Respiratory Manifestations and Their Physical, Psychological, and Social Impacts in Ehlers-Danlos Syndromes and Generalized Hypermobility Spectrum Disorders: A Narrative Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Literature Search
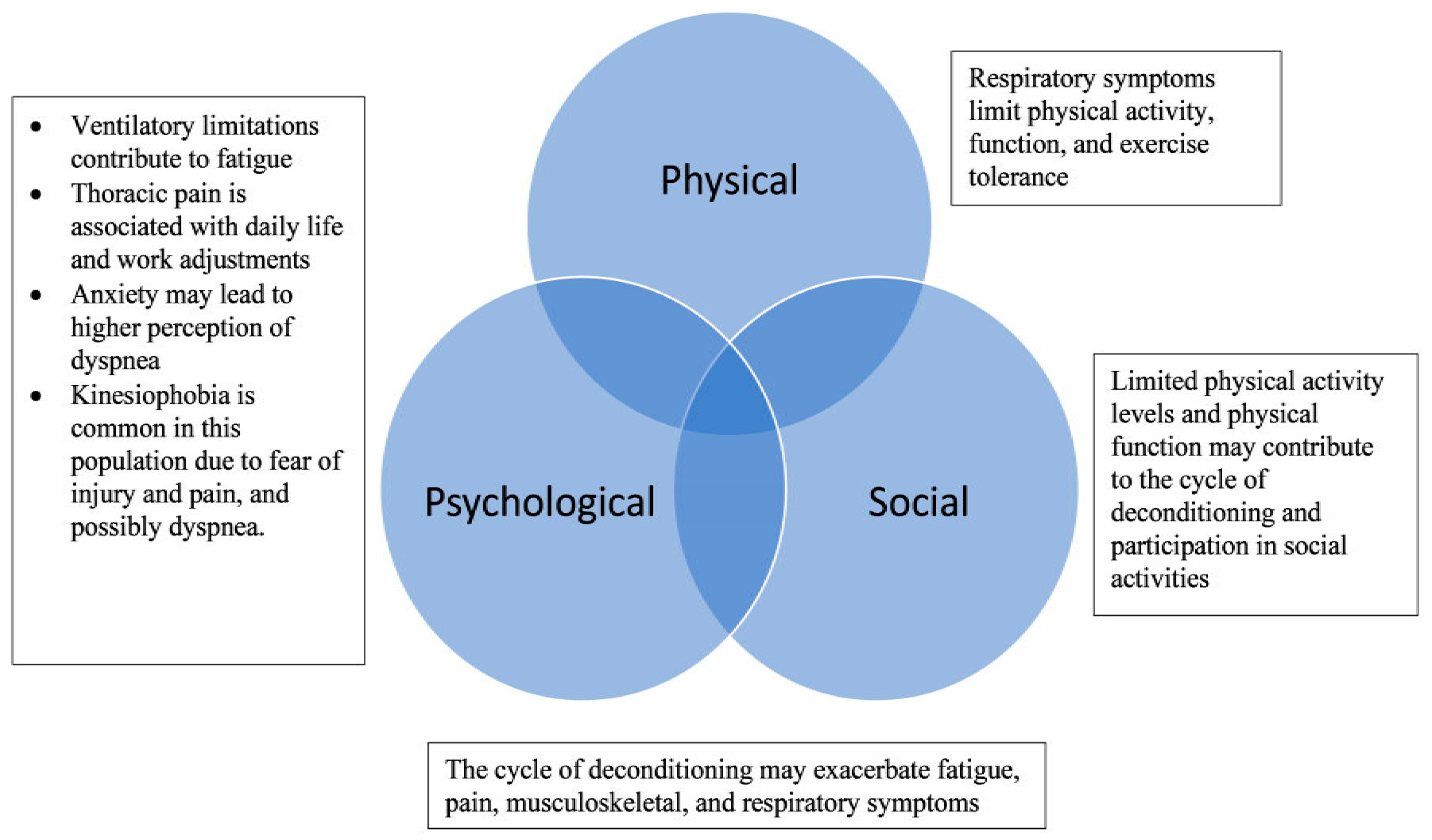
2.2. Health-Related Quality of Life: Physical, Psychological, and Social Guiding Framework
3. Results
3.1. Identified Articles
3.2. Physical Domains of Health-Related Quality of Life (HRQL): Physical Functioning, Activity, and Exercise Tolerance
3.3. Physical Health-Related Quality of Life (HRQL): Pain and Fatigue
3.4. Psychological Health-Related Quality of Life (HRQL): Anxiety and Kinesiophobia
3.5. Social Health-Related Quality of Life (HRQL): Daily Function, Social Participation, and Work Disruptions
4. Discussion
4.1. Physical Domain
4.2. Psychological and Social Domain
4.3. Future Directions
4.4. Clinical Implication for Research and Practice
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Acronyms
| cEDS | Classical Ehlers-Danlos Syndrome |
| EDS | Ehlers-Danlos Syndrome |
| G-HSD | Generalized Hypermobility Spectrum Disorders |
| hEDS | Hypermobile Ehlers-Danlos Syndrome |
| HRQL | Health-Related Quality of Life |
| PROMs | Patient-Reported Outcome Measures |
| POTS | Postural Orthostatic Tachycardia Syndrome |
| MCAS | Mast Cell Activation Syndrome |
References
- Bogart, K.R.; Irvin, V.L. Health-related quality of life among adults with diverse rare disorders. Orphanet J. Rare Dis. 2017, 12, 177. [Google Scholar] [CrossRef] [PubMed]
- Castori, M.; Camerota, F.; Celletti, C.; Grammatico, P.; Padua, L. Quality of life in the classic and hypermobility types of Ehlers-Danlos syndrome [corrected]. Ann. Neurol. 2010, 67, 145–146, author reply 146–147. [Google Scholar] [CrossRef]
- Malfait, F.; Francomano, C.; Byers, P.; Belmont, J.; Berglund, B.; Black, J.; Bloom, L.; Bowen, J.M.; Brady, A.F.; Burrows, N.P.; et al. The 2017 international classification of the Ehlers-Danlos syndromes. Am. J. Med. Genet. Part C Semin. Med. Genet. 2017, 175, 8–26. [Google Scholar] [CrossRef]
- Demmler, J.C.; Atkinson, M.D.; Reinhold, E.J.; Choy, E.; Lyons, R.A.; Brophy, S.T. Diagnosed prevalence of Ehlers-Danlos syndrome and hypermobility spectrum disorder in Wales, UK: A national electronic cohort study and case-control comparison. BMJ Open 2019, 9, e031365. [Google Scholar] [CrossRef]
- De Wandele, I.; Rombaut, L.; Malfait, F.; De Backer, T.; De Paepe, A.; Calders, P. Clinical heterogeneity in patients with the hypermobility type of Ehlers-Danlos syndrome. Res. Dev. Disabil. 2013, 34, 873–881. [Google Scholar] [CrossRef] [PubMed]
- Mittal, N.; Mina, D.S.; McGillis, L.; Weinrib, A.; Slepian, P.M.; Rachinsky, M.; Buryk-Iggers, S.; Laflamme, C.; Lopez-Hernandez, L.; Hussey, L.; et al. The GoodHope Ehlers Danlos Syndrome Clinic: Development and implementation of the first interdisciplinary program for multi-system issues in connective tissue disorders at the Toronto General Hospital. Orphanet J. Rare Dis. 2021, 16, 357. [Google Scholar] [CrossRef] [PubMed]
- Scheper, M.C.; de Vries, J.E.; Verbunt, J.; Engelbert, R.H. Chronic pain in hypermobility syndrome and Ehlers-Danlos syndrome (hypermobility type): It is a challenge. J. Pain Res. 2015, 8, 591–601. [Google Scholar] [CrossRef]
- Voermans, N.C.; Knoop, H.; van de Kamp, N.; Hamel, B.C.; Bleijenberg, G.; van Engelen, B.G. Fatigue is a frequent and clinically relevant problem in Ehlers-Danlos Syndrome. Semin. Arthritis Rheum. 2010, 40, 267–274. [Google Scholar] [CrossRef]
- Bulbena-Cabre, A.; Rojo, C.; Pailhez, G.; Buron Maso, E.; Martin-Lopez, L.M.; Bulbena, A. Joint hypermobility is also associated with anxiety disorders in the elderly population. Int. J. Geriatr. Psychiatry 2018, 33, e113–e119. [Google Scholar] [CrossRef]
- Berglund, B.; Pettersson, C.; Pigg, M.; Kristiansson, P. Self-reported quality of life, anxiety and depression in individuals with Ehlers-Danlos syndrome (EDS): A questionnaire study. BMC Musculoskelet. Disord. 2015, 16, 89. [Google Scholar] [CrossRef]
- Murray, B.; Yashar, B.M.; Uhlmann, W.R.; Clauw, D.J.; Petty, E.M. Ehlers-Danlos syndrome, hypermobility type: A characterization of the patients’ lived experience. Am. J. Med. Genet. Part A 2013, 161A, 2981–2988. [Google Scholar] [CrossRef]
- Bascom, R.; Dhingra, R.; Francomano, C.A. Respiratory manifestations in the Ehlers-Danlos syndromes. Am. J. Med. Genet. Part C Semin. Med. Genet. 2021, 187, 533–548. [Google Scholar] [CrossRef] [PubMed]
- Chohan, K.; Mittal, N.; McGillis, L.; Lopez-Hernandez, L.; Camacho, E.; Rachinsky, M.; Mina, D.S.; Reid, W.D.; Ryan, C.M.; Champagne, K.A.; et al. A review of respiratory manifestations and their management in Ehlers-Danlos syndromes and hypermobility spectrum disorders. Chronic Respir. Dis. 2021, 18, 14799731211025313. [Google Scholar] [CrossRef] [PubMed]
- Leganger, J.; Fonnes, S.; Kulas Soborg, M.L.; Rosenberg, J.; Burcharth, J. The most common comorbidities in patients with Ehlers-Danlos syndrome: A 15-year nationwide population-based cohort study. Disabil. Rehabil. 2022, 44, 189–193. [Google Scholar] [CrossRef]
- Miller, A.J.; Stiles, L.E.; Sheehan, T.; Bascom, R.; Levy, H.P.; Francomano, C.A.; Arnold, A.C. Prevalence of hypermobile Ehlers-Danlos syndrome in postural orthostatic tachycardia syndrome. Auton. Neurosci. Basic Clin. 2020, 224, 102637. [Google Scholar] [CrossRef]
- De Wandele, I.; Calders, P.; Peersman, W.; Rimbaut, S.; De Backer, T.; Malfait, F.; De Paepe, A.; Rombaut, L. Autonomic symptom burden in the hypermobility type of Ehlers-Danlos syndrome: A comparative study with two other EDS types, fibromyalgia, and healthy controls. Semin. Arthritis Rheum. 2014, 44, 353–361. [Google Scholar] [CrossRef]
- Kohn, A.; Chang, C. The Relationship Between Hypermobile Ehlers-Danlos Syndrome (hEDS), Postural Orthostatic Tachycardia Syndrome (POTS), and Mast Cell Activation Syndrome (MCAS). Clin. Rev. Allergy Immunol. 2020, 58, 273–297. [Google Scholar] [CrossRef]
- Monaco, A.; Choi, D.; Uzun, S.; Maitland, A.; Riley, B. Association of mast-cell-related conditions with hypermobile syndromes: A review of the literature. Immunol. Res. 2022, 70, 419–431. [Google Scholar] [CrossRef]
- Mathias, C.J.; Owens, A.; Iodice, V.; Hakim, A. Dysautonomia in the Ehlers-Danlos syndromes and hypermobility spectrum disorders—With a focus on the postural tachycardia syndrome. Am. J. Med. Genet. Part C Semin. Med. Genet. 2021, 187, 510–519. [Google Scholar] [CrossRef]
- Mihaltan, F.; Adir, Y.; Antczak, A.; Porpodis, K.; Radulovic, V.; Pires, N.; de Vries, G.J.; Horner, A.; De Bontridder, S.; Chen, Y.; et al. Importance of the relationship between symptoms and self-reported physical activity level in stable COPD based on the results from the SPACE study. Respir. Res. 2019, 20, 89. [Google Scholar] [CrossRef]
- Alahmari, A.D.; Patel, A.R.; Kowlessar, B.S.; Mackay, A.J.; Singh, R.; Wedzicha, J.A.; Donaldson, G.C. Daily activity during stability and exacerbation of chronic obstructive pulmonary disease. BMC Pulm. Med. 2014, 14, 98. [Google Scholar] [CrossRef]
- Gruenberger, J.B.; Vietri, J.; Keininger, D.L.; Mahler, D.A. Greater dyspnea is associated with lower health-related quality of life among European patients with COPD. Int. J. Chronic Obstr. Pulm. Dis. 2017, 12, 937–944. [Google Scholar] [CrossRef] [PubMed]
- Hanania, N.A.; O’Donnell, D.E. Activity-related dyspnea in chronic obstructive pulmonary disease: Physical and psychological consequences, unmet needs, and future directions. Int. J. Chronic Obstr. Pulm. Dis. 2019, 14, 1127–1138. [Google Scholar] [CrossRef]
- The World Health Organization Quality of Life Assessment (WHOQOL): Development and general psychometric properties. Soc. Sci. Med. 1998, 46, 1569–1585. [CrossRef]
- Clark, N.L.; Johnson, M.; Rangan, A.; Kottam, L.; Swainston, K. The biopsychosocial impact of hypermobility spectrum disorders in adults: A scoping review. Rheumatol. Int. 2023, 43, 985–1014. [Google Scholar] [CrossRef] [PubMed]
- Sage, L.; Russo, M.L.; Byers, P.H.; Demasi, J.; Morris, S.A.; Puryear, L.N.; Fulton, D.S.; Shalhub, S.; Vascular Ehlers-Danlos Syndrome Research Collaborative. Setting a research agenda for vascular Ehlers-Danlos syndrome using a patient and stakeholder engagement model. J. Vasc. Surg. 2020, 72, 1436–1444.e2. [Google Scholar] [CrossRef] [PubMed]
- Sarna, L.; Evangelista, L.; Tashkin, D.; Padilla, G.; Holmes, C.; Brecht, M.L.; Grannis, F. Impact of respiratory symptoms and pulmonary function on quality of life of long-term survivors of non-small cell lung cancer. Chest 2004, 125, 439–445. [Google Scholar] [CrossRef]
- Seemungal, T.A.; Donaldson, G.C.; Paul, E.A.; Bestall, J.C.; Jeffries, D.J.; Wedzicha, J.A. Effect of exacerbation on quality of life in patients with chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 1998, 157, 1418–1422. [Google Scholar] [CrossRef]
- Hurst, J.R.; Wilkinson, T.M.; Donaldson, G.C.; Wedzicha, J.A. Upper airway symptoms and quality of life in chronic obstructive pulmonary disease (COPD). Respir. Med. 2004, 98, 767–770. [Google Scholar] [CrossRef]
- Ruffin, R.E.; Wilson, D.H.; Chittleborough, C.R.; Southcott, A.M.; Smith, B.; Christopher, D.J. Multiple respiratory symptoms predict quality of life in chronic lung disease: A population-based study of Australian adults. Qual. Life Res. 2000, 9, 1031–1039. [Google Scholar] [CrossRef]
- Reina-Gutierrez, S.; Caty, G.; Torres-Costoso, A.; Pitance, L.; Manicourt, D.H.; Reychler, G. Assessment of functional respiratory complaints and related factors in people with hypermobile Ehlers-Danlos syndrome: Cross-sectional study. Respir. Med. Res. 2023, 83, 101017. [Google Scholar] [CrossRef]
- Engel, G.L. The need for a new medical model: A challenge for biomedicine. Science 1977, 196, 129–136. [Google Scholar] [CrossRef]
- Megari, K. Quality of Life in Chronic Disease Patients. Health Psychol. Res. 2013, 1, e27. [Google Scholar] [CrossRef]
- Rombaut, L.; Malfait, F.; Cools, A.; De Paepe, A.; Calders, P. Musculoskeletal complaints, physical activity and health-related quality of life among patients with the Ehlers-Danlos syndrome hypermobility type. Disabil. Rehabil. 2010, 32, 1339–1345. [Google Scholar] [CrossRef]
- Jeffery, T.; Postavaru, G.I.; Matei, R.; Meizel, K. ‘I Have Had to Stop Singing Because I Can’t Take the Pain’: Experiences of Voice, Ability, and Loss in Singers with Hypermobility Spectrum Disorders. J. Voice 2024, 38, 966.e919–966.e929. [Google Scholar] [CrossRef]
- Hakimi, A.; Bergoin, C.; Mucci, P. Evidence of ventilatory constraints during exercise in hypermobile Ehlers-Danlos syndrome. Eur. J. Appl. Physiol. 2022, 122, 2367–2374. [Google Scholar] [CrossRef]
- De Baets, S.; Cruyt, E.; Calders, P.; Dewandele, I.; Malfait, F.; Vanderstraeten, G.; Van Hove, G.; van De Velde, D. Societal participation in ehlers-danlos syndromes and hypermobility spectrum disorder, compared to fibromyalgia and healthy controls. PLoS ONE 2022, 17, e0269608. [Google Scholar] [CrossRef]
- Ruiz Maya, T.; Fettig, V.; Mehta, L.; Gelb, B.D.; Kontorovich, A.R. Dysautonomia in hypermobile Ehlers-Danlos syndrome and hypermobility spectrum disorders is associated with exercise intolerance and cardiac atrophy. Am. J. Med. Genet. Part A 2021, 185, 3754–3761. [Google Scholar] [CrossRef]
- Simmonds, J.V.; Herbland, A.; Hakim, A.; Ninis, N.; Lever, W.; Aziz, Q.; Cairns, M. Exercise beliefs and behaviours of individuals with Joint Hypermobility syndrome/Ehlers-Danlos syndrome—Hypermobility type. Disabil. Rehabil. 2019, 41, 445–455. [Google Scholar] [CrossRef] [PubMed]
- Schubart, J.R.; Schaefer, E.; Hakim, A.J.; Francomano, C.A.; Bascom, R. Use of Cluster Analysis to Delineate Symptom Profiles in an Ehlers-Danlos Syndrome Patient Population. J. Pain Symptom Manag. 2019, 58, 427–436. [Google Scholar] [CrossRef] [PubMed]
- Krahe, A.M.; Adams, R.D.; Nicholson, L.L. Features that exacerbate fatigue severity in joint hypermobility syndrome/Ehlers-Danlos syndrome—Hypermobility type. Disabil. Rehabil. 2018, 40, 1989–1996. [Google Scholar] [CrossRef]
- Morgan, A.W.; Pearson, S.B.; Davies, S.; Gooi, H.C.; Bird, H.A. Asthma and airways collapse in two heritable disorders of connective tissue. Ann. Rheum. Dis. 2007, 66, 1369–1373. [Google Scholar] [CrossRef]
- Berglund, B.; Nordstrom, G.; Lutzen, K. Living a restricted life with Ehlers-Danlos syndrome (EDS). Int. J. Nurs. Stud. 2000, 37, 111–118. [Google Scholar] [CrossRef]
- Rombaut, L.; Malfait, F.; De Wandele, I.; Taes, Y.; Thijs, Y.; De Paepe, A.; Calders, P. Muscle mass, muscle strength, functional performance, and physical impairment in women with the hypermobility type of Ehlers-Danlos syndrome. Arthritis Care Res. 2012, 64, 1584–1592. [Google Scholar] [CrossRef]
- Coussens, M.; Lapauw, B.; Banica, T.; De Wandele, I.; Pacey, V.; Rombaut, L.; Malfait, F.; Calders, P. Muscle Strength, Muscle Mass and Physical Impairment in Women with hypermobile Ehlers-Danlos syndrome and Hypermobility Spectrum Disorder. J. Musculoskelet. Neuronal Interact. 2022, 22, 5–14. [Google Scholar]
- Ayres, J.G.; Pope, F.M.; Reidy, J.F.; Clark, T.J. Abnormalities of the lungs and thoracic cage in the Ehlers-Danlos syndrome. Thorax 1985, 40, 300–305. [Google Scholar] [CrossRef]
- De Baets, S.; Vanhalst, M.; Coussens, M.; Rombaut, L.; Malfait, F.; Van Hove, G.; Calders, P.; Vanderstraeten, G.; van de Velde, D. The influence of Ehlers-Danlos syndrome—Hypermobility type, on motherhood: A phenomenological, hermeneutical study. Res. Dev. Disabil. 2017, 60, 135–144. [Google Scholar] [CrossRef]
- Reychler, G.; Liistro, G.; Pierard, G.E.; Hermanns-Le, T.; Manicourt, D. Inspiratory muscle strength training improves lung function in patients with the hypermobile Ehlers-Danlos syndrome: A randomized controlled trial. Am. J. Med. Genet. Part A 2019, 179, 356–364. [Google Scholar] [CrossRef]
- Hakim, A.; De Wandele, I.; O’Callaghan, C.; Pocinki, A.; Rowe, P. Chronic fatigue in Ehlers-Danlos syndrome-Hypermobile type. Am. J. Med. Genet. Part C Semin. Med. Genet. 2017, 175, 175–180. [Google Scholar] [CrossRef] [PubMed]
- Hsu, L. Ehlers-Danlos syndrome and chronic pain. J. Pain Palliat. Care Pharmacother. 2012, 26, 178–179. [Google Scholar] [CrossRef]
- Molander, P.; Novo, M.; Hallstam, A.; Lofgren, M.; Stalnacke, B.M.; Gerdle, B. Ehlers-Danlos Syndrome and Hypermobility Syndrome Compared with Other Common Chronic Pain Diagnoses-A Study from the Swedish Quality Registry for Pain Rehabilitation. J. Clin. Med. 2020, 9, 2143. [Google Scholar] [CrossRef]
- Benistan, K.; Martinez, V. Pain in hypermobile Ehlers-Danlos syndrome: New insights using new criteria. Am. J. Med. Genet. Part A 2019, 179, 1226–1234. [Google Scholar] [CrossRef]
- Levine, M.; Adler, J. Acute diaphragmatic rupture in a patient with Ehlers-Danlos syndrome. J. Emerg. Med. 2011, 41, 366–368. [Google Scholar] [CrossRef]
- Leier, C.V.; Call, T.D.; Fulkerson, P.K.; Wooley, C.F. The spectrum of cardiac defects in the Ehlers-Danlos syndrome, types I and III. Ann. Intern. Med. 1980, 92, 171–178. [Google Scholar] [CrossRef]
- Dolan, A.L.; Mishra, M.B.; Chambers, J.B.; Grahame, R. Clinical and echocardiographic survey of the Ehlers-Danlos syndrome. Br. J. Rheumatol. 1997, 36, 459–462. [Google Scholar] [CrossRef]
- Castori, M.; Morlino, S.; Celletti, C.; Celli, M.; Morrone, A.; Colombi, M.; Camerota, F.; Grammatico, P. Management of pain and fatigue in the joint hypermobility syndrome (a.k.a. Ehlers-Danlos syndrome, hypermobility type): Principles and proposal for a multidisciplinary approach. Am. J. Med. Genet. Part A 2012, 158A, 2055–2070. [Google Scholar] [CrossRef]
- Sedky, K.; Gaisl, T.; Bennett, D.S. Prevalence of Obstructive Sleep Apnea in Joint Hypermobility Syndrome: A Systematic Review and Meta-Analysis. J. Clin. Sleep Med. JCSM Off. Publ. Am. Acad. Sleep Med. 2019, 15, 293–299. [Google Scholar] [CrossRef]
- Gaisl, T.; Giunta, C.; Bratton, D.J.; Sutherland, K.; Schlatzer, C.; Sievi, N.; Franzen, D.; Cistulli, P.A.; Rohrbach, M.; Kohler, M. Obstructive sleep apnoea and quality of life in Ehlers-Danlos syndrome: A parallel cohort study. Thorax 2017, 72, 729–735. [Google Scholar] [CrossRef]
- Moyer, C.A.; Sonnad, S.S.; Garetz, S.L.; Helman, J.I.; Chervin, R.D. Quality of life in obstructive sleep apnea: A systematic review of the literature. Sleep Med. 2001, 2, 477–491. [Google Scholar] [CrossRef] [PubMed]
- Wasim, S.; Suddaby, J.S.; Parikh, M.; Leylachian, S.; Ho, B.; Guerin, A.; So, J. Pain and gastrointestinal dysfunction are significant associations with psychiatric disorders in patients with Ehlers-Danlos syndrome and hypermobility spectrum disorders: A retrospective study. Rheumatol. Int. 2019, 39, 1241–1248. [Google Scholar] [CrossRef] [PubMed]
- Reilly, C.C.; Floyd, S.V.; Lee, K.; Warwick, G.; James, S.; Gall, N.; Rafferty, G.F. Breathlessness and dysfunctional breathing in patients with postural orthostatic tachycardia syndrome (POTS): The impact of a physiotherapy intervention. Auton. Neurosci. Basic Clin. 2020, 223, 102601. [Google Scholar] [CrossRef] [PubMed]
- Bulbena, A.; Baeza-Velasco, C.; Bulbena-Cabre, A.; Pailhez, G.; Critchley, H.; Chopra, P.; Mallorqui-Bague, N.; Frank, C.; Porges, S. Psychiatric and psychological aspects in the Ehlers-Danlos syndromes. Am. J. Med. Genet. Part C Semin. Med. Genet. 2017, 175, 237–245. [Google Scholar] [CrossRef] [PubMed]
- Bennett, S.E.; Walsh, N.; Moss, T.; Palmer, S. Understanding the psychosocial impact of joint hypermobility syndrome and Ehlers-Danlos syndrome hypermobility type: A qualitative interview study. Disabil. Rehabil. 2021, 43, 795–804. [Google Scholar] [CrossRef] [PubMed]
- Buryk-Iggers, S.; Mittal, N.; Santa Mina, D.; Adams, S.C.; Englesakis, M.; Rachinsky, M.; Lopez-Hernandez, L.; Hussey, L.; McGillis, L.; McLean, L.; et al. Exercise and Rehabilitation in People with Ehlers-Danlos Syndrome: A Systematic Review. Arch. Rehabil. Res. Clin. Transl. 2022, 4, 100189. [Google Scholar] [CrossRef]
- Palomo-Toucedo, I.C.; Leon-Larios, F.; Reina-Bueno, M.; Vazquez-Bautista, M.D.C.; Munuera-Martinez, P.V.; Dominguez-Maldonado, G. Psychosocial Influence of Ehlers-Danlos Syndrome in Daily Life of Patients: A Qualitative Study. Int. J. Environ. Res. Public Health 2020, 17, 6425. [Google Scholar] [CrossRef]
- Painter, P.; Stewart, A.L.; Carey, S. Physical functioning: Definitions, measurement, and expectations. Adv. Ren. Replace. Ther. 1999, 6, 110–123. [Google Scholar] [CrossRef]
- Geddes, E.L.; Reid, W.D.; Crowe, J.; O’Brien, K.; Brooks, D. Inspiratory muscle training in adults with chronic obstructive pulmonary disease: A systematic review. Respir. Med. 2005, 99, 1440–1458. [Google Scholar] [CrossRef]
- Baeza-Velasco, C.; Bulbena, A.; Polanco-Carrasco, R.; Jaussaud, R. Cognitive, emotional, and behavioral considerations for chronic pain management in the Ehlers-Danlos syndrome hypermobility-type: A narrative review. Disabil. Rehabil. 2019, 41, 1110–1118. [Google Scholar] [CrossRef]
- Ghosh, A.K. Anaerobic threshold: Its concept and role in endurance sport. Malays. J. Med. Sci. MJMS 2004, 11, 24–36. [Google Scholar]
- Sheel, A.W.; Foster, G.E.; Romer, L.M. Exercise and its impact on dyspnea. Curr. Opin. Pharmacol. 2011, 11, 195–203. [Google Scholar] [CrossRef]
- Celletti, C.; Castori, M.; La Torre, G.; Camerota, F. Evaluation of kinesiophobia and its correlations with pain and fatigue in joint hypermobility syndrome/Ehlers-Danlos syndrome hypermobility type. BioMed Res. Int. 2013, 2013, 580460. [Google Scholar] [CrossRef] [PubMed]
- Dube, B.P.; Vermeulen, F.; Laveneziana, P. Exertional Dyspnoea in Chronic Respiratory Diseases: From Physiology to Clinical Application. Arch. Bronconeumol. 2017, 53, 62–70. [Google Scholar] [CrossRef] [PubMed]
- Janssens, T.; De Peuter, S.; Stans, L.; Verleden, G.; Troosters, T.; Decramer, M.; Van den Bergh, O. Dyspnea perception in COPD: Association between anxiety, dyspnea-related fear, and dyspnea in a pulmonary rehabilitation program. Chest 2011, 140, 618–625. [Google Scholar] [CrossRef]
- Schuler, M.; Wittmann, M.; Faller, H.; Schultz, K. The interrelations among aspects of dyspnea and symptoms of depression in COPD patients—A network analysis. J. Affect. Disord. 2018, 240, 33–40. [Google Scholar] [CrossRef]
- Assassi, S.; Leyva, A.L.; Mayes, M.D.; Sharif, R.; Nair, D.K.; Fischbach, M.; Nguyen, N.; Reveille, J.D.; Gonzalez, E.B.; McNearney, T.A.; et al. Predictors of fatigue severity in early systemic sclerosis: A prospective longitudinal study of the GENISOS cohort. PLoS ONE 2011, 6, e26061. [Google Scholar] [CrossRef]
- von Leupoldt, A.; Chan, P.Y.; Bradley, M.M.; Lang, P.J.; Davenport, P.W. The impact of anxiety on the neural processing of respiratory sensations. Neuroimage 2011, 55, 247–252. [Google Scholar] [CrossRef] [PubMed]
- O’Donnell, D.E.; Banzett, R.B.; Carrieri-Kohlman, V.; Casaburi, R.; Davenport, P.W.; Gandevia, S.C.; Gelb, A.F.; Mahler, D.A.; Webb, K.A. Pathophysiology of dyspnea in chronic obstructive pulmonary disease: A roundtable. Proc. Am. Thorac. Soc. 2007, 4, 145–168. [Google Scholar] [CrossRef]
- Reijnders, T.; Troosters, T.; Janssens, W.; Gosselink, R.; Langer, D.; Davenport, P.W.; von Leupoldt, A. Brain Activations to Dyspnea in Patients with COPD. Front. Physiol. 2020, 11, 7. [Google Scholar] [CrossRef]
- O’Donnell, D.E.; Milne, K.M.; James, M.D.; de Torres, J.P.; Neder, J.A. Dyspnea in COPD: New Mechanistic Insights and Management Implications. Adv. Ther. 2020, 37, 41–60. [Google Scholar] [CrossRef]
- Arthur, K.; Caldwell, K.; Forehand, S.; Davis, K. Pain control methods in use and perceived effectiveness by patients with Ehlers-Danlos syndrome: A descriptive study. Disabil. Rehabil. 2016, 38, 1063–1074. [Google Scholar] [CrossRef]
- Levine, D.; Work, B.; McDonald, S.; Harty, N.; Mabe, C.; Powell, A.; Sanford, G. Occupational Therapy Interventions for Clients with Ehlers-Danlos Syndrome (EDS) in the Presence of Postural Orthostatic Tachycardia Syndrome (POTS). Occup. Ther. Health Care 2022, 36, 253–270. [Google Scholar] [CrossRef] [PubMed]
- Rocchetti, M.; Bassotti, A.; Corradi, J.; Damiani, S.; Pasta, G.; Annunziata, S.; Guerrieri, V.; Mosconi, M.; Gentilini, D.; Brondino, N. Is the Pain Just Physical? The Role of Psychological Distress, Quality of Life, and Autistic Traits in Ehlers-Danlos Syndrome, an Internet-Based Survey in Italy. Healthcare 2021, 9, 1472. [Google Scholar] [CrossRef]
- Jolley, C.J.; Moxham, J. A physiological model of patient-reported breathlessness during daily activities in COPD. Eur. Respir. Rev. Off. J. Eur. Respir. Soc. 2009, 18, 66–79. [Google Scholar] [CrossRef] [PubMed]
- Hallstrand, T.S.; Bates, P.W.; Schoene, R.B. Aerobic conditioning in mild asthma decreases the hyperpnea of exercise and improves exercise and ventilatory capacity. Chest 2000, 118, 1460–1469. [Google Scholar] [CrossRef] [PubMed]
- Lim, K.E.; Kim, S.R.; Kim, H.K.; Kim, S.R. Symptom Clusters and Quality of Life in Subjects with COPD. Respir. Care 2017, 62, 1203–1211. [Google Scholar] [CrossRef]
- Langhinrichsen-Rohling, J.; Lewis, C.L.; McCabe, S.; Lathan, E.C.; Agnew, G.A.; Selwyn, C.N.; Gigler, M.E. They’ve been BITTEN: Reports of institutional and provider betrayal and links with Ehlers-Danlos Syndrome patients’ current symptoms, unmet needs and healthcare expectations. Ther. Adv. Rare Dis. 2021, 2, 26330040211022033. [Google Scholar] [CrossRef]
- Halverson, C.M.E.; Clayton, E.W.; Garcia Sierra, A.; Francomano, C. Patients with Ehlers-Danlos syndrome on the diagnostic odyssey: Rethinking complexity and difficulty as a hero’s journey. Am. J. Med. Genet. Part C Semin. Med. Genet. 2021, 187, 416–424. [Google Scholar] [CrossRef]
- Morel, T.; Cano, S.J. Measuring what matters to rare disease patients—Reflections on the work by the IRDiRC taskforce on patient-centered outcome measures. Orphanet J. Rare Dis. 2017, 12, 171. [Google Scholar] [CrossRef]
- Slade, A.; Isa, F.; Kyte, D.; Pankhurst, T.; Kerecuk, L.; Ferguson, J.; Lipkin, G.; Calvert, M. Patient reported outcome measures in rare diseases: A narrative review. Orphanet J. Rare Dis. 2018, 13, 61. [Google Scholar] [CrossRef]
- Anthoine, E.; Moret, L.; Regnault, A.; Sebille, V.; Hardouin, J.B. Sample size used to validate a scale: A review of publications on newly-developed patient reported outcomes measures. Health Qual. Life Outcomes 2014, 12, 2. [Google Scholar] [CrossRef]
- Rozenberg, D.; Al Kaabi, N.; Camacho Perez, E.; Nourouzpour, S.; Lopez-Hernandez, L.; McGillis, L.; Goligher, E.; Reid, W.D.; Chow, C.W.; Ryan, C.M.; et al. Evaluation and Management of Dyspnea in Hypermobile Ehlers-Danlos Syndrome and Generalized Hypermobility Spectrum Disorder: Protocol for a Pilot and Feasibility Randomized Controlled Trial. JMIR Res. Protoc. 2023, 12, e44832. [Google Scholar] [CrossRef] [PubMed]
- Henderson, F.C., Sr.; Austin, C.; Benzel, E.; Bolognese, P.; Ellenbogen, R.; Francomano, C.A.; Ireton, C.; Klinge, P.; Koby, M.; Long, D.; et al. Neurological and spinal manifestations of the Ehlers-Danlos syndromes. Am. J. Med. Genet. Part C Semin. Med. Genet. 2017, 175, 195–211. [Google Scholar] [CrossRef]
- Lohkamp, L.N.; Marathe, N.; Fehlings, M.G. Craniocervical Instability in Ehlers-Danlos Syndrome-A Systematic Review of Diagnostic and Surgical Treatment Criteria. Glob. Spine J. 2022, 12, 1862–1871. [Google Scholar] [CrossRef]
- Ikenouchi, H.; Takagi, M.; Nishimura, A.; Yamaguchi, E.; Koge, J.; Saito, K.; Toyoda, K.; Koga, M. Bilateral carotid artery dissection due to Eagle syndrome in a patient with vascular Ehlers-Danlos syndrome: A case report. BMC Neurol. 2020, 20, 285. [Google Scholar] [CrossRef] [PubMed]
- Badhey, A.; Jategaonkar, A.; Anglin Kovacs, A.J.; Kadakia, S.; De Deyn, P.P.; Ducic, Y.; Schantz, S.; Shin, E. Eagle syndrome: A comprehensive review. Clin. Neurol. Neurosurg. 2017, 159, 34–38. [Google Scholar] [CrossRef] [PubMed]
- Berglund, B.; Anne-Cathrine, M.; Randers, I. Dignity not fully upheld when seeking health care: Experiences expressed by individuals suffering from Ehlers-Danlos syndrome. Disabil. Rehabil. 2010, 32, 1–7. [Google Scholar] [CrossRef]
- Forsythe, L.P.; Szydlowski, V.; Murad, M.H.; Ip, S.; Wang, Z.; Elraiyah, T.A.; Fleurence, R.; Hickam, D.H. A systematic review of approaches for engaging patients for research on rare diseases. J. Gen. Intern. Med. 2014, 29 (Suppl. S3), S788–S800. [Google Scholar] [CrossRef]
| Disease-Related Terms | HRQL Terms | Measure-Related Terms | Other-Related Terms |
|---|---|---|---|
| Ehlers-Danlos Syndrome OR Ehlers-Danlos OR Hypermobility Syndrome OR Hypermobility Spectrum Disorder | Quality of Life OR Activities of Daily Living OR Functional Capacity OR Social Participation | Patient-reported Outcome Measures | Lived Experience OR English Language |
| Authors and Year | Aim(s) | Study Population | Study Design/Measures | Key Findings |
|---|---|---|---|---|
| Jeffery (2024) [35] | To explore the voice experience, singing ability, and well-being of singers diagnosed with HSD or hEDS | N = 276 completed the survey across Europe, America, Africa, and Australia including n = 71 professionally trained singers, age range = 18–60, 96% female Inclusion:
Exclusion:
| Mixed-method study Online surveys: written closed and open-ended questions
|
|
| Reina-Gutierrez (2023) [31] | To assess functional respiratory complaints and their relationship with mental health | N = 186 hEDS from Belgium; 57% were between 36 and 55 years, 86% female Inclusion:
Exclusion: Inability to read or comprehend questionnaires | Cross-sectional study Online surveys: emailed to participants
|
|
| Hakimi (2022) [36] | To explore lung function during exercise in hEDS patients | N = 12 hEDS from France; Mean age = 41 ± 14 years, 92% female Inclusion:
| Exploratory study Tests: Spirometry, incremental cardio-pulmonary exercise test, constant load exercise test
|
|
| De Baets (2022) [37] | To investigate differences in societal participation between EDS/G-HSD and healthy controls | N = 69 EDS/G-HSD (20 hEDS, 4 cEDS, 18 vEDS, 27 G-HSD), Mean age: 41 ± 17 years, 79% female Inclusion:
Exclusion:
| Retrospective case-control study Ghent Participation Scale—Societal participation (self-performed and delegated activities) |
|
| Ruiz Maya (2021) [38] | To assess the prevalence of dysautonomia, associated symptoms, and physical activity/exercise levels in EDS/G-HSD | N = 144 EDS/G-HSD; 42% hEDS 58% G-HSD Median age = 31 years, 94% female Inclusion:
Exclusion: Alternative genetic or rheumatologic diagnosis | Retrospective chart review study
|
|
| Simmonds (2019) [39] | To explore exercise perceptions, behaviours, and experiences with physiotherapy in EDS | N = 946 hEDS; 72.1% were between 18 and 40 years of age, 96% female Inclusion:
Exclusion:
| Cross-sectional study Open-ended questionnaires: Exercise barriers and thematic description of exercise beliefs |
|
| Schubart (2019) [40] | To identify phenotypic EDS subgroups with distinct symptom profiles | N = 175 EDS, Median age = 42 years, 7% female Inclusion:
| Exploratory study Data abstracted from the National Institute on Aging Intramural Research Program
|
|
| Krahe (2018) [41] | To investigate predictors of fatigue and its prevalence, severity, and impact in EDS | N = 117 EDS, Mean age = 35 ± 21 years, 94% female Inclusion:
Exclusion:
| Cross-sectional study Questionnaires:
| Fatigue predictors:
|
| Voermans et al. (2010) [8] | To measure fatigue, its clinical relevance, and possible determinants | N = 273 from Dutch patient organization, Mean age = 40.7, 89% female Inclusion:
Exclusion:
| Cross-sectional study Questionnaires:
|
|
| Morgan et al. (2007) [42] | To investigate respiratory manifestations in HSD/Benign Joint Hypermobility Syndrome (BJHS) and EDS | N = 126 BJHS N = 162 EDS Median age: 37 years, 78% female Inclusion:
| Cross-sectional study Questionnaire: St. George’s Respiratory Questionnaire Pulmonary function tests—respiratory manifestations and lung volumes |
|
| Berglund et al. (2000) [43] | To explore the daily life experiences of people with EDS | N = 10 EDS from Sweden, Age range = 21–67 years, 63% female Inclusion:
| Exploratory study Individual Interviews: Thematic description of daily life | Daily functioning:
|
| Identified Gaps in the Literature | Future Research Directions |
|---|---|
| Current research utilizes patient-reported outcome measures (PROMs) that are not disease specific and have not been validated in EDS or G-HSD. | Validation of respiratory and non-respiratory PROMs in EDS and G-HSD. |
| Development of respiratory and disease-specific PROMs and instruments for using both quantitative and qualitative methodologies to ensure symptoms and outcomes are appropriately evaluated. | |
| Lack of patient-centered approaches in guiding research studies and care. | Utilization of qualitative methodology to capture prioritized outcomes for individuals with EDS/G-HSD. |
| Identify respiratory symptom management needs and priorities for advancement of patient-centered care. | |
| Establish patient advisory boards and opportunities for knowledge dissemination meetings. | |
| Identify key research questions and priorities that have a significant impact on the EDS/G-HSD population. | |
| Respiratory symptom profile development and identification of symptom clusters given multi-morbidity. | Utilize qualitative and quantitative methods to characterize respiratory symptoms and recognition of symptom clusters. |
| Limited information on the mechanisms of respiratory symptoms and their relationship to key outcomes. | Investigate the pathophysiology of key respiratory symptoms (e.g., dyspnea) and assess the association of respiratory symptoms to key outcomes such as health-related quality of life. |
| The majority of respiratory research in EDS/G-HSD focuses on the physical domains of health. | Investigate the psychosocial domains related to respiratory manifestations in EDS/G-HSD, as part of comprehensive evaluation and symptom management. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaabi, N.A.; Camacho, E.; Orchanian-Cheff, A.; Silano, V.; McGillis, L.; Truong, W.T.; Reid, W.D.; Chow, C.-W.; Ryan, C.M.; Slepian, M.; et al. Respiratory Manifestations and Their Physical, Psychological, and Social Impacts in Ehlers-Danlos Syndromes and Generalized Hypermobility Spectrum Disorders: A Narrative Review. J. Clin. Med. 2025, 14, 4126. https://doi.org/10.3390/jcm14124126
Kaabi NA, Camacho E, Orchanian-Cheff A, Silano V, McGillis L, Truong WT, Reid WD, Chow C-W, Ryan CM, Slepian M, et al. Respiratory Manifestations and Their Physical, Psychological, and Social Impacts in Ehlers-Danlos Syndromes and Generalized Hypermobility Spectrum Disorders: A Narrative Review. Journal of Clinical Medicine. 2025; 14(12):4126. https://doi.org/10.3390/jcm14124126
Chicago/Turabian StyleKaabi, Noor Al, Encarna Camacho, Ani Orchanian-Cheff, Vanessa Silano, Laura McGillis, Wing Ting Truong, W. Darlene Reid, Chung-Wai Chow, Clodagh M. Ryan, Maxwell Slepian, and et al. 2025. "Respiratory Manifestations and Their Physical, Psychological, and Social Impacts in Ehlers-Danlos Syndromes and Generalized Hypermobility Spectrum Disorders: A Narrative Review" Journal of Clinical Medicine 14, no. 12: 4126. https://doi.org/10.3390/jcm14124126
APA StyleKaabi, N. A., Camacho, E., Orchanian-Cheff, A., Silano, V., McGillis, L., Truong, W. T., Reid, W. D., Chow, C.-W., Ryan, C. M., Slepian, M., Santa Mina, D., Clarke, H., Mittal, N., & Rozenberg, D. (2025). Respiratory Manifestations and Their Physical, Psychological, and Social Impacts in Ehlers-Danlos Syndromes and Generalized Hypermobility Spectrum Disorders: A Narrative Review. Journal of Clinical Medicine, 14(12), 4126. https://doi.org/10.3390/jcm14124126





