A Systematic Review and Meta-Analysis on the Efficacy and Safety of Concomitant Laparoscopic Cholecystectomy and Sleeve Gastrectomy in Patients with Morbid Obesity
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Measures
2.1.1. Primary Outcomes
2.1.2. Secondary Outcome
2.2. Inclusion and Exclusion Criteria
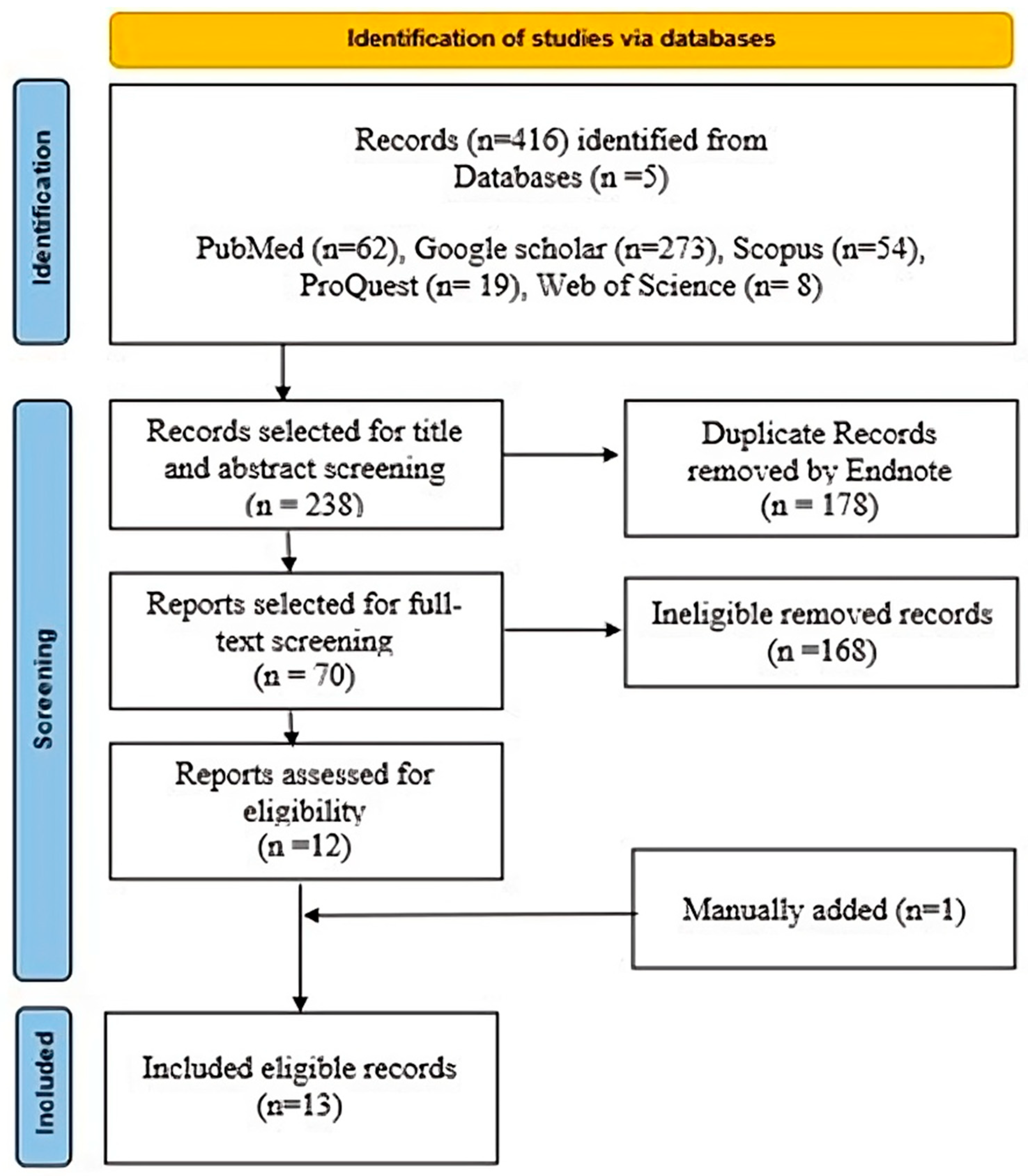
2.3. Search Strategy
2.4. Title and Abstract Screening
2.5. Full Text Screening
2.6. Data Extraction and Study Selection
2.7. Quality Assessment
2.8. Statistical Analysis
Heterogeneity Assessment
2.9. Sensitivity Analysis
2.10. Meta-Regression
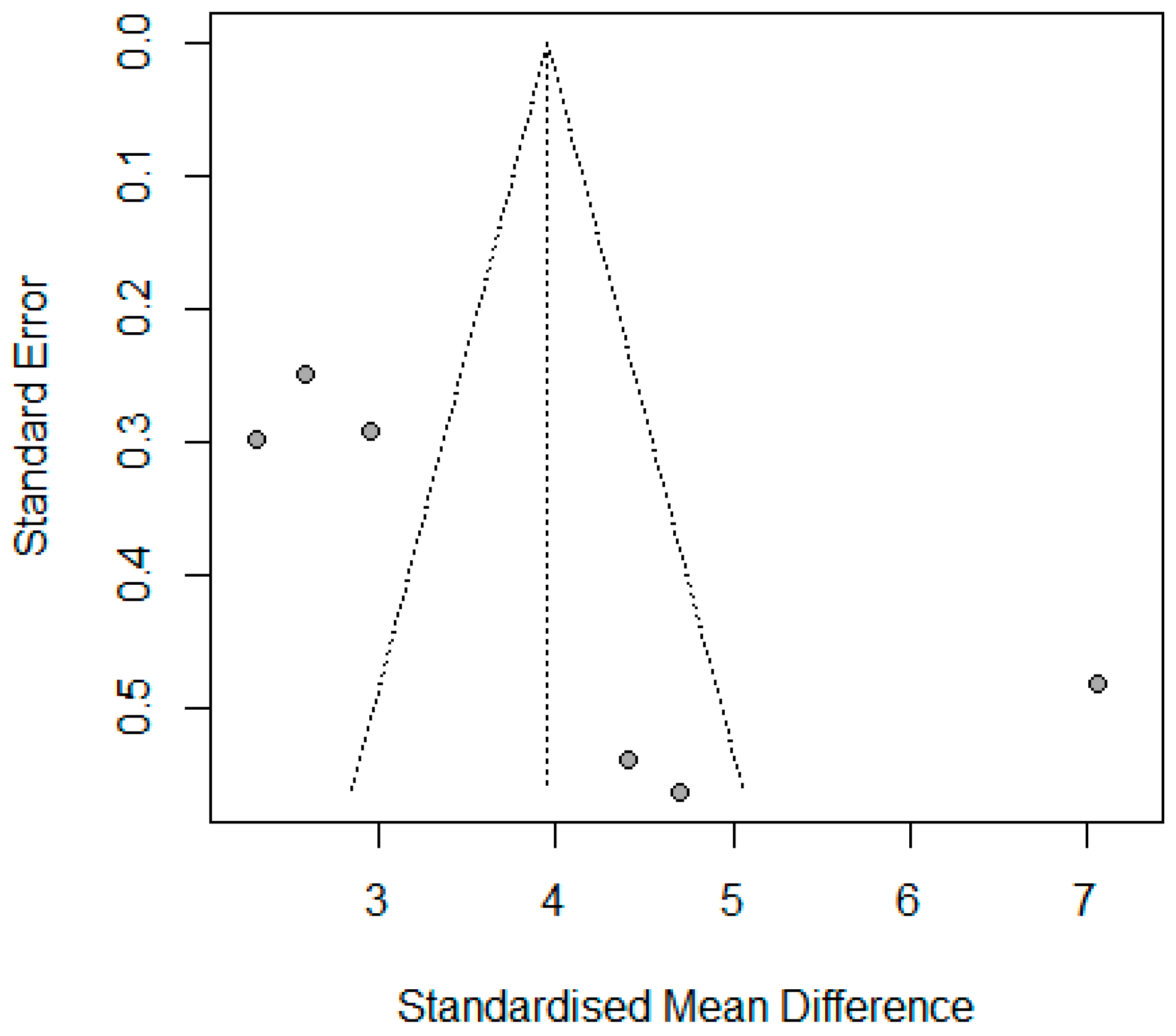
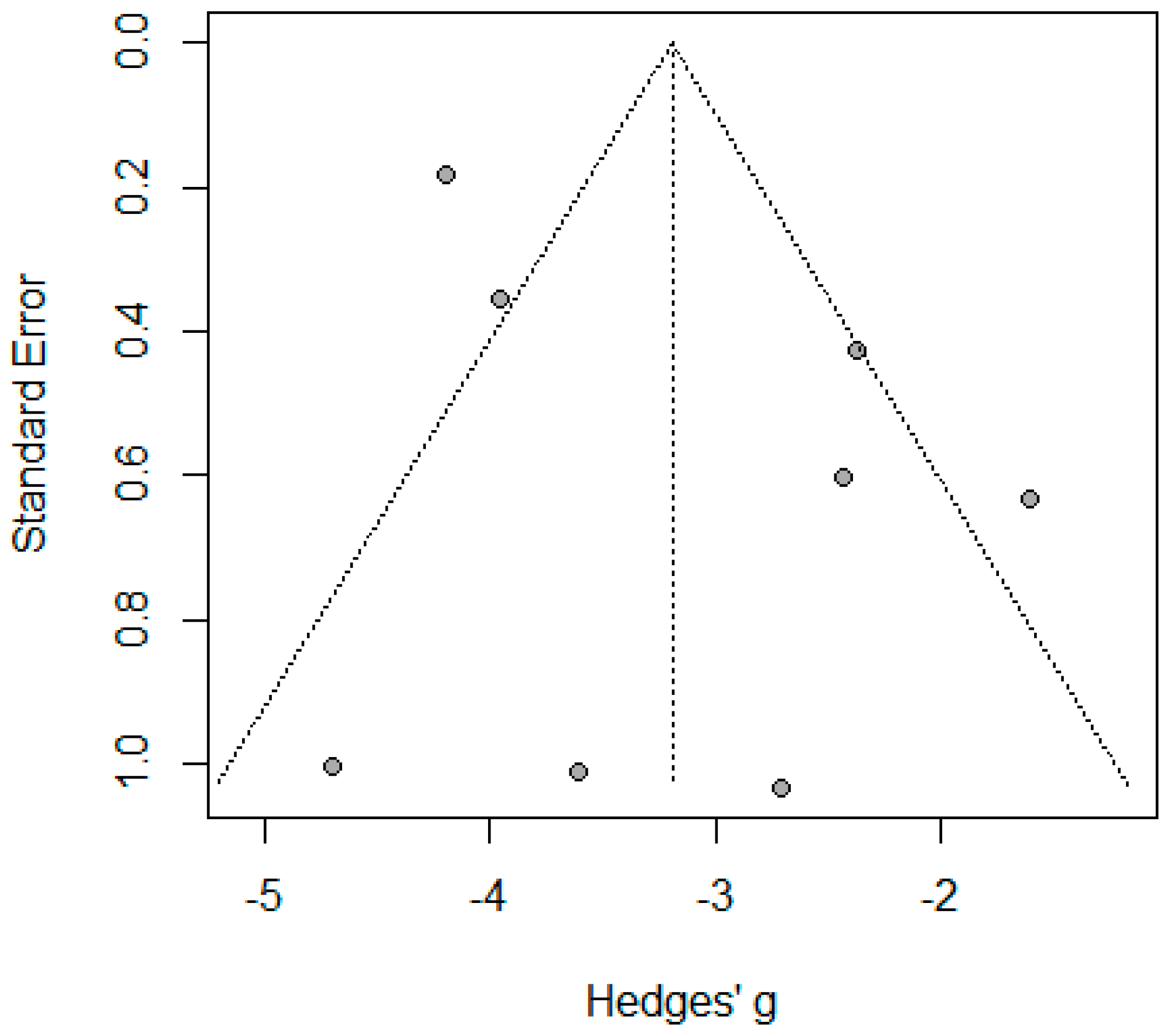
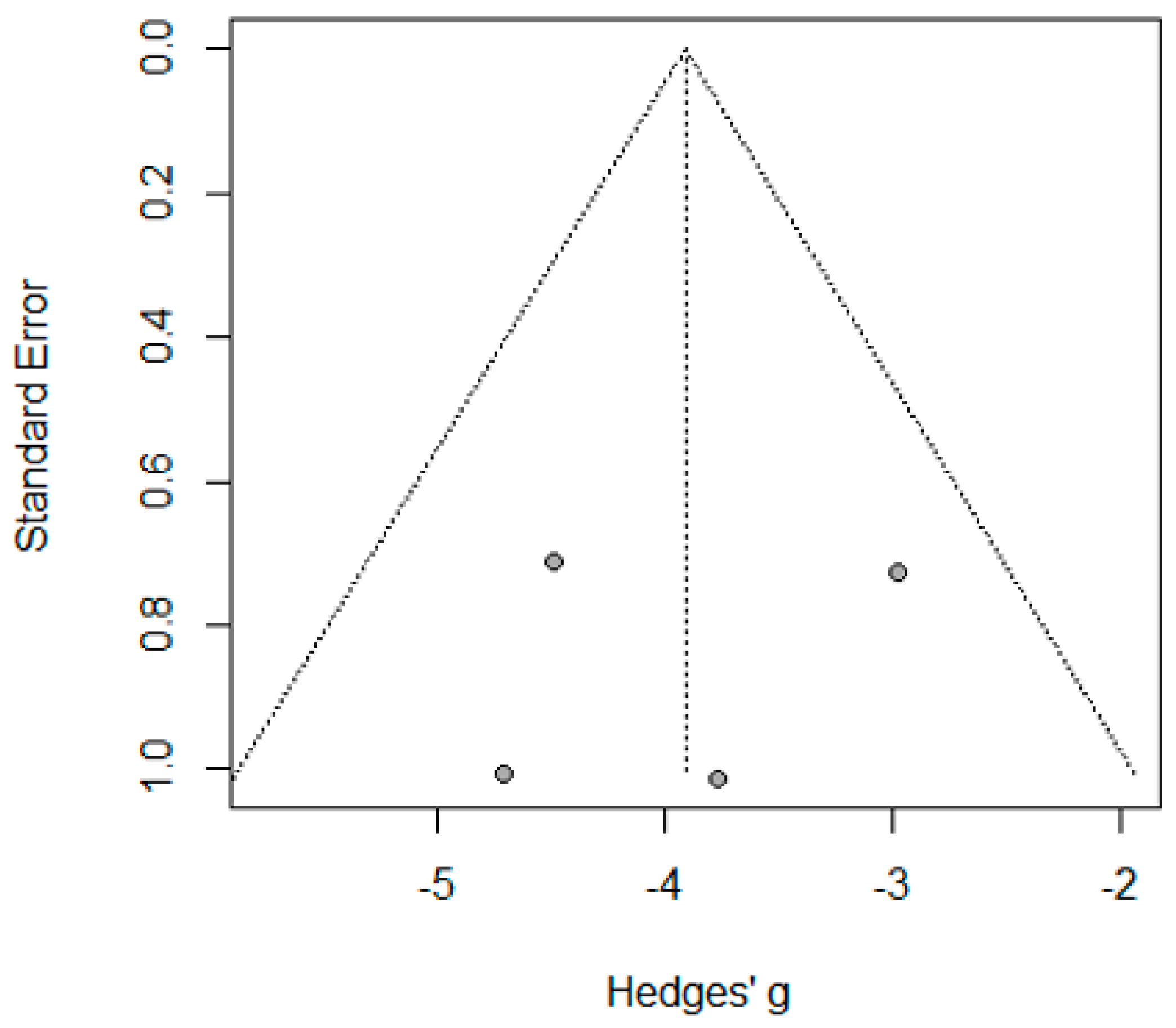
2.11. Publication Bias
3. Results
3.1. The Efficacy
BMI Standardized Mean Difference (SMD) Peri-Operation
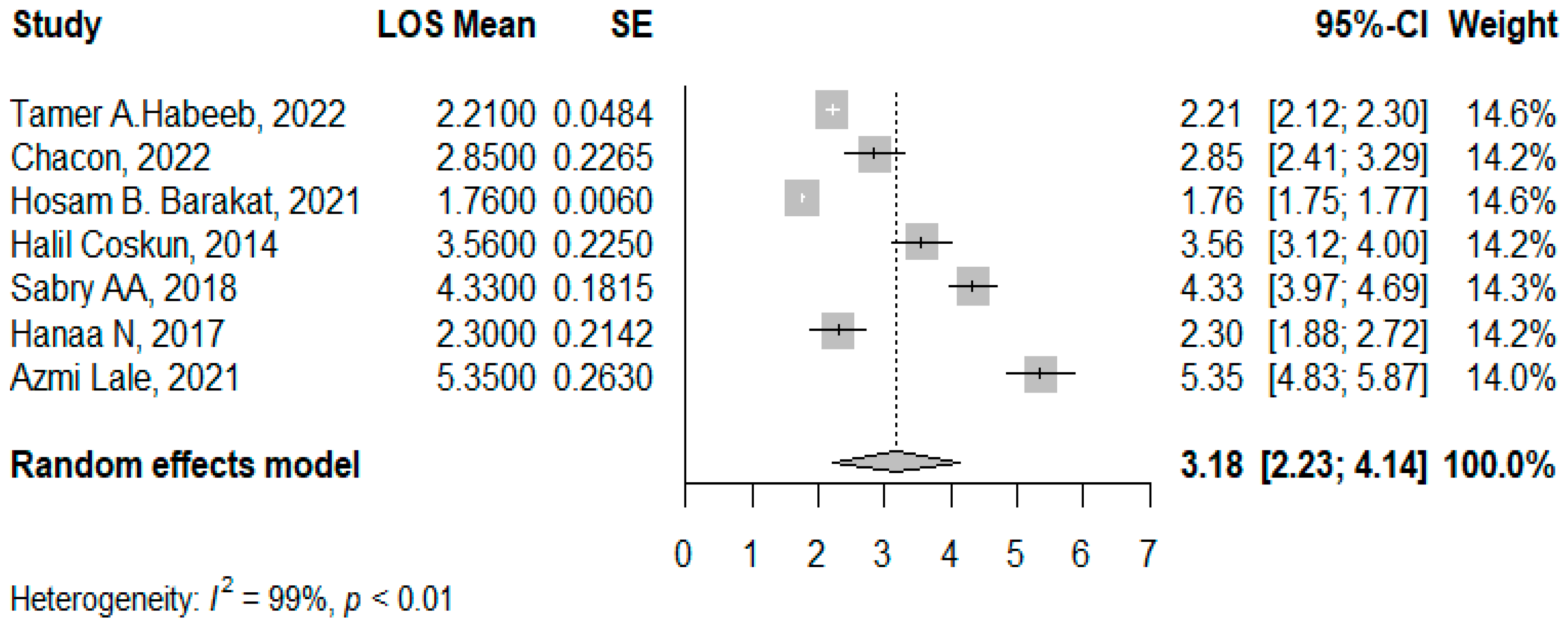
3.2. LOS Post the Concomitant Operations
3.3. Complications and Safety
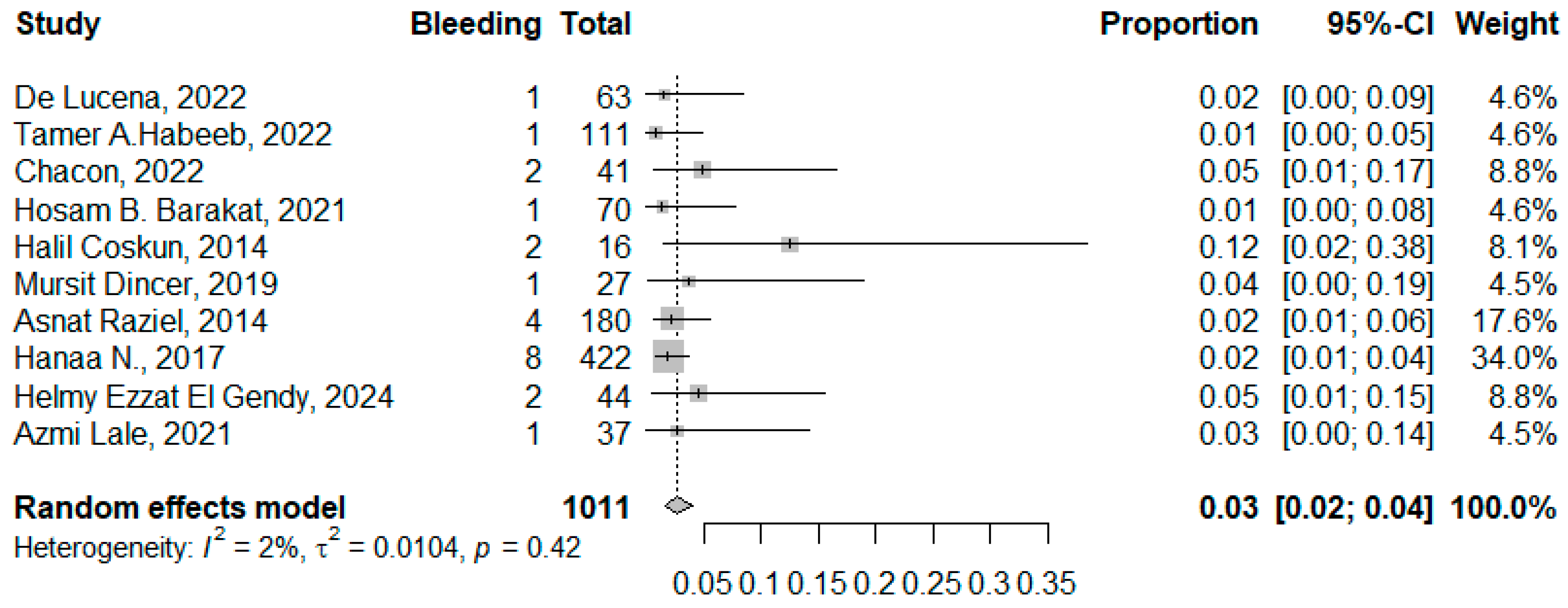
3.3.1. Bleeding
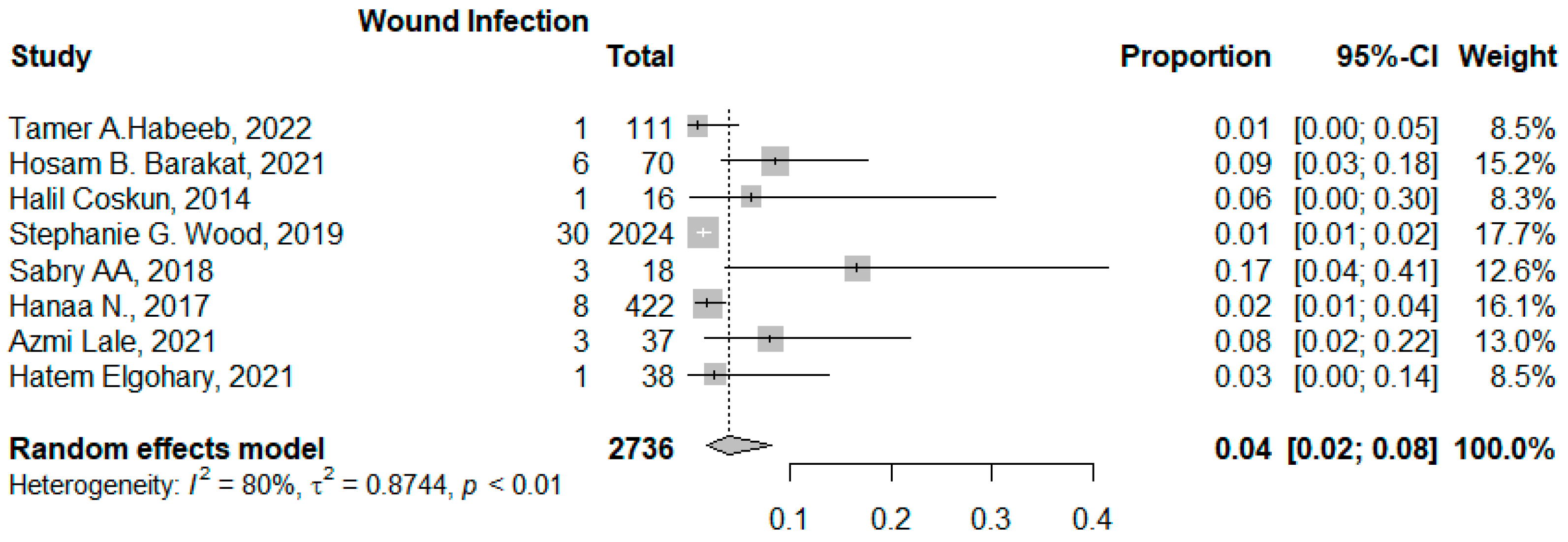
3.3.2. Wound Infection
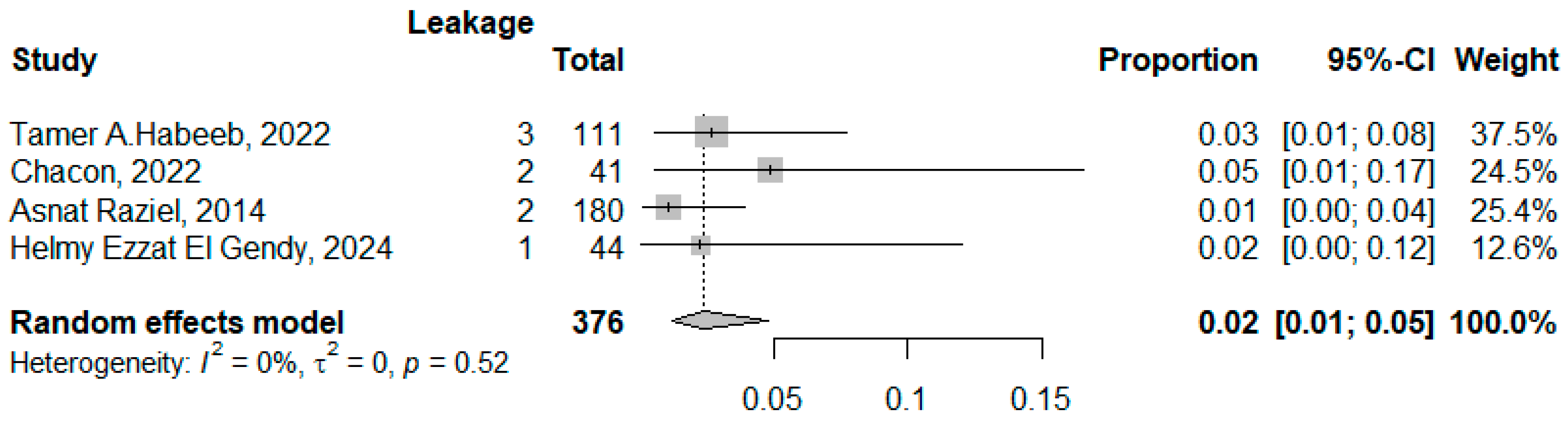
3.3.3. The Biliary/Gastric Leakage
4. Discussion
4.1. Effectiveness of Concurrent LC and SG
4.2. Postoperative Safety and Complications
4.3. Bleeding and Biliary/Gastric Leak
4.4. Length of Hospital Stay
4.5. Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Disclaimer
Abbreviations
| LC | Laparoscopic Cholecystectomy |
| SG | Sleeve Gastrectomy |
| BMI | Body Mass Index |
| LOS | Length of Stay |
| GD | Gallstone Disease |
| PI/ECO | Population, Intervention, Comparison, Outcome |
| RCT | Randomized Controlled Trial |
| RoB 2 | Risk of Bias Tool 2 (Cochrane tool for assessing risk of bias) |
| PRISMA | Preferred Reporting Items for Systematic Reviews and Meta-Analyses |
| MeSH | Medical Subject Headings |
| ASA | American Society of Anesthesiologists (classification) |
| SMD | Standardized Mean Difference |
| I2 | I-squared Statistic (heterogeneity measure) |
| SE | Standard Error |
| UDCA | Ursodeoxycholic Acid |
| IFSO | International Federation for the Surgery of Obesity and Metabolic Disorders |
| MBSAQIP | Metabolic and Bariatric Surgery Accreditation and Quality Improvement Program |
| GERD | Gastroesophageal Reflux Disease |
| CI | Confidence Interval |
References
- de Lucena, A.V.; Cordeiro, G.G.; Leão, L.H.; Kreimer, F.; de Siqueira, L.T.; da Conti Oliveira Sousa, G.; de Lucena, L.H.; Ferraz, Á.A. Cholecystectomy concomitant with bariatric surgery: Safety and metabolic effects. Obes. Surg. 2022, 32, 1093–1102. [Google Scholar] [CrossRef] [PubMed]
- Alimoğulları, M.; Buluş, H.J.S.T. Predictive factors of gallstone formation after sleeve gastrectomy: A multivariate analysis of risk factors. Surg. Today 2020, 50, 1002–1007. [Google Scholar] [CrossRef]
- Adams, L.B.; Chang, C.; Pope, J.; Kim, Y.; Liu, P.; Yates, A. Randomized, prospective comparison of ursodeoxycholic acid for the prevention of gallstones after sleeve gastrectomy. Obes. Surg. 2016, 26, 990–994. [Google Scholar] [CrossRef]
- Abo-Ryia, M.H.; Abd-Allah, H.S.; El-Khadrawy, O.H.; Moussa, G.I. Predictors of gallstone formation in morbidly obese patients after bariatric surgery: A retrospective observational study. Surg. Sci. 2014, 5, 1–5. [Google Scholar] [CrossRef]
- Sattaratnamai, A.; Samankatiwat, N.; Udomsawaengsup, S.; Ratchaburi Hospital; King Chulalongkorn Memorial Hospital. Subsequent Cholecystectomy Rate After Bariatric Surgery in Morbid Obesity Patients: A Systematic Review and Meta-Analysis; SAGE: Newcastle upon Tyne, UK, 2015.
- Amorim-Cruz, F.; Santos-Sousa, H.; Ribeiro, M.; Nogueiro, J.; Pereira, A.; Resende, F.; Costa-Pinho, A.; Preto, J.; Lima-Da-Costa, E.; Sousa-Pinto, B. Risk and prophylactic management of gallstone disease in bariatric surgery: A systematic review and a Bayesian meta-analysis. J. Gastrointest. Surg. 2023, 27, 433–448. [Google Scholar] [CrossRef]
- Manatsathit, W.; Leelasinjaroen, P.; Al-Hamid, H.; Szpunar, S.; Hawasli, A. The incidence of cholelithiasis after sleeve gastrectomy and its association with weight loss: A two-centre retrospective cohort study. Int. J. Surg. 2016, 30, 13–18. [Google Scholar] [CrossRef] [PubMed]
- Wood, S.G.; Kumar, S.B.; Dewey, E.; Lin, M.Y.; Carter, J.T. Safety of concomitant cholecystectomy with laparoscopic sleeve gastrectomy and gastric bypass: A MBSAQIP analysis. Surg. Obes. Relat. Dis. 2019, 15, 864–870. [Google Scholar] [CrossRef]
- Elgohary, H.; El Azawy, M.; Elbanna, M.; Elhossainy, H.; Omar, W. Concomitant versus delayed cholecystectomy in bariatric surgery. J. Obes. 2021, 2021, 9957834. [Google Scholar] [CrossRef] [PubMed]
- Habeeb, T.A.A.M.; Kermansaravi, M.; Giménez, M.E.; Manangi, M.N.; Elghadban, H.; Abdelsalam, S.A.; Metwalli, A.M.; Baghdadi, M.A.; Sarhan, A.A.; Moursi, A.M.; et al. Sleeve gastrectomy and cholecystectomy are safe in obese patients with asymptomatic cholelithiasis. A Multicenter Randomized Trial. World J. Surg. 2022, 46, 1721–1733. [Google Scholar] [CrossRef]
- Barakat, H.B.; El-Sherpiny, W.Y.; Ghazaly, M.; Elmahdy, T.M. Concomitant cholecystectomy during laparoscopic sleeve gastrectomy through the same four ports: Feasibility and early results. Egypt. J. Surg. 2021, 40, 509–514. [Google Scholar]
- Coskun, H.; Hasbahceci, M.; Bozkurt, S.; Cipe, G.; Malya, F.U.; Memmi, N.; Karatepe, O.; Akcakaya, A.; Muslumanoglu, M. Is concomitant cholecystectomy with laparoscopic sleeve gastrectomy safe. Turk. J. Gastroenterol. 2014, 25, 624–627. [Google Scholar] [CrossRef] [PubMed]
- Iannelli, A.; Treacy, P.; Sebastianelli, L.; Schiavo, L.; Martini, F. Perioperative complications of sleeve gastrectomy: Review of the literature. J. Minimal Access Surg. 2019, 15, 1–7. [Google Scholar]
- Dincer, M.; Doğan, F.J.V.; Techniques, O.M. The effect of concomitant cholecystectomy and sleeve gastrectomy on morbidity in high-risk obese patients with symptomatic gallstones. Videosurgery Other Miniinvasive Tech. 2019, 14, 237–241. [Google Scholar] [CrossRef] [PubMed]
- Cochrane Handbook for Systematic Reviews of Interventions. Available online: https://training.cochrane.org/handbook (accessed on 15 January 2025).
- PRISMA 2020 Checklist. Available online: https://www.prisma-statement.org/prisma-2020-checklist (accessed on 15 January 2025).
- Cochrane Methods Bias. RoB 2: A Revised Cochrane Risk-of-Bias Tool for Randomized Trials. Available online: https://methods.cochrane.org/bias/resources/rob-2-revised-cochrane-risk-bias-tool-randomized-trials (accessed on 15 January 2025).
- Stang, A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur. J. Epidemiol. 2010, 25, 603–605. [Google Scholar] [CrossRef]
- Higgins, J.P. Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.1; The Cochrane Collaboration: London, UK, 2008; Available online: http://www.cochrane-handbook.org (accessed on 15 January 2025).
- Baker, W.L.; White, C.M.; Cappelleri, J.C.; Kluger, J.; Coleman, C.I.; on behalf of the Health Outcomes, Policy, and Economics (HOPE) Collaborative Group. Understanding heterogeneity in meta-analysis: The role of meta-regression. Int. J. Clin. Pract. 2009, 63, 1426–1434. [Google Scholar]
- Higgins, J.P.T.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.J.; Welch, V.A. Cochrane Handbook for Systematic Reviews of and N. Interventions Version 6.3; John Wiley & Sons: Hoboken, NJ, USA, 2022. [Google Scholar]
- El Gendy, H.E.; Asar, H.E.H.H.; Abuzeid, M.M.M. Feasibility and safety of concomitant laparoscopic cholecystectomy and sleeve gastrectomy: A retrospective study. Egypt. J. Hosp. Med. 2024, 96, 3014–3018. [Google Scholar]
- Lale, A.; Yur, M.; Do Aygen, E. Safety of the concomitant cholecystectomy during laparoscopic sleeve gastrectomy in patients with symptomatic gallstone: A single-center experience. Laparosc. Endosc. Surg. Sci. 2021, 28, 44. [Google Scholar] [CrossRef]
- Raziel, A.; Sakran, N.; Szold, A.; Goitein, D. Concomitant cholecystectomy during laparoscopic sleeve gastrectomy. Surg. Endosc. 2015, 29, 2789–2793. [Google Scholar] [CrossRef]
- Chacón, C.G.P.; Vilallonga, R.; López, Ó.G.; de Gordejuela, A.G.R.; Beisani, M.; Busquet, E.C.; Fort, J.M.; Carrasco, M.A. Analysis of the management of cholelithiasis in bariatric surgery patients: A single-center experience. Obes. Surg. 2022, 32, 704–711. [Google Scholar] [CrossRef]
- Dakour-Aridi, H.N.; El-Rayess, H.M.; Abou-Abbass, H.; Abu-Gheida, I.; Habib, R.H.; Safadi, B.Y. Safety of concomitant cholecystectomy at the time of laparoscopic sleeve gastrectomy: Analysis of the American College of Surgeons National Surgical Quality Improvement Program database. Surg. Obes. Relat. Dis. 2017, 13, 934–941. [Google Scholar] [CrossRef]
- Barakat, H.; Hassan, A.; Elsheikh, M.; Abdelhamid, A. Laparoscopic single anastomosis sleeve ileal bypass in the surgical management of morbid obesity: A single-centre experience. Surg. Pract. 2024, 28, 68–75. [Google Scholar] [CrossRef]
- Sabry, A.A.; Alkarmouty, A.F. Cholecystectomy during laparoscopic sleeve gastrectomy in morbidly obese patients. Int. J. Adv. Res. 2018, 6, 1042–1045. [Google Scholar]
- Shaffer, E.A. Gallstone disease: Epidemiology of gallbladder stone disease. Best Pract. Res. Clin. Gastroenterol. 2006, 20, 981–996. [Google Scholar] [CrossRef]
- Gatta, D.R.; Huidobro, L.; Petermann-Rocha, F.; Van de Wyngard, V.; Godoy, F.; Cid, V.; Garrido, M.; Cook, P.; Roa, J.C.; Vargas, C.; et al. Sex disparities in gallstone disease: Insights from the MAUCO prospective population-based cohort study. BMJ Open Gastroenterol. 2024, 11, e001457. [Google Scholar] [CrossRef]
- Fobi, M.; Lee, H.; Igwe, D.; Felahy, B.; James, E.; Stanczyk, M.; Fobi, N. Prophylactic cholecystectomy with gastric bypass operation: Incidence of gallbladder disease. Obes. Surg. 2002, 12, 350–353. [Google Scholar] [CrossRef]
- Worni, M.; Guller, U.; Shah, A.; Gandhi, M.; Shah, J.; Rajgor, D.; Pietrobon, R.; Jacobs, D.O.; Østbye, T. Cholecystectomy concomitant with laparoscopic gastric bypass: A trend analysis of the nationwide inpatient sample from 2001 to 2008. Obes. Surg. 2012, 22, 220–229. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wudel, L.; Wright, J.; Debelak, J.P.; Allos, T.M.; Shyr, Y.; Chapman, W.C. Prevention of Gallstone Formation in Morbidly Obese Patients Undergoing Rapid Weight Loss: Results of a Randomized Controlled Pilot Study. J. Surg. Res. 2002, 102, 50–56. [Google Scholar] [CrossRef]
- Chang, J.; Corcelles, R.; Boules, M.; Jamal, M.H.; Schauer, P.R.; Kroh, M.D. Predictive factors of biliary complications after bariatric surgery. Surg. Obes. Relat. Dis. 2016, 12, 1706–1710. [Google Scholar] [CrossRef]
- Talha, A.; Abdelbaki, T.; Farouk, A.; Hasouna, E.; Azzam, E.; Shehata, G. Cholelithiasis after bariatric surgery, incidence, and prophylaxis: Randomized controlled trial. Surg. Endosc. 2020, 34, 5331–5337. [Google Scholar] [CrossRef]
- Şen, O.; Türkçapar, A.G.; Yerdel, M.A. Cholelithiasis After Sleeve Gastrectomy and Effectiveness of Ursodeoxycholic Acid Treatment. J. Laparoendosc. Adv. Surg. Tech. 2020, 30, 1150–1152. [Google Scholar] [CrossRef]
- Magouliotis, D.E.; Tasiopoulou, V.S.; Svokos, A.A.; Svokos, K.A.; Chatedaki, C.; Sioka, E.; Zacharoulis, D. Ursodeoxycholic Acid in the Prevention of Gallstone Formation After Bariatric Surgery: An Updated Systematic Review and Meta-analysis. Obes. Surg. 2017, 27, 3021–3030. [Google Scholar] [CrossRef] [PubMed]
- Morais, M.; Faria, G.; Preto, J.; Costa-Maia, J. Gallstones and Bariatric Surgery: To Treat or Not to Treat? World J. Surg. 2016, 40, 2904–2910. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]



| Study | Design | Sample Size | Inclusion and Exclusion Criteria | Study Quality Score |
|---|---|---|---|---|
| Asnat Raziel, 2014 [24] Israel | Retrospective cohort | 180 |
| 5 (Moderate) |
| Azmi Lale, 2021 [23] Turkey | Retrospective cohort | 37 |
| 8 (High) |
| Chacón, 2022 [25] Spain | Retrospective cohort study | 41 |
| 8 (High) |
| de Lucena, 2022 [1] Brazil | Retrospective cohort | 63 |
| 8 (High) |
| Halil Coşkun, 2014 [12] Turkey | Case-control | 16 |
| 7 (High) |
| Hanaa N.i, 2017 [26] American database | Retrospective cohort | 422 |
| 8 (High) |
| Hatem Elgohary, 2021 [9] Egypt | Prospective cohort | 38 |
| 7 (High) |
| Helmy Ezzat El Gendy, 2024 [22] Egypt | Retrospective cohort | 44 |
| 6 (Moderate) |
| Hosam B. Barakat, 2021 [27] Egypt | Prospective cohort | 70 |
| 6 (Moderate) |
| Mürşit Dincer, 2019 [14] Turkey | Retrospective cohort | 27 |
| 5 (Moderate) |
| Sabry AA, 2018 [28] Egypt | Prospective cohort | 18 |
| 5 (Moderate) |
| Stephanie G. Wood, 2019 [8] United States and Canada | Retrospective cohort | 2024 |
| 8 (High) |
| Tamer A. Habeeb, 2022 [10] Egypt | Multicenter randomized trial | 111 |
| High quality (low RoB) |
| Study | Initial BMI | Follow-Up Period | Comorbidities | Operative Time (min) | Age (Mean ± SD) Median (IQR) Age Group | Gender N (%) |
|---|---|---|---|---|---|---|
| Asnat Raziel, 2014 [24] Israel | 43.1 | 28 months | Previous cholelithiasis 180 (100%) | 46 | Female 79% | |
| Azmi Lale, 2021 [23] Turkey | 45.5 ± 5.9 | 17.5 ± 8.1 months | Hypertension 7 (18.9%) Diabetes mellitus 10 (27.0%) Dyslipidemia 6 (16.2%) Heart diseases 2 (5.4%) Others 8 (21.6%) | 82.7 ± 19.6 | 38.6 ± 9.3 | 4 Males (5.5%) 33 Females (18.5%) |
| Chacón, 2022 [25] Spain | - | - | Previous cholelithiasis 12 (15.58%) | 152.44 ± 46.45 | 45.30 ± 10.49 | |
| de Lucena, 2022 [1] Brazil | 40.82 ± 4.47 | 2.95 ± 2.37 months | Hypertension 34 (54.0) Diabetes mellitus 10 (15.9) Dyslipidemia 24 (38.1) | - | Age group (16–29) 12 (19.0%) (30–39) 17 (27.0%) (40–49) 21 (33.3%) (50–67) 13 (20.6%) | Male 6 (9.5%) Female 57 (90.5%) |
| Halil Coşkun, 2014 [12] Turkey | 51.1 ± 5.6 | - | - | 157.2 ± 40.0 | 39.6 ± 10.2 | Male 1 (6.25%) Female 15 (93.75%) |
| Hanaa N.i, 2017 [26] American database | 46.5 ± 7.6 | 30-day | Hypertension 202 (47.9) Diabetes mellitus 95 (22.5%) Dyspnea 56 (13.2%) Current smoker 38 (9%) Others 24 (5.6%) | 128.2 ± 53.9 | 45.2 ± 11.1 | Male 80 (19%) Females 342 (81%) |
| Hatem Elgohary, 2021 [9] Egypt | 48.21 ± 8.57 | 12 months | Hypertension 7 (18.4%) Diabetes mellitus 4 (10.5%) Dyslipidemia 11 (28.9%) | 84.19 ± 19.62 | 37.65 ± 10.15 | Male 7 (18.4%) Female 31 (81.6%) |
| Helmy Ezzat el Gendy, 2024 [22] Egypt | 50.10 ± 9.91 | 6 months | - | >60 to 120 (59.1%) >120–180 (29.5%) ≤60 min (4.5%) >180 to 240 (4.5%) >240 to 300 (2.3%) | 36.48 ± 10.62 | Male 3 (6.8%) Female 41 (93.2%) |
| Hosam B. Barakat, 2021 [11] Egypt | 46.2 ± 9.95 | 2 years | Hypertension 8 (11.4%) Diabetes mellitus 6 (8.6%) Dyslipidemia 33 (25.7%) Previous cholelithiasis 70 (100%) Obstructive sleep apnea 2 (2.8%) Others 17 (24.3%) | 76.82 ± 17.22 min | 42.44 ± 10.32 | Female 61 (87%) Male 9 (13%) |
| Mürşit Dincer, 2019 [14] Turkey | 42.9 (40.8–47.5) | - | Hypertension 6 (22.22%) Diabetes mellitus 15 (55.56%) GERD 3 (11.11%) Obstructive sleep apnea 1 (3.7%) Others 6 (22.22%) | 65.7 ± 8.5 | 40.7 ± 8.2 | Male 5 (18.5%) Female 22 (81.5%) |
| Sabry AA, 2018 [28] Egypt | 43.42 ± 2.57 | 30 days | - | 151.56 ± 21.11 | 32.72 ± 6.25 | Male 3 (16.7%) Females 15 (83.3%) |
| Stephanie G. Wood, 2019 [8] United States and Canada | 44.9 (7.9) | 30 days | Hypertension 978 (48%) Diabetes mellitus 422 (21%) Dyslipidemia 419 (21%) GERD 602 (30%) Obstructive sleep apnea 658 (32%) Others 428 (21.14%) | 103.7 (46.2) | 45.2 (12) | Females 1710 (84.5%) males 314 (15.5%) |
| Tamer A. Habeeb, 2022 [10] Egypt | 42.7 ± 2.92 | 2 years | Hypertension 17 (15%) Diabetes mellitus 30 (27%) | 50.13 ± 1.99 | Age group (18–35) 34 (30.5%) (36–45) 69 (62%) >45 8 (7.5%) | Male 21 (19%) Female 90 (81%) |
| Study | BMI Difference (Peri-Operation After One Year of the Operation) | Bleeding | Wound Infection | Leakage (Biliary/Gastric) | Other Complications | Length of Stay (Days) |
|---|---|---|---|---|---|---|
| Asnat raziel, 2014 [24] Israel | - | 4 (2.22%) | - | 2 (1.11%) | Incisional hernia 4 (2.2%) Others 3 (1.6%) | 2 days |
| Azmi lale, 2021 [23] Turkey | 92.7 ± 21.0 | 1 (2.7%) | 3 (8.1%) | 1 (1.6%) | Blood transfusion 1 (2.7%) Portal venous embolism: 2 (5.4%) Others 2 (5.4%) | 5.35 ± 1.6 |
| Chacón, 2022 [25] * Spain | 58.30 ± 25.26 | 2 (4.8%) | - | 2 (4.8%) | - | 2.85 ± 1.45 |
| de Lucena, 2022 [1] Brazil | 28.15 ± 9.55 | 1 (1.4%) | - | - | - | - |
| Halil Coşkun, 2014 [12] Turkey | - | 2 (12.5%) | 1 (6.25%) | - | - | 3.56 ± 0.9 |
| Hanaa N.i, 2017 [26] American database | - | 8 (1.9%) | 8 (1.9%) | - | Venous thromboembolism 2 (0.47%) Sepsis/septic shock 2 (0.5%) Progressive renal insufficiency 24 (5.7%) Others 10 (2.3%) | 2.3 ± 4.4 |
| Hatem Elgohary, 2021 [9] Egypt | 78.04 ± 16.6 | 6 (15.8%) | 1 (2.6%) | - | Others 1 (2.6%) | - |
| Helmy ezzat el gendy, 2024 [22] Egypt | - | 2 (4.5%) | Postoperative gastric leakage 1 (2.3%) | Intraabdominal collection 1 (2.3%) Extensive tissue adhesions 2 (2.3%) Postoperative subcutaneous collection1 (2.3%) | 2 | |
| Hosam B. Barakat, 2021 [11] Egypt | 62.48 ± 5.12 | 1 (1.4%) | 6 (8.5%) | - | Incisional hernia 2 (2.8%) Intraabdominal collection 1 (1.4%) Others 2 (2.8%) | 1.76 ± 0.05 |
| Mürşit dincer, 2019 [14] Turkey | - | 1 (3.7%) | - | - | Tissue adhesion 22 (81.48) | 4 (3–4) |
| Sabry AA, 2018 Egypt | - | 4 (22.2%) | 2 (11%) | - | Intraabdominal collection 1 (5.5%) Gall bladder rupture 3 (16.7%) Others 13 (72.2%) | 4.33 ± 0.77 |
| Stephanie G. Wood, 2019 [8] United States and Canada | - | - | 30 (1.5%) | - | Reoperation 33 (1.6%) Blood transfusion 10 (0.5%) Others = 3518 | 1.9 (1.8) |
| Tamer A. Habeeb, 2022 [10] Egypt | 24.63 ± 1.26 | 1 (1.6%) | 1 (1.6%) | 1 (1.6%) | Reoperation 2 (3.2%) Incisional hernia 2 (3.6%) | 2.21 ± 0.51 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abd El Maksoud, W.M.; Abbas, K.S.; Al Amri, F.S.; Alzahrani, H.A.; Dalboh, A.; Alshandeer, M.H.; Alghamdi, M.A.; Yahya, F.H.; Bawahab, A.M.; Fayed, H.M.; et al. A Systematic Review and Meta-Analysis on the Efficacy and Safety of Concomitant Laparoscopic Cholecystectomy and Sleeve Gastrectomy in Patients with Morbid Obesity. J. Clin. Med. 2025, 14, 4108. https://doi.org/10.3390/jcm14124108
Abd El Maksoud WM, Abbas KS, Al Amri FS, Alzahrani HA, Dalboh A, Alshandeer MH, Alghamdi MA, Yahya FH, Bawahab AM, Fayed HM, et al. A Systematic Review and Meta-Analysis on the Efficacy and Safety of Concomitant Laparoscopic Cholecystectomy and Sleeve Gastrectomy in Patients with Morbid Obesity. Journal of Clinical Medicine. 2025; 14(12):4108. https://doi.org/10.3390/jcm14124108
Chicago/Turabian StyleAbd El Maksoud, Walid M., Khaled S. Abbas, Fahad S. Al Amri, Hassan A. Alzahrani, Abdullah Dalboh, Marei H. Alshandeer, Maha A. Alghamdi, Fadhl H. Yahya, Abdullrahman M. Bawahab, Haytham M. Fayed, and et al. 2025. "A Systematic Review and Meta-Analysis on the Efficacy and Safety of Concomitant Laparoscopic Cholecystectomy and Sleeve Gastrectomy in Patients with Morbid Obesity" Journal of Clinical Medicine 14, no. 12: 4108. https://doi.org/10.3390/jcm14124108
APA StyleAbd El Maksoud, W. M., Abbas, K. S., Al Amri, F. S., Alzahrani, H. A., Dalboh, A., Alshandeer, M. H., Alghamdi, M. A., Yahya, F. H., Bawahab, A. M., Fayed, H. M., Bosaily, A. J. M., & Bawahab, M. A. (2025). A Systematic Review and Meta-Analysis on the Efficacy and Safety of Concomitant Laparoscopic Cholecystectomy and Sleeve Gastrectomy in Patients with Morbid Obesity. Journal of Clinical Medicine, 14(12), 4108. https://doi.org/10.3390/jcm14124108





