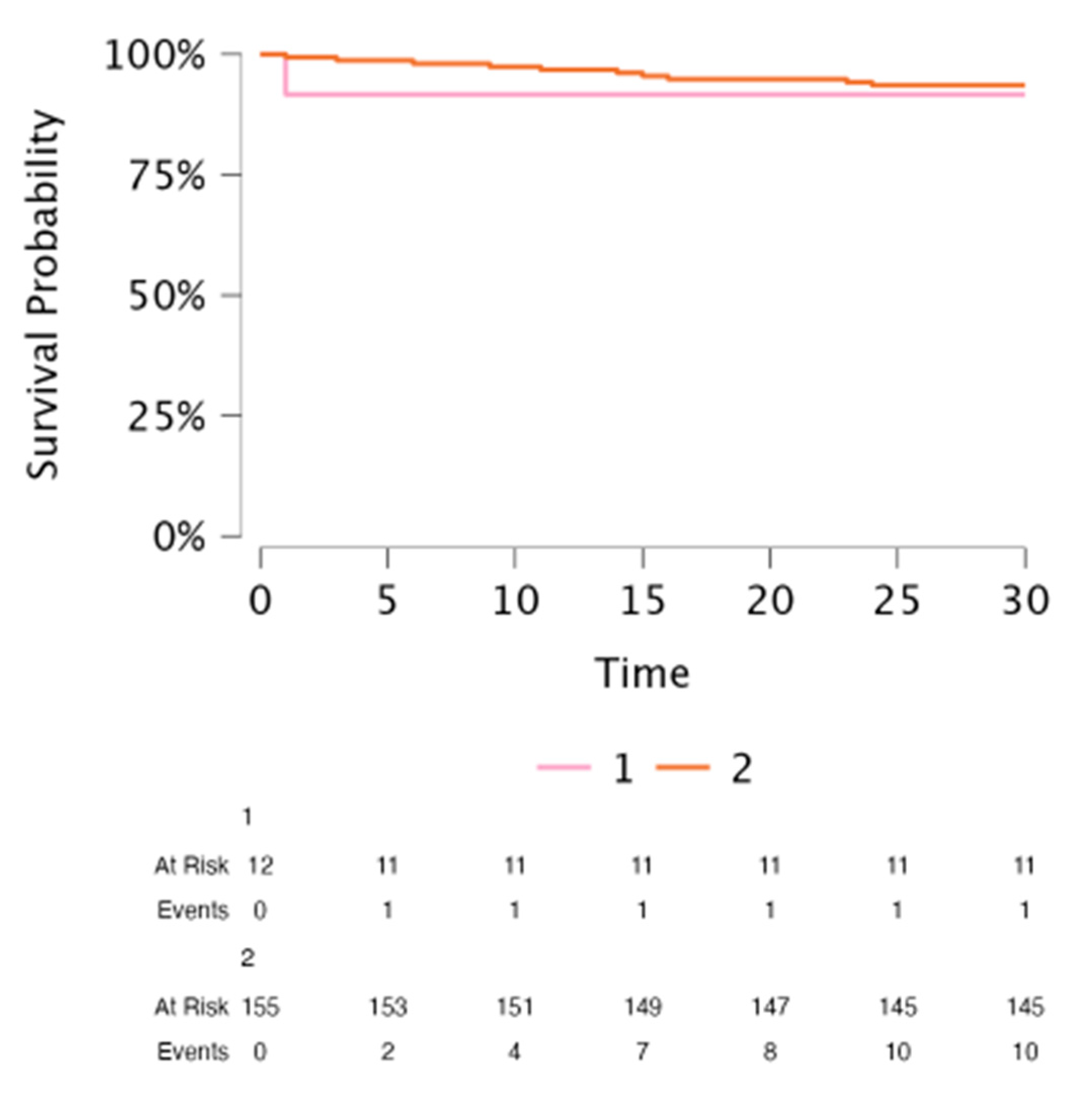
3.3. TLE Outcomes in CHD Patients: Success Rate, Complication Rate, Survival and Rate of Advanced Technique Use
3.4. Comparison of TLE Outcomes in CHD Patients with Non-CHD Patients
4. Discussion
4.1. TLEs in Surgically Repaired CHD Patients
4.2. Use of Mechanically Powered Sheaths in CHD Patients: Efficacy
4.3. Use of Mechanically Powered Sheaths in CHD Patients: Safety
4.4. Abandoned Leads
4.5. Study Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CHD | Congenital heart disease |
| ICD | Implantable cardioverter defibrillator |
| PM | Pacemaker |
| TLE | Transvenous lead extraction |
References
- Van Der Linde, D.; Konings, E.E.; Slager, M.A.; Witsenburg, M.; Helbing, W.A.; Takkenberg, J.J.; Roos-Hesselink, J.W. Birth prevalence of congenital heart disease worldwide: A systematic review and meta-analysis. J. Am. Coll. Cardiol. 2011, 58, 2241–2247. [Google Scholar] [CrossRef] [PubMed]
- Khairy, P.; Ionescu-Ittu, R.; Mackie, A.S.; Abrahamowicz, M.; Pilote, L.; Marelli, A.J. Changing mortality in congenital heart disease. J. Am. Coll. Cardiol. 2010, 56, 1149–1157.f. [Google Scholar] [CrossRef] [PubMed]
- Khairy, P.; Van Hare, G.F.; Balaji, S.; Berul, C.I.; Cecchin, F.; Cohen, M.I.; Daniels, C.J.; Deal, B.J.; Dearani, J.A.; de Groot, N.; et al. PACES/HRS expert consensus statement on the recognition and management of arrhyth mias in adult congenital heart disease: Developed in partnership between the Pediatric and Congenital Electrophysiology devel oped in partnership between the Pediatric and Congenital Electrophysiology Society (PACES) and the Heart Rhythm Society (HRS). Endorsed by the governing bodies of PACES, HRS, the American College of Cardiology (ACC), the American Heart Association (AHA), the European Heart Rhythm Association (EHRA), the Canadian Heart Rhythm Society (CHRS), and the International Society for Adult Congenital Heart Disease (ISACHD). Can. J. Cardiol. 2014, 30, e1–e63. [Google Scholar]
- Koyak, Z.; Harris, L.; de Groot, J.R.; Silversides, C.K.; Oechslin, E.N.; Bouma, B.J.; Budts, W.; Zwinderman, A.H.; Van Gelder, I.C.; Mulder, B.J.M.; et al. Sudden cardiac death in adult congenital heart disease. Circulation 2012, 126, 1944–1954. [Google Scholar] [CrossRef] [PubMed]
- Czosek, R.J.; Meganathan, K.; Anderson, J.B.; Knilans, T.K.; Marino, B.S.; Heaton, P.C. Cardiac rhythm devices in the pediatric population: Utilization and complications. Heart Rhythm 2012, 9, 199–208. [Google Scholar] [CrossRef]
- Wilkoff, B.L.; Love, C.J.; Byrd, C.L.; Bongiorni, M.G.; Carrillo, R.G.; Crossley, G.H.; Epstein, L.M.; Friedman, R.A.; Kennergren, C.E.; Mitkowski, P.; et al. Transvenous lead extraction: Heart Rhythm Society expert consensus on facilities, training, indications, and patient management. Heart Rhythm 2009, 6, 1085–1104. [Google Scholar]
- Khairy, P.; Landzberg, M.J.; Gatzoulis, M.A.; Mercier, L.A.; Fernandes, S.M.; Côté, J.M.; Lavoie, J.P.; Fournier, A.; Guerra, P.G.; Frogoudaki, A.; et al. Epicardial Versus Endocardial pacing and Thromboembolic events Investigators. Transvenous pacing leads and systemic thromboemboli in patients with intracardiac shunts: A multicenter study. Circulation 2006, 113, 2391–2397. [Google Scholar] [CrossRef]
- Bongiorni, M.G.; Kennergren, C.; Butter, C.; Deharo, J.C.; Kutarski, A.; Rinaldi, C.; Romano, S.L.; Maggioni, A.P.; Andarala, M.; Auricchio, A.; et al. The European Lead Extraction ConTRolled (ELECTRa) study: A European Heart Rhythm Association (EHRA) Registry of Transvenous Lead Extraction Outcomes. Eur. Heart J. 2017, 3, 2995–3005. [Google Scholar] [CrossRef]
- Starck, C.T.; Gonzalez, E.; Al-Razzo, O.; Mazzone, P.; Delnoy, P.-P.; Breitenstein, A.; Steffel, J.; Eulert-Grehn, J.; Lanmüller, P.; Melillo, F.; et al. Results of the Patient-Related Outcomes of Mechanical lead Extraction Techniques (PROMET) study: A multicentre retrospective study on advanced mechanical lead extraction techniques. Europace 2020, 22, 1103–1110. [Google Scholar] [CrossRef]
- Sharma, S.; Lee, B.K.; Garg, A.; Peyton, R.; Schuler, B.T.; Mason, P.; Delnoy, P.P.; Gallagher, M.M.; Hariharan, R.; Schaerf, R.; et al. Performance and outcomes of transvenous rotational lead extraction: Results from a prospective, monitored, international clinical study. Heart Rhythm O2 2021, 2, 113–121. [Google Scholar] [CrossRef]
- Zsigmond, E.-J.; Saghy, L.; Benak, A.; Miklos, M.; Makai, A.; Hegedus, Z.; Alacs, E.; Agocs, S.; Vamos, M. A head-to-head comparison of laser vs. powered mechanical sheaths as first choice and second line extraction tools. Europace 2023, 25, 591–599. [Google Scholar] [CrossRef]
- Yap, S.-C.; Bhagwandien, R.E.; Theuns, D.A.M.J.; Yasar, Y.E.; de Heide, J.; Hoogendijk, M.G.; Kik, C.; Szili-Torok, T. Efficacy and safety of transvenous lead extraction using a liberal combined superior and femoral approach. J. Interv. Card. Electrophysiol. 2021, 62, 239–248. [Google Scholar] [CrossRef] [PubMed]
- Pham, T.D.N.; Cecchin, F.; O’Leary, E.; Fynn-Thompson, F.; Triedman, J.K.; Gauvreau, K.; Mah, D.Y. Lead Extraction at a Pediatric/Congenital Heart Disease Center: The Importance of Patient Age at Implant. JACC Clin. Electrophysiol. 2022, 8, 343–353. [Google Scholar] [CrossRef] [PubMed]
- Bongiorni, M.G.; Burri, H.; Deharo, J.C.; Starck, C.; Kennergren, C.; Saghy, L.; Rao, A.; Tascini, C.; Lever, N.; Kutarski, A.; et al. 2018 EHRA expert consensus statement on lead extraction: Recommendations on definitions, endpoints, research trial design, and data collection requirements for clinical scientific studies and registries: Endorsed by APHRS/HRS/LAHRS. Europace 2018, 20, 1217. [Google Scholar] [CrossRef]
- Blomström-Lundqvist, C.; Traykov, V.; Erba, P.A.; Burri, H.; Nielsen, J.C.; Bongiorni, M.G.; Poole, J.; Boriani, G.; Costa, R.; Deharo, J.-C.; et al. European Heart Rhythm Association (EHRA) international consensus document on how to prevent, diagnose, and treat cardiac implantable electronic device infections-endorsed by the Heart Rhythm Society (HRS), the Asia Pacific Heart Rhythm Society (APHRS), the Latin American Heart Rhythm Society (LAHRS), International Society for Cardiovascular Infectious Diseases (ISCVID), and the European Society of Clinical Microbiology and Infectious Diseases (ESCMID) in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS). Eur. Heart J. 2020, 41, 2012–2032. [Google Scholar]
- Baumgartner, H.; De Backer, J.; Babu-Narayan, S.V.; Budts, W.; Chessa, M.; Diller, G.-P.; Lung, B.; Kluin, J.; Lang, I.M.; Meijboom, F.; et al. 2020 ESC Guidelines for the management of adult congenital heart disease. Eur. Heart J. 2021, 42, 563–645. [Google Scholar] [CrossRef]
- Albertini, L.; Kawada, S.; Nair, K.; Harris, L. Incidence and Clinical Predictors of Early and Late Complications of Implantable Cardioverter-Defibrillators in Adults With Congenital Heart Disease. Can. J. Cardiol. 2023, 39, 236–245. [Google Scholar] [CrossRef] [PubMed]
- Fender, E.A.; Killu, A.M.; Cannon, B.C.; Friedman, P.A.; Mcleod, C.J.; Hodge, D.O.; Broberg, C.S.; Henrikson, C.A.; Cha, Y.-M. Lead extraction outcomes in patients with congenital heart disease. Europace 2017, 19, 441–446. [Google Scholar] [CrossRef]
- Khairy, P.; Roux, J.; Dubuc, M.; Thibault, B.; Guerra, P.G.; Macle, L.; Mercier, L.; Dore, A.; Roy, D.; Talajic, M.; et al. Laser lead extraction in adult congenital heart disease. J. Cardiovasc. Electrophysiol. 2007, 18, 507–511. [Google Scholar] [CrossRef]
- McCanta, A.C.; Kong, M.H.; Carboni, M.P.; Greenfield, R.A.; Hranitzky, P.M.; Kanter, R.J. Laser lead extraction in congenital heart disease: A case-controlled study. Pacing Clin. Electrophysiol. 2013, 36, 372–380. [Google Scholar] [CrossRef]
- Gourraud, J.B.; Chaix, M.A.; Shohoudi, A.; Pagé, P.; Dubuc, M.; Thibault, B.; Poirier, N.C.; Dore, A.; Marcotte, F.; Mongeon, F.-P.; et al. Transvenous Lead Extraction in Adults With Congenital Heart Disease: Insights From a 20-Year Single-Center Experience. Circ. Arrhythm. Electrophysiol. 2018, 11, e005409. [Google Scholar] [CrossRef]
- Cecchin, F.; Atallah, J.; Walsh, E.P.; Triedman, J.K.; Alexander, M.E.; Berul, C.I. Lead extraction in pediatric and congenital heart disease patients. Circ. Arrhythm. Electrophysiol. 2010, 3, 437–444. [Google Scholar] [CrossRef] [PubMed]
- Gianni, C.; Elchouemi, M.; Helmy, R.; Spinetta, L.; La Fazia, V.M.; Pierucci, N.; Asfour, I.; Della Rocca, D.G.; Mohanty, S.; Bassiouny, M.A.; et al. Safety and feasibility of same-day discharge following uncomplicated transvenous lead extraction. J. Cardiovasc. Electrophysiol. 2024, 35, 278–287. [Google Scholar] [CrossRef] [PubMed]
- Esposito, M.; Kennergren, C.; Holmström, N.; Nilsson, S.; Eckerdal, J.; Thomsen, P. Morphologic and immunohistochemical observations of tissues surrounding retrieved transvenous pacemaker leads. J. Biomed. Mater. Res. 2002, 63, 548–558. [Google Scholar] [CrossRef] [PubMed]
- Moak, J.P.; Freedenberg, V.; Ramwell, C.; Skeete, A. Effectiveness of excimer laser-assisted pacing and ICD lead extraction in children and young adults. Pacing Clin. Electrophysiol. 2000, 29, 461–466. [Google Scholar] [CrossRef]
- Hussein, A.A.; Tarakji, K.G.; Martin, D.O.; Gadre, A.; Fraser, T.; Kim, A.; Brunner, M.P.; Barakat, A.F.; Saliba, W.I.; Kanj, M.; et al. Cardiac Implantable Electronic Device Infections: Added Complexity and Suboptimal Outcomes With Previously Abandoned Leads. JACC Clin. Electrophysiol. 2017, 3, 1–9. [Google Scholar] [CrossRef]
- Kolodzinska, K.; Kutarski, A.; Grabowski, M.; Jarzyna, I.; Małecka, B.; Opolski, G. Abrasions of the outer silicone insulation of endocardial leads in their intracardiac part: A new mechanism of lead-dependent endocarditis. Europace 2012, 14, 903–910. [Google Scholar] [CrossRef]
- Issa, Z. An Approach to Transvenous Lead Extraction in Patients with Malfunctioning or Superfluous Leads EP LabDigest 2022. Available online: https://www.hmpgloballearningnetwork.com/site/eplab/approach-transvenous-lead-extraction-patients-malfunctioning-or-superfluous-leads (accessed on 21 May 2025).
- Segreti, L.; Rinaldi, C.A.; Claridge, S.; Svendsen, J.H.; Blomstrom-Lundqvist, C.; Auricchio, A.; Butter, C.; Dagres, N.; Deharo, J.-C.; Maggioni, A.P.; et al. Procedural outcomes associated with transvenous lead extraction in patients with abandoned leads: An ESC-EHRA ELECTRa (European Lead Extraction ConTRolled) Registry Sub-Analysis. Europace 2019, 21, 645–654. [Google Scholar] [CrossRef]
- Kusumoto, F.M.; Schoenfeld, M.H.; Wilkoff, B.L.; Berul, C.I.; Birgersdotter-Green, U.M.; Carrillo, R.; Cha, Y.-M.; Clancy, J.; Deharo, J.-C.; Ellenbogen, K.A.; et al. 2017 HRS expert consensus statement on cardiovascular implantable electronic device lead management and extraction. Heart Rhythm 2017, 14, e503–e551. [Google Scholar] [CrossRef]
- Strachinaru, M.; Kievit, C.M.; Yap, S.C.; Hirsch, A.; Geleijnse, M.L.; Szili-Torok, T. Multiplane/3D transesophageal echocardiography monitoring to improve the safety and outcome of complex transvenous lead extractions. Echocardiography 2019, 36, 980–986. [Google Scholar] [CrossRef]
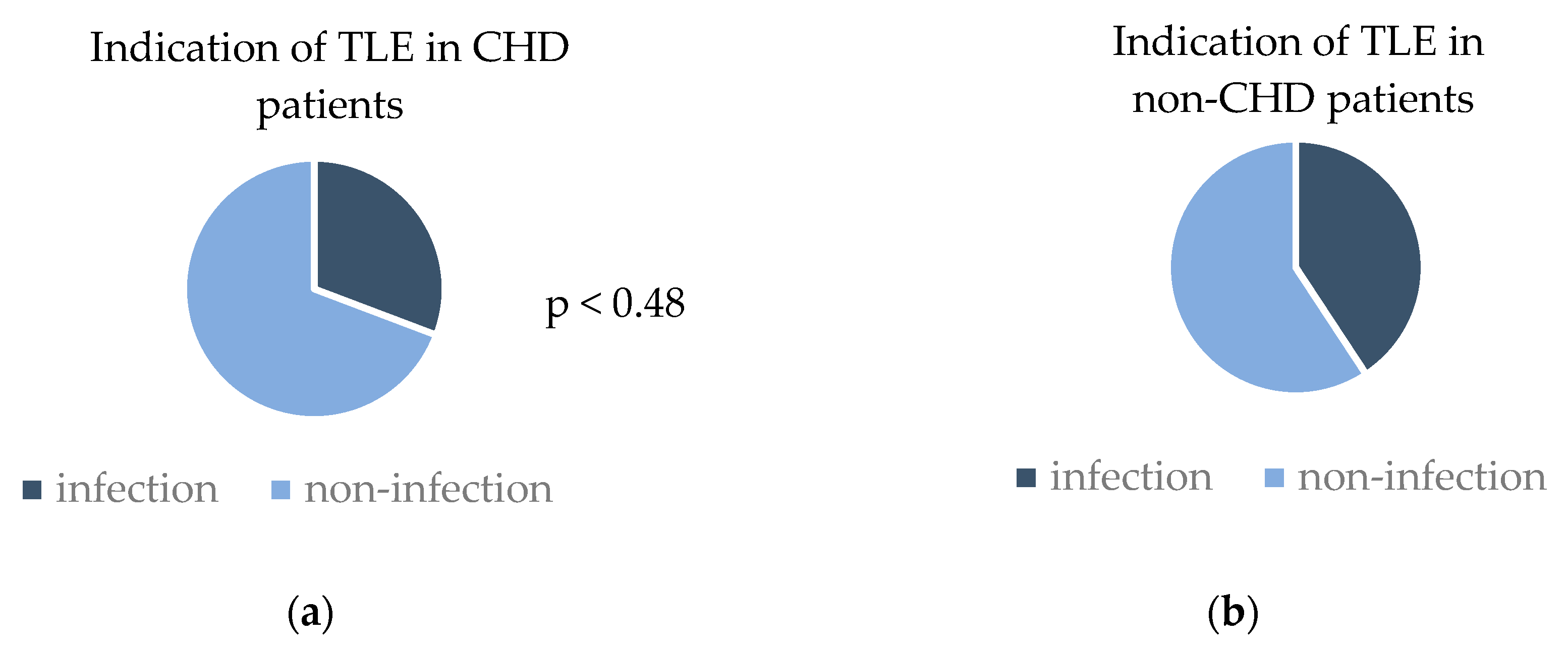
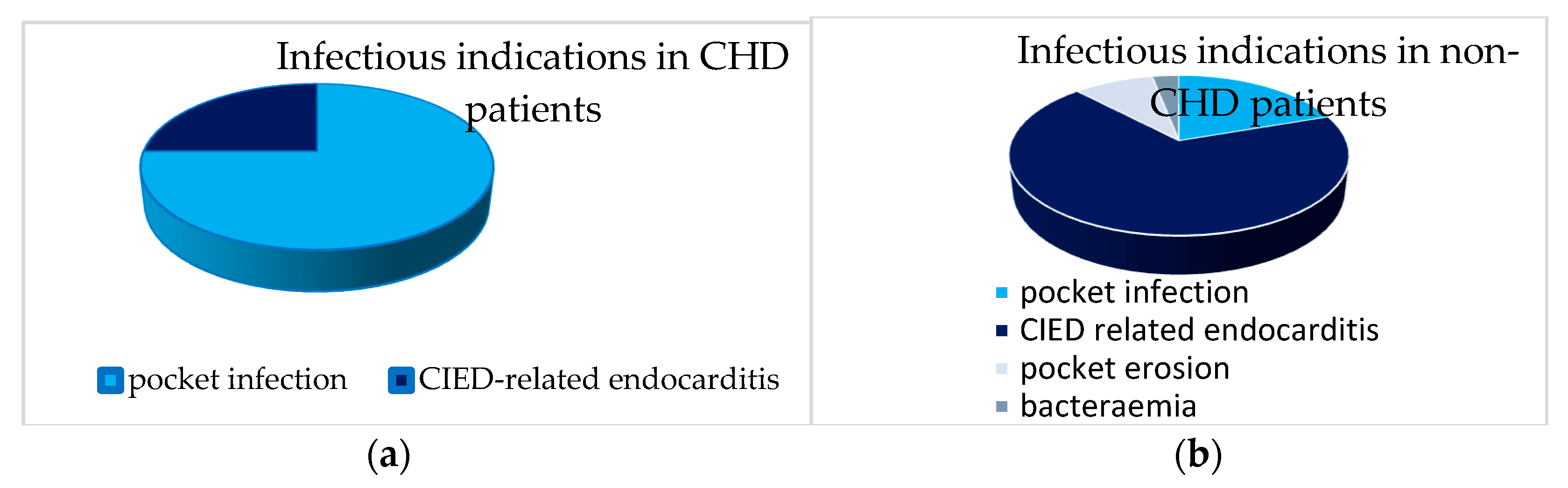
| CHD (n = 13) | Non-CHD (n = 162) | p-Value | |
|---|---|---|---|
| Patient age at time of lead implantation (years) | 21.2 ± 17 | 57.1 ± 18 | <0.01 |
| Patient age at time of extraction (years) | 33.4 ± 13 | 63.3 ± 16 | <0.01 |
| Female | 9 (69%) | 49 (30%) | <0.01 |
| HFrEF and HFmrEF | 3 (23%) | 97 (60%) | 0.06 |
| Beta-blocker therapy | 2 (66%) | 80 (84%) | 0.418 |
| ACEI/ARB/ARNI therapy | 2 (66%) | 71 (74%) | 0.752 |
| MRA therapy | 1 (33%) | 59 (62%) | 0.314 |
| Prior cardiac surgery | 13 (100%) | 21 (13%) | <0.01 |
| Previous valve prothesis implantation | 4 (30.7%) | 12 (7.4%) | <0.01 |
| Diabetes mellitus | 1 (7.7%) | 45 (27.75) | 0.113 |
| n | |||
| Underlying CHD heart disease | d-Transposition of the great arteries St.p. op. Senning | 3 (23%) | |
| Double outlet right ventricle St.p. op. Fontan | 2 (15%) | ||
| Tetralogy of Fallot | 3 (23%) | ||
| Coarctation of the aorta | 4 (30%) | ||
| Ventricular septal defect | 1 (7%) |
| Extraction No | Age | Sex | EF % | CHD Complexity Classification | Basic CHD | Additional Cardiac Abnormality | Extracardiac Abnormality | Cardiac Repair Surgery | Number of Extracted Leads | Implant Duration of Oldest Extracted Lead (years) | RV Diam. (mm) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 24 | male | 55 | mild | ventricular septal defect | none | none | patch repair | 3 | 23 | 24 |
| 2 | 14 | female | 73 | moderate | coarctation of the aorta | incomplete AV septal defect and cleft mitral valve | none | extended end-to-end anastomosis | 1 | 6 | 31 |
| 3 | 41 | female | 51 | moderate | tetralogy of Fallot | none | none | complete reconstruction | 2 | 6 | 40 |
| 4 | 35 | male | 31 | severe | d-transposition of the great arteries | none | none | Senning operation | 1 | 6 | 54 |
| 5 | 39 | female | 55 | severe | double outlet right ventricle | ASD, pulmonary stenosis | none | Fontan operation | 1 | 23 | 31 |
| 6 | 27 | male | 50 | severe | d-transposition of the great arteries | ASD | none | Senning operation | 3 | 25 | 42 |
| 7 | 16 | female | 69 | moderate | coarctation of the aorta | incomplete AV septal defect and cleft mitral valve | none | end-to-end anastomosis | 2 | 8 | 31 |
| 8 | 57 | female | 63 | moderate | tetralogy of Fallot | none | none | complete reconstruction | 2 | 16 | 46 |
| 9 | 24 | female | 74 | moderate | coarctation of the aorta | VSD, ASD | none | end-to-end anastomosis, ASD and VSD patch repair | 1 | 14 | 29 |
| 10 | 48 | female | 27 | moderate | coarctation of the aorta | persistent vena cava sup.sin. | Turner syndrome | Bentall operation | 1 | 1.4 | 31 |
| 11 | 37 | male | 30 | severe | d-transposition of the great arteries | none | none | Senning operation | 1 | 6 | 30 |
| 12 | 24 | female | 65 | severe | double outlet right ventricle | VSD, pulmonary stenosis | none | Fontan operation | 3 | 21 | 41 |
| 13 | 48 | female | 64 | moderate | tetralogy of Fallot | none | none | complete reconstruction | 1 | 1 | 51 |
| Devices: | CHD Patients (n = 13) | Non-CHD Patients (n = 162) | p-Value |
|---|---|---|---|
| Single chamber (atrial) PM | 1 (7.7%) | 2 (1.23%) | 0.004 |
| Single chamber (ventricular) PM | 1 (7.7%) | 10 (6.17%) | |
| Dual chamber PM | 8 (61.5%) | 45 (27.7%) | |
| Single chamber VDD PM | 0 | 3 (1.85%) | |
| CRT-P | 0 | 16 (9.9%) | |
| Single chamber ICD | 0 | 26 (16%) | |
| Single chamber VDD ICD | 0 | 8 (4.9%) | |
| Dual chamber ICD | 3 (23%) | 7 (4.3%) | |
| CRT-D | 0 | 45 (27.7%) | |
| Leads: | CHD patients (n = 22) | Non-CHD patients (n = 242) | p-value |
| Lead age at time of extraction (median) (years) | 8 | 4 | <0.01 |
| Lead age: min max (years) | 1.4–25 | 1–28 | |
| Number of leads present per patient | 0.448 | ||
| 1 lead | 2 (15%) | 44 (27%) | |
| 2 leads | 8 (61%) | 58 (35%) | |
| 3 leads | 3 (23%) | 52 (32%) | |
| 4 leads | 0 | 6 (3.7%) | |
| 5 leads | 0 | 2 (1.2%) | |
| Number of leads treated per patient | 0.594 | ||
| 1 lead | 7 (54%) | 109 (67%) | |
| 2 leads | 3 (23%) | 31 (19%) | |
| 3 leads | 3 (23%) | 19 (12%) | |
| 4 leads | 0 | 3 (2%) | |
| Number of ICD lead extractions | 2 (15%) | 65 (40%) | 0.07 |
| Number of abandoned lead extractions | 3 (23%) | 10 (6%) | 0.025 |
| Outcomes: | CHD patients (n = 13) | Non-CHD patients (n = 162) | p-value |
| Complete procedural success | 12 (92%) | 140 (87%) | 0.581 |
| Clinical success | 13 (100%) | 148 (91%) | 0.269 |
| Procedural complications | 0 | 19 (11%) | 0.191 |
| 30-day mortality | 1 (8.3%) | 10 (6.6%) | 0.825 |
| Use of simple manual traction | 4 (30%) | 79 (48%) | 0.211 |
| Use of locking stylet and manual traction | 1 (8.3%) | 18 (11%) | 0.641 |
| Use of powered sheath | 8 (61%) | 63 (38%) | 0.110 |
| use of Evolution powered sheath | 3 (23%) | 17 (10%) | 0.191 |
| use of TightRail powered sheath | 2 (15%) | 37 (22%) | |
| use of femoral snare | 3 (23%) | 8 (4.9%) | 0.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Csillik, A.; Gagyi, R.B.; Kardos, A.; Földesi, C.; Som, Z.; Vamos, M.; Szili-Torok, T. Transvenous Lead Extraction in Patients with Congenital Heart Disease. J. Clin. Med. 2025, 14, 4178. https://doi.org/10.3390/jcm14124178
Csillik A, Gagyi RB, Kardos A, Földesi C, Som Z, Vamos M, Szili-Torok T. Transvenous Lead Extraction in Patients with Congenital Heart Disease. Journal of Clinical Medicine. 2025; 14(12):4178. https://doi.org/10.3390/jcm14124178
Chicago/Turabian StyleCsillik, Andrea, Rita Beata Gagyi, Attila Kardos, Csaba Földesi, Zoltán Som, Mate Vamos, and Tamas Szili-Torok. 2025. "Transvenous Lead Extraction in Patients with Congenital Heart Disease" Journal of Clinical Medicine 14, no. 12: 4178. https://doi.org/10.3390/jcm14124178
APA StyleCsillik, A., Gagyi, R. B., Kardos, A., Földesi, C., Som, Z., Vamos, M., & Szili-Torok, T. (2025). Transvenous Lead Extraction in Patients with Congenital Heart Disease. Journal of Clinical Medicine, 14(12), 4178. https://doi.org/10.3390/jcm14124178





