Coronary Revascularization in Patients with Hemophilia and Acute Coronary Syndrome: Case Report and Brief Literature Review
Abstract
1. Introduction
2. Materials and Methods
3. Clinical Case Description
- (1)
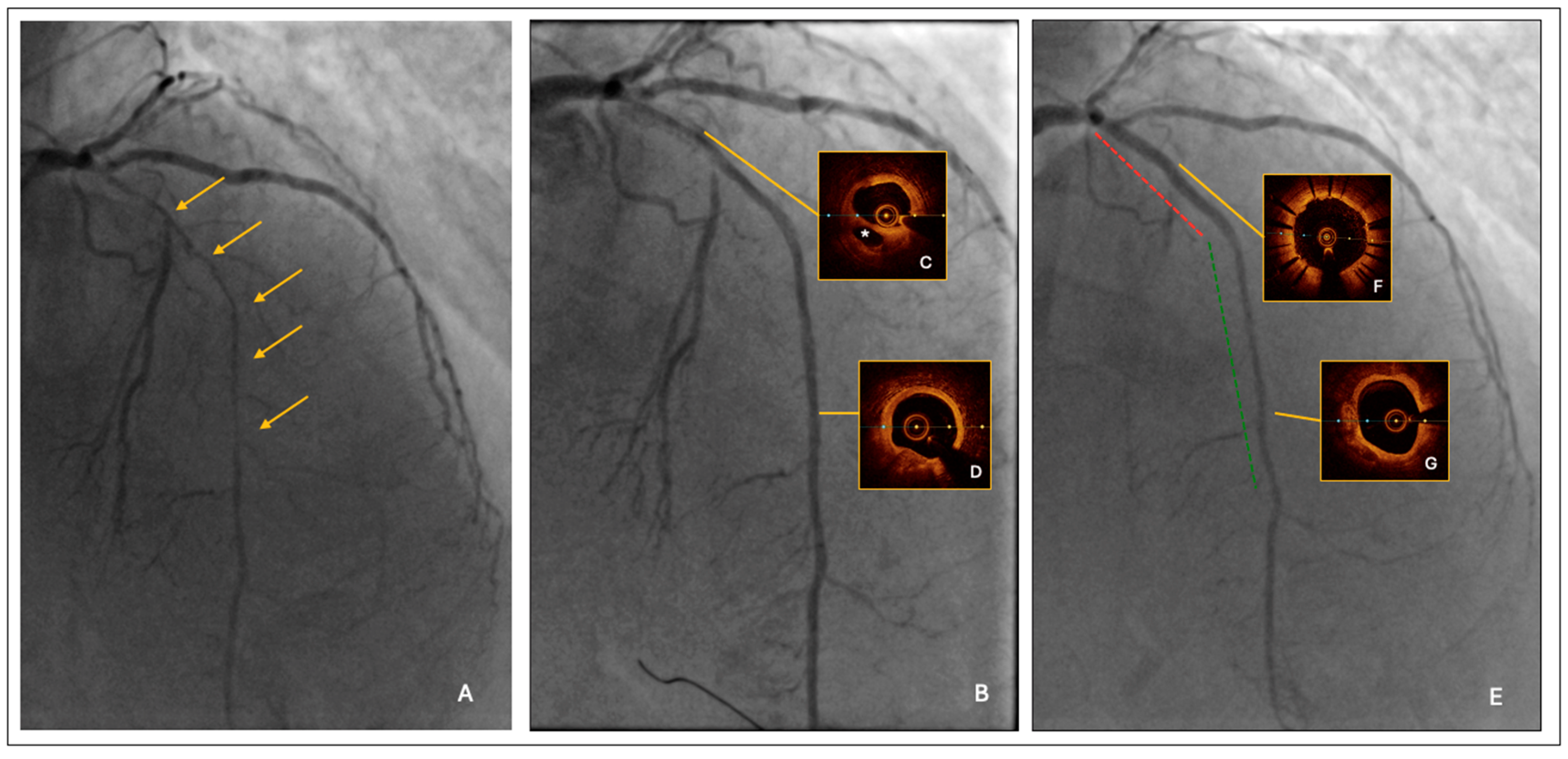
- Lesion predilatation by a semi-compliant balloon, sized 1:1 to the reference vessel diameter; it was 2.0 mm at the distal segment and increased progressively up to 3.0 mm at the proximal segment, respectively. After lesion predilatation, the result was optimal in the middle-distal LAD, but suboptimal in the proximal segment, where a long linear dissection was observed (Figure 1B).
- (2)
- Intravascular imaging by optical coherence tomography (OCT) confirmed an adequate overall luminal gain and confirmed the presence of a large linear dissection in the proximal LAD, with significant residual stenosis and a moderate burden of calcium (Figure 1C,D).
- (3)
- The procedure was completed by a 2.25/20 mm and 2.5/25 mm drug-coated balloon (DCB) angioplasty of mid-distal LAD and by proximal LAD stenting using a 3.0/33 mm polymer-free Biolimus eluting stent.
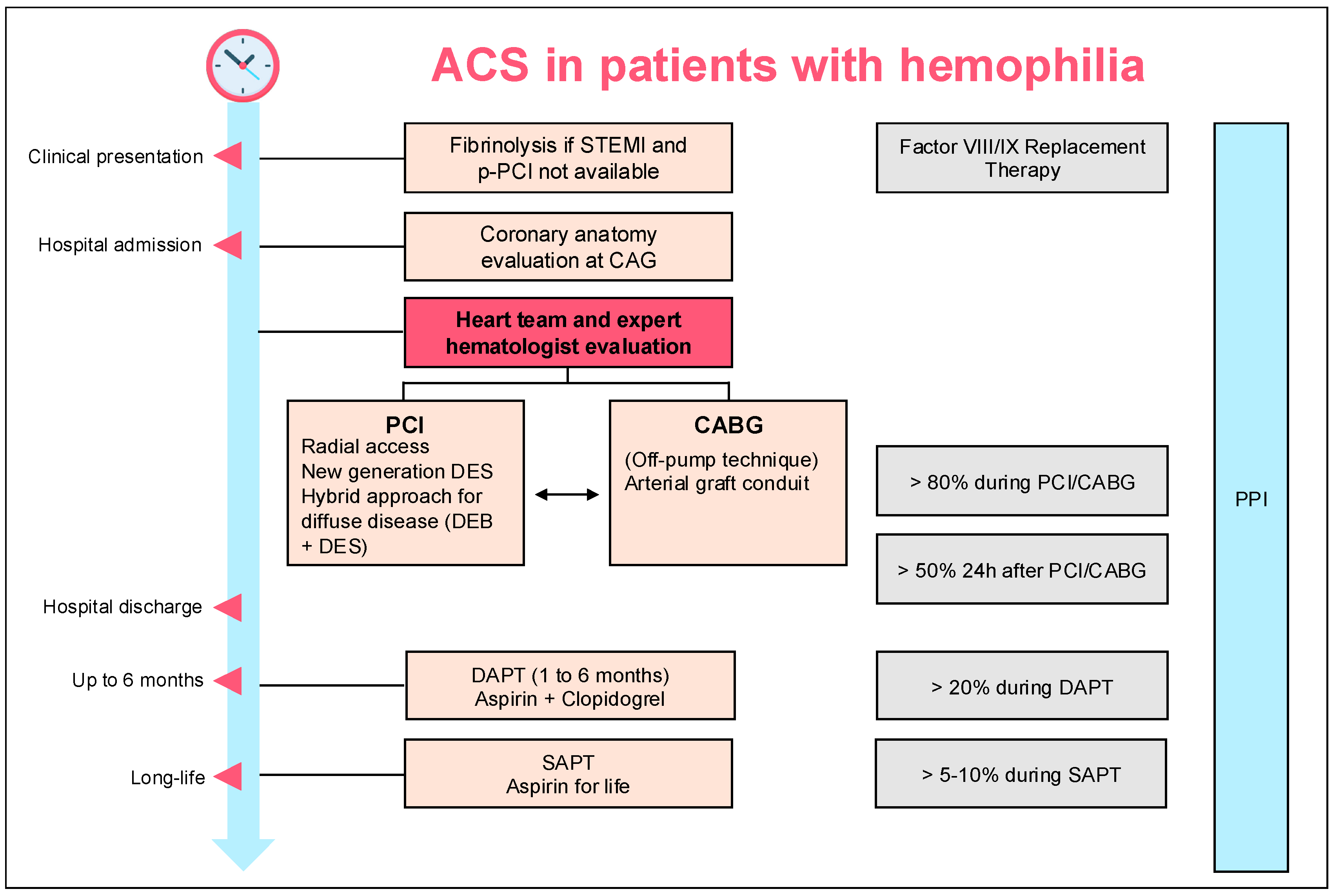
4. Discussion
- (1)
- The presence of a multivessel disease imposes a choice between surgical and percutaneous revascularization and between complete and incomplete revascularization.
- (2)
- The treatment of a long and diffuse CAD represents a great challenge, regardless of the revascularization modality, either surgical or percutaneous.
- (3)
- When the chosen revascularization modality is PCI, it must encompass a strategy suitable for a short DAPT.
4.1. Revascularization Modality
4.2. Issues Related to the Treatment of Diffuse Atherosclerotic Disease
4.3. DAPT Duration After Revascularization
5. Conclusions
Funding
Informed Consent Statement
Conflicts of Interest
Abbreviations
| ACS | acute coronary syndrome |
| AF | atrial fibrillation |
| BMS | bare metal stent |
| CABG | coronary artery bypass grafting |
| CAD | coronary artery disease |
| DAPT | dual antiplatelet therapy |
| DES | drug-eluting stent |
| ESC | European Society of Cardiology |
| FFR | fractional flow reserve |
| HBR | high bleeding risk |
| HCV | hepatitis C virus |
| LAD | left anterior descending artery |
| LVEF | left ventricular ejection fraction |
| NSTE-ACS | non-ST elevation acute coronary syndrome |
| OAC | oral anticoagulant |
| OCT | optical coherence tomography |
| PCI | percutaneous coronary intervention |
| PPI | proton-pump inhibitors |
| STEMI | ST-elevation myocardial infarction |
| VKA | vitamin K antagonist |
References
- Berntorp, E.; Fischer, K.; Hart, D.P.; Mancuso, M.E.; Stephensen, D.; Shapiro, A.D.; Blanchette, V. Haemophilia. Nat. Rev. Dis. Primers 2021, 7, 45. [Google Scholar] [CrossRef] [PubMed]
- Thachil, J.; Connors, J.M.; Mahlangu, J.; Sholzberg, M. Reclassifying hemophilia to include the definition of outcomes and phenotype as new targets. J. Thromb. Haemost. 2023, 21, 1737–1740. [Google Scholar] [CrossRef]
- Chen, H.; Yang, S. Acute coronary syndrome management in hemophiliacs: How to maintain balance?: A review. Medicine 2023, 102, E33298. [Google Scholar] [CrossRef] [PubMed]
- Dima, N.; Rezus, E.; Ganceanu-Rusu, A.R.; Popescu, D.; Isac, P.N.S.; Badescu, M.C.; Badulescu, O.V.; Tanase, D.M.; Ouatu, A.; Genes, T.-M.; et al. Current Therapeutic Approach to Acute Myocardial Infarction in Patients with Congenital Hemophilia. Life 2021, 11, 1072. [Google Scholar] [CrossRef] [PubMed]
- Tuinenburg, A.; Mauser-Bunschoten, E.P.; Verhaar, M.C.; Biesma, D.H.; Schutgens, R.E.G. Cardiovascular disease in patients with hemophilia. J. Thromb. Haemost. 2009, 7, 247–254. [Google Scholar] [CrossRef]
- Mannucci, P.M.; Schutgens, R.E.G.; Santagostino, E.; Mauser-Bunschoten, E.P. How I treat age-related morbidities in elderly persons with hemophilia. Blood 2009, 114, 5256–5263. [Google Scholar] [CrossRef]
- Dobreanu, S.; Ciocoiu, M.; Badulescu, O.V.; Filip, N.; Badescu, M.C.; Bojan, I.B.; Vladeanu, M.; Ciuntu, B.-M.; Tanevski, A.; Tudor, R. Thrombotic Disease in Hemophilic Patients: Is This a Paradox in a State of Hypocoagulability? Diagnostics 2024, 14, 286. [Google Scholar] [CrossRef]
- Coppola, A.; Franchini, M.; Makris, M.; Santagostino, E.; Di Minno, G.; Mannucci, P.M. Thrombotic adverse events to coagulation factor concentrates for treatment of patients with haemophilia and von Willebrand disease: A systematic review of prospective studies. Haemophilia 2012, 18, e173–e187. [Google Scholar] [CrossRef]
- Theodoropoulos, K.C.; Vakalopoulou, S.; Oikonomou, M.; Stavropoulos, G.; Ziakas, A.; Kanonidis, I.; Kassimis, G. How to manage a patient with haemophilia and ACS requiring PCI: A battle between bleeding and thrombosis. Medicina 2021, 57, 352. [Google Scholar] [CrossRef]
- Ferraris, V.A.; Boral, L.I.; Cohen, A.J.; Smyth, S.S.; White, G.C. Consensus review of the treatment of cardiovascular disease in people with hemophilia A and B. Cardiol. Rev. 2015, 23, 53–68. [Google Scholar] [CrossRef]
- Srivastava, A.; Santagostino, E.; Dougall, A.; Kitchen, S.; Sutherland, M.; Pipe, S.W.; Carcao, M.; Mahlangu, J.; Ragni, M.V.; Windyga, J.; et al. WFH Guidelines for the Management of Hemophilia, 3rd ed.; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2020; Volume 26, pp. 1–158. [Google Scholar]
- Byrne, R.A.; Rossello, X.; Coughlan, J.J.; Barbato, E.; Berry, C.; Chieffo, A.; Claeys, M.J.; Dan, G.-A.; Dweck, M.R.; Galbraith, M.; et al. 2023 ESC Guidelines for the management of acute coronary syndromes. Eur. Heart J. 2023, 44, 3720–3826. [Google Scholar] [CrossRef] [PubMed]
- Windyga, J.; Marquardt, N.; Dolan, G.; Rodgers, R.; Nunez, R.; Carvalho, M.; Zupan, I.P.; Hermans, C.; De Moerloose, P.; Mancuso, M.E.; et al. Applicability of the European Society of Cardiology Guidelines on the management of acute coronary syndromes to older people with haemophilia A—A modified Delphi consensus by the ADVANCE Working Group. Haemophilia 2023, 29, 21–32. [Google Scholar] [CrossRef]
- Cohen, O.C.; Bertelli, M.; Manmathan, G.; Little, C.; Riddell, A.; Pollard, D.; Aradom, E.; Mussara, M.; Harrington, C.; Kanagasabapathy, P.; et al. Challenges of antithrombotic therapy in the management of cardiovascular disease in patients with inherited bleeding disorders: A single-centre experience. Haemophilia 2021, 27, 425–433. [Google Scholar] [CrossRef] [PubMed]
- Staritz, P.; de Moerloose, P.; Schutgens, R.; Dolan, G. Applicability of the European Society of Cardiology guidelines on management of acute coronary syndromes to people with haemophilia—An assessment by the ADVANCE Working Group. Haemophilia 2013, 19, 833–840. [Google Scholar] [CrossRef]
- Walsh, M.; Tran, H.; Shaw, J.; McCarthy, P.; Davis, A.K.; McGiffin, D.; Bhave, P. Guide to performing cardiac surgery in patients with hereditary bleeding disorders. J. Card. Surg. 2015, 30, 61–69. [Google Scholar]
- Shaefi, S.; Mittel, A.; Loberman, D.; Ramakrishna, H. Off-Pump Versus On-Pump Coronary Artery Bypass Grafting—A Systematic Review and Analysis of Clinical Outcomes. J. Cardiothorac. Vasc. Anesth. 2019, 33, 232–244. [Google Scholar] [CrossRef]
- Tang, M.; Wierup, P.; Terp, K.; Ingerslev, J.; Sørensen, B. Cardiac surgery in patients with haemophilia. Haemophilia 2009, 15, 101–107. [Google Scholar] [CrossRef]
- Vaz, C.; Sousa, M.; Fernandes, S.; Oliveira, M.; Carvalho, M.; Lopes, M.; Koch, C. Myocardial infarction and severe haemophilia B: A challenging balanced management. Haemophilia 2021, 27, 100. [Google Scholar]
- Gundabolu, K.; Goldsweig, A.; Bhatt, V.R.; Koepsell, S.A.; Harper, J.L. ST-Segment Elevation Myocardial Infarction (STEMI) and Pulmonary Embolism in a Hemophilia A Patient Receiving Emicizumab and recombinant Activated Factor VII. Haemophilia 2020, 26, e5–e8. [Google Scholar] [CrossRef]
- Kacprzak, M.; Brzeczek, M.; Koniarek, W.; Zielinska, M.; Chojnowski, K. Haemophilia and acute coronary syndrome–should we still be afraid, should we avoid new antiplatelet drugs? Clin. Pract. 2018, 15, 635–638. [Google Scholar] [CrossRef]
- Bailly, J.; Mahlangu, J. Myocardial infarction in severe haemophilia. J. Haemoph. Pract. 2018, 5, 8–11. [Google Scholar] [CrossRef]
- Brugaletta, S.; Moretti, C.; Omerovic, E.; Tamburino, C.; Cortese, B.; Presbitero, P.; Sardella, G.; Briguori, C.; Picchi, A.; De Caterina, R.; et al. Radial versus femoral access and bivalirudin versus unfractionated heparin in invasively managed patients with acute coronary syndrome (MATRIX): Final 1-year results of a multicentre, randomised controlled trial. Lancet 2018, 392, 835–848. [Google Scholar]
- de Andrade, P.B.; de Ribamar Costa, J.; Rinaldi, F.S.; de Castro Bienert, I.R.; Barbosa, R.A.; Esteves, V.; Tebet, M.; Zukowski, C.; Maia, F.; Piva, E.M.L.A. Vascular Closure Devices Attenuate Femoral Access Complications of Primary Percutaneous Coronary Intervention. J. Invasive Cardiol. 2020, 32, 364–370. [Google Scholar]
- Sun, M.; Gao, J.; Guo, M.; Wang, R.; Pang, N.; Zhang, B.; Zhang, N. Vascular Closure Devices versus Manual Compression in Cardiac Interventional Procedures: Systematic Review and Meta-Analysis. Cardiovasc. Ther. 2022, 2022, 8569188. [Google Scholar]
- Alhaddad, Z.; Al Khouri, Z.A.; Alhaddad, I.A. Percutaneous Coronary Intervention for Acute Coronary Syndrome in a 44-Year-Old Man with Hemophilia A. Tex. Heart Inst. J. 2022, 49, e207436. [Google Scholar] [CrossRef]
- Aguiar-Ricardo, I.; Agostinho, J.; Pereira, A.; Rodrigues, F.; Brito, D.; Pinto, F.J.; Catarino, C.; Pedro, M.M. Acute Coronay Syndrome in a Patient with Severe Hemophilia A: Dificult Decisions. Rev. Port. Cardiologia 2021, 40, 985.e1–985.e5. [Google Scholar] [CrossRef]
- Abraham, M.R.; Leavitt, A.D.; Ports, T.A.; Soni, K.; Tanriverdi, T.; Mayfield, J.J. Acute coronary syndrome in patients with hemophilia: A delicate balancing act. J. Thromb. Thrombolysis 2022, 54, 323–329. [Google Scholar]
- Shoji, S.; Cuisset, T.; Ueyama, H.; Bangalore, S.; Kohsaka, S.; Mehran, R.; Bhatt, D.L.; Takagi, H.; Stone, G.W.; Fujisaki, T.; et al. Short-Term DAPT and DAPT De-Escalation Strategies for Patients with Acute Coronary Syndromes: A Systematic Review and Network Meta-Analysis. Circ. Cardiovasc. Interv. 2023, 16, 557–569. [Google Scholar]
- Ariotti, S.; Adamo, M.; Costa, F.; Patialiakas, A.; Briguori, C.; Thury, A.; Colangelo, S.; Campo, G.; Tebaldi, M.; Ungi, I.; et al. Is Bare-Metal Stent Implantation Still Justifiable in High Bleeding Risk Patients Undergoing Percutaneous Coronary Intervention?: A Pre-Specified Analysis From the ZEUS Trial. JACC Cardiovasc. Interv. 2016, 9, 426–436. [Google Scholar] [CrossRef]
- Ferrone, M.; Russo, M.; Tesorio, T.; Di Gioia, G.; Cioppa, A.; Popusoi, G.; Pittorino, L.; Franzese, M.; Verde, N.; Salemme, L.; et al. Contemporary Use of Drug-Coated Balloons for Coronary Angioplasty: A Comprehensive Review. J. Clin. Med. 2024, 13, 243. [Google Scholar] [CrossRef]
- Mangieri, A.; Rossi, M.L.; Tartaglia, F.; Stefanini, G.G.; Novelli, L.; Regazzoli, D.; Sticchi, A.; Leone, P.P.; Cozzi, O.; Gitto, M.; et al. Immediate and follow-up outcomes of drug-coated balloon angioplasty in de novo long lesions on large coronary arteries. EuroIntervention 2023, 19, e923. [Google Scholar]
- Ben-Dor, I.; Waksman, R.; Hill, A.P.; Merdler, I.; Bhogal, S.; Wermers, J.P. Drug-coated balloons for coronary artery disease: An updated review with future perspectives. Cardiovasc. Revascularization Med. 2024, 69, 79–89. [Google Scholar]
- Cortese, B.; Ribichini, F.; Fezzi, S.; Malakouti, S.; Sivalingam, J.; Khater, J. Drug-Coated Balloon in Acute Coronary Syndromes: Ready for the Prime Time? Curr. Cardiol. Rep. 2024, 26, 359–372. [Google Scholar]
- Martin, K.; Key, N.S. How I treat patients with inherited bleeding disorders who need anticoagulant therapy. Blood 2016, 128, 178–184. [Google Scholar] [CrossRef]
- De Potter, T.J.R.; Lumbers, R.T. 2024 ESC Guidelines for the management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS). Piotr Suwalski 2024, 45, 3314–3414. [Google Scholar]
- Corballis, N.H.; Wickramarachchi, U.; Vassiliou, V.S.; Eccleshall, S.C. Duration of dual antiplatelet therapy in elective drug-coated balloon angioplasty. Catheter. Cardiovasc. Interv. 2020, 96, 1016–1020. [Google Scholar] [CrossRef] [PubMed]
- Claassens, D.M.; Bergmeijer, T.O.; Vos, G.J.; Hermanides, R.S.; van’t Hof, A.W.; Van Der Harst, P.; Barbato, E.; Morisco, C.; Tjon Joe Gin, R.M.; Asselbergs, F.W.; et al. Clopidogrel Versus Ticagrelor or Prasugrel After Primary Percutaneous Coronary Intervention According to CYP2C19 Genotype: A POPular Genetics Subanalysis. Circ. Cardiovasc. Interv. 2021, 14, E009434. [Google Scholar] [CrossRef]
- Lenting, P.J. Laboratory monitoring of hemophilia A treatments: New challenges. Blood Adv. 2020, 4, 2111–2118. [Google Scholar] [CrossRef]
- Boehnel, C.; Rickli, H.; Graf, L.; Maeder, M.T. Coronary angiography with or without percutaneous coronary intervention in patients with hemophilia-Systematic review. Catheter. Cardiovasc. Interv. 2018, 92, 1–15. [Google Scholar] [CrossRef]
- Huber, K.; Andreotti, F.; Kjeldsen, K.; Pathak, A.; Collet, J.; Lip, G.; Rosano, G.; Atar, D.; Husted, S.; Wassmann, S.; et al. Expert position paper on the use of proton pump inhibitors in patients with cardiovascular disease and antithrombotic therapy. Eur. Heart J. 2013, 34, 1708–1713. [Google Scholar]
- Gimotty, P.A.; Matthai, W.H.; Reilley, M.J.; Fogarty, P.F.; Vega, R.; Blair, A.; Buckley, M. Revascularization strategies and in-hospital management in acute coronary syndromes complicated by hemophilia A or hemophilia B. Blood Coagul. Fibrinolysis 2017, 28, 650–657. [Google Scholar]
- Ruel, M.; Deb, S.; Patrono, C.; Glineur, D.; Tranbaugh, R.; Di Giammarco, G.; Di Franco, A.; Puskas, J.; Taggart, D.P.; Girardi, L.N.; et al. Mechanisms, consequences, and prevention of coronary graft failure. Circulation 2017, 136, 1749–1764. [Google Scholar]
- Tanaka, A.; Ino, Y.; Yamaguchi, T.; Yamano, T.; Kuroi, A.; Okamura, Y.; Honda, K.; Nishimura, Y.; Aoki, H.; Matsuo, Y.; et al. Impact of functional focal versus diffuse coronary artery disease on bypass graft patency. Int. J. Cardiol. 2016, 222, 16–21. [Google Scholar]
- Ullrich, H.; Olschewski, M.; Münzel, T.; Gori, T. Coronary In-Stent Restenosis: Predictors and Treatment. Dtsch. Arztebl. Int. 2021, 118, 637. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, M.; Yamaji, K.; Nakamura, S.; Ikari, Y.; Kohsaka, S.; Amano, T.; Ako, J.; Morino, Y.; Tanabe, K.; Kozuma, K.; et al. Clinical expert consensus document on drug-coated balloon for coronary artery disease from the Japanese Association of Cardiovascular Intervention and Therapeutics. Cardiovasc. Interv. Ther. 2023, 38, 166–176. [Google Scholar]
- Arslani, K.; Jeger, R. Drug-coated Balloons for Small Coronary Disease—A Literature Review. Curr. Cardiol. Rep. 2021, 23, 173. [Google Scholar] [CrossRef]
- Pocock, S.; Kim, H.-S.; Mehran, R.; Capodanno, D.; Farb, A.; Gregson, J.; Morice, M.-C.; Haude, M.; Eikelboom, J.; Cutlip, D.; et al. Defining high bleeding risk in patients undergoing percutaneous coronary intervention: A consensus document from the Academic Research Consortiumfor High Bleeding Risk. Ukr. J. Cardiol. 2021, 28, 53–72. [Google Scholar]
- Erriquez, A.; Campo, G.; Guiducci, V.; Escaned, J.; Moreno, R.; Casella, G.; Menozzi, M.; Cerrato, E.; Sacchetta, G.; Menozzi, A.; et al. Complete vs Culprit-Only Revascularization in Older Patients with Myocardial Infarction and High Bleeding Risk: A Randomized Clinical Trial. JAMA Cardiol. 2024, 9, 565–573. [Google Scholar] [CrossRef]
- Picchi, A.; Cerrato, E.; Cocco, M.; Cavazza, C.; Guiducci, V.; Varbella, F.; Menozzi, A.; Vadalà, G.; Sacchetta, G.; Menozzi, M.; et al. Complete vs Culprit-Only Revascularization in Older Patients with Myocardial Infarction with or Without ST-Segment Elevation. J. Am. Coll. Cardiol. 2024, 84, 2014–2022. [Google Scholar]
- Herrman, J.-P.R.; Kiemeneij, F.; Slagboom, T.; Amoroso, G.; Vink, M.A.; Dirksen, M.T.; Patterson, M.S.; Vos, N.S.; van der Schaaf, R.J.; van Nooijen, F.C. Safety and feasibility of a PAclitaxel-eluting balloon angioplasty in Primary Percutaneous coronary intervention in Amsterdam (PAPPA): One-year clinical outcome of a pilot study. EuroIntervention 2014, 10, 584–590. [Google Scholar]
- Mustonen, J.; Eränen, J.; Mäntylä, P.; Kärkkäinen, J.M.; Uskela, S.; Siljander, A.; Rissanen, T.T. Percutaneous coronary intervention with drug-coated balloon-only strategy in stable coronary artery disease and in acute coronary syndromes: An all-comers registry study. Catheter. Cardiovasc. Interv. 2019, 93, 893–900. [Google Scholar]
- James, S.; Wallentin, L.; Horrow, J.; Husted, S.; Emanuelsson, H.; Skene, A.; Katus, H.; Harrington, R.A.; Mahaffey, K.W.; Becker, R.C.; et al. Ticagrelor versus clopidogrel in patients with acute coronary syndromes. N. Engl. J. Med. 2009, 361, 1045–1057. [Google Scholar]
- Madaudo, C.; Parlati, A.L.M.; Di Lisi, D.; Carluccio, R.; Sucato, V.; Vadalà, G.; Nardi, E.; Macaione, F.; Cannata, A.; Manzullo, N.; et al. Artificial intelligence in cardiology: A peek at the future and the role of ChatGPT in cardiology practice. J. Cardiovasc. Med. 2024, 25, 766–771. [Google Scholar] [CrossRef] [PubMed]
| Author (Year) | Sex, Age | Hemophilia Type and Severity | Comorbidities | Type of AMI | Case Management |
|---|---|---|---|---|---|
| Theodoropoulos et al., 2021 [9] | Male, 70 yrs | B, mild | Hypertension, COPD | NSTEMI | Aspirin and clopidogrel (loading doses) PCI via radial access Zotarolimus-eluting stent UFH (peri-procedural) DAPT × 1 month (aspirin and clopidogrel) Prophylactic FIX administration before PCI and during DAPT |
| Vaz et al., 2021 [19] | Male, 56 yrs | B, severe | Hypertension, dyslipidemia, smoking | STEMI | Aspirin (loading dose) PCI via radial access Ticagrelor 180 mg + UFH 5000 IU i.v. DES implanted DAPT with aspirin and ticagrelor, then SAPT with aspirin Symptoms occurred 8 h after FIX concentrate infusion Prophylactic FIX during DAPT |
| Gundabolu et al., 2019 [20] | Male, 40 yrs | A, severe (with inhibitors) | Smoking | STEMI | Medical management Low-dose rFVIIa 5–10 IU/kg/h × 4 days DAPT (aspirin + ticagrelor × 3 months, then SAPT with aspirin) Emicizumab 1.5 mg/kg × 3 days before STEMI cFVIII 10 IU/kg × 6 h before During hospitalization: emicizumab continued, rFVIIa not used |
| Kacprzak et al., 2018 [21] | Male, 67 yrs | A, severe | Chronic hepatitis C | STEMI | Aspirin and clopidogrel (loading doses) 500 IU UFH i.v. PCI via radial access 5 DES: 4 everolimus-eluting stents + 1 Biolimus A9-eluting stent |
| Bailly et al., 2021 [22] | Male, 54 yrs | A, severe | Dyslipidemia, smoking, HIV infection | STEMI | After stenting: switched clopidogrel to ticagrelor (180 mg loading dose) DAPT (aspirin + ticagrelor × 12 months), then SAPT with aspirin FVIII prophylaxis during hospitalization + 3 months of follow-up No bleeding episodes |
| Vascular access site |
|
| Revascularization strategy | |
| Anticoagulant |
|
| Antiplatelet | |
| Replacement therapy |
|
| PPI |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vadalà, G.; Mingoia, G.; Astuti, G.; Madaudo, C.; Sucato, V.; Adorno, D.; D’Agostino, A.; Novo, G.; Corrado, E.; Galassi, A.R. Coronary Revascularization in Patients with Hemophilia and Acute Coronary Syndrome: Case Report and Brief Literature Review. J. Clin. Med. 2025, 14, 4130. https://doi.org/10.3390/jcm14124130
Vadalà G, Mingoia G, Astuti G, Madaudo C, Sucato V, Adorno D, D’Agostino A, Novo G, Corrado E, Galassi AR. Coronary Revascularization in Patients with Hemophilia and Acute Coronary Syndrome: Case Report and Brief Literature Review. Journal of Clinical Medicine. 2025; 14(12):4130. https://doi.org/10.3390/jcm14124130
Chicago/Turabian StyleVadalà, Giuseppe, Giulia Mingoia, Giuseppe Astuti, Cristina Madaudo, Vincenzo Sucato, Daniele Adorno, Alessandro D’Agostino, Giuseppina Novo, Egle Corrado, and Alfredo Ruggero Galassi. 2025. "Coronary Revascularization in Patients with Hemophilia and Acute Coronary Syndrome: Case Report and Brief Literature Review" Journal of Clinical Medicine 14, no. 12: 4130. https://doi.org/10.3390/jcm14124130
APA StyleVadalà, G., Mingoia, G., Astuti, G., Madaudo, C., Sucato, V., Adorno, D., D’Agostino, A., Novo, G., Corrado, E., & Galassi, A. R. (2025). Coronary Revascularization in Patients with Hemophilia and Acute Coronary Syndrome: Case Report and Brief Literature Review. Journal of Clinical Medicine, 14(12), 4130. https://doi.org/10.3390/jcm14124130








