Lamotrigine Therapy: Relation Between Treatment of Bipolar Affective Disorder and Incidence of Stevens–Johnson Syndrome—A Narrative Review of the Existing Literature
Abstract
1. Introduction
What Is Bipolar Affective Disorder and Its Potential Hazards?
2. Lamotrigine—A New Option in the Prevention of Depressive Episodes
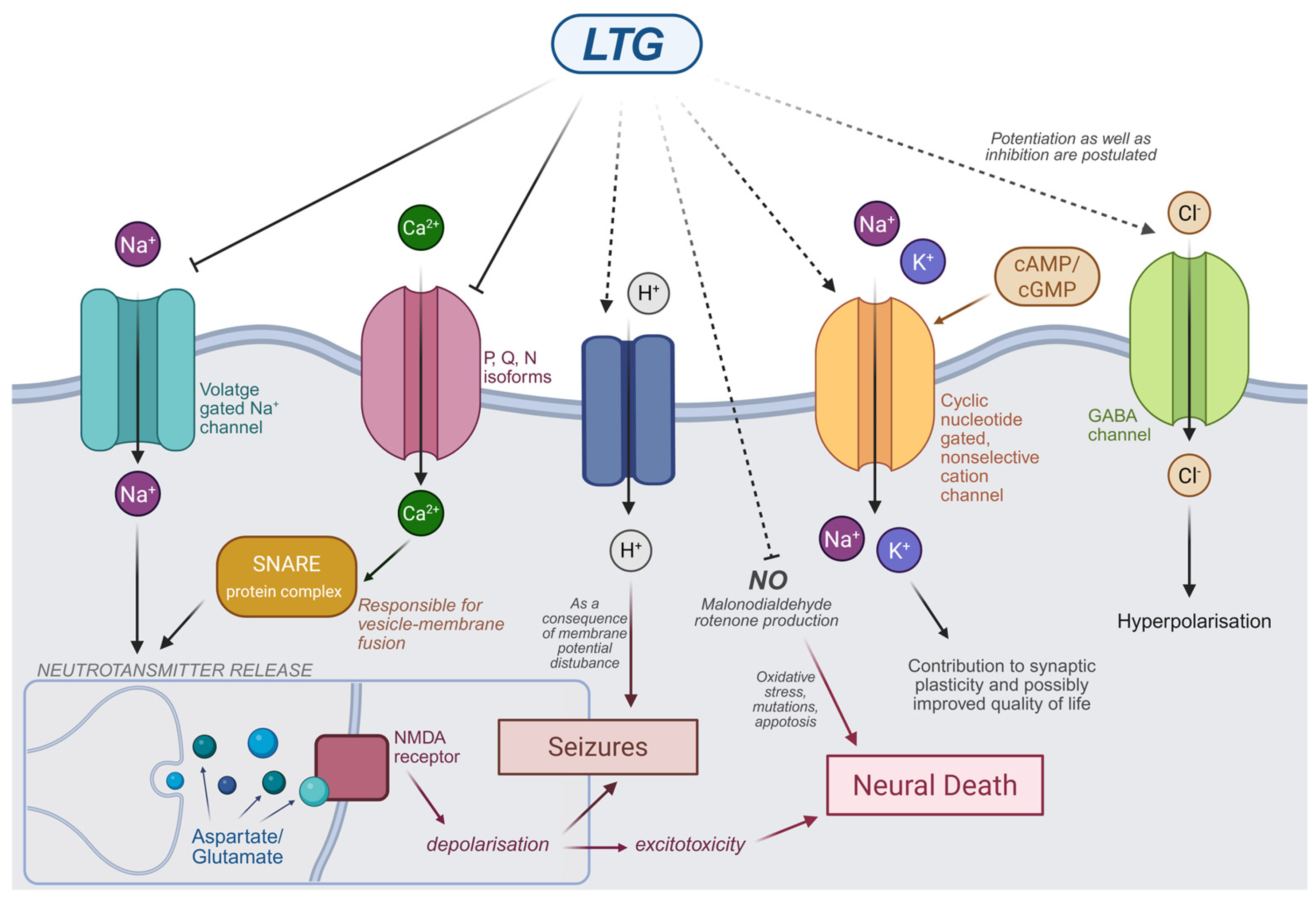
2.1. Mechanism of Action of LTG
2.2. Pharmacokinetics of Lamotrigine
2.3. Prevalence of Lamotrigine Use in Medical Treatment
2.3.1. Therapeutic Application of Lamotrigine in the Management of Epilepsy
2.3.2. Therapeutic Application of Lamotrigine in the Management of Bipolar Affective Disorder
2.3.3. Therapeutic Application of Lamotrigine in the Management of Personality Disorders
2.3.4. Off-Label Treatment with Lamotrigine
3. Principles of Pharmacotherapy in Bipolar Disorder (BD)
3.1. Lamotrigine Monotherapy
3.2. Combination Therapy as a Response to Clinical Diversity
3.3. Combination Therapy with Lithium
3.4. Combination Therapy with Valproic Acid
3.5. Combination Therapy with Antidepressants
4. Dosage, Serum Concentration and Therapeutic Efficacy
4.1. Monotherapy or Combination Therapy with Non-Inducers/Inhibitors of Glucuronidation
4.2. Combination Therapy with Valproate
4.3. Combination Therapy with Carbamazepine
4.4. Therapeutic Dose Ranges and Efficacy
4.5. Lamotrigine Side Effects
4.6. Incidence of SJS as an Adverse Effect of LTG Administration
5. Stevens–Johnson Syndrome Characteristics
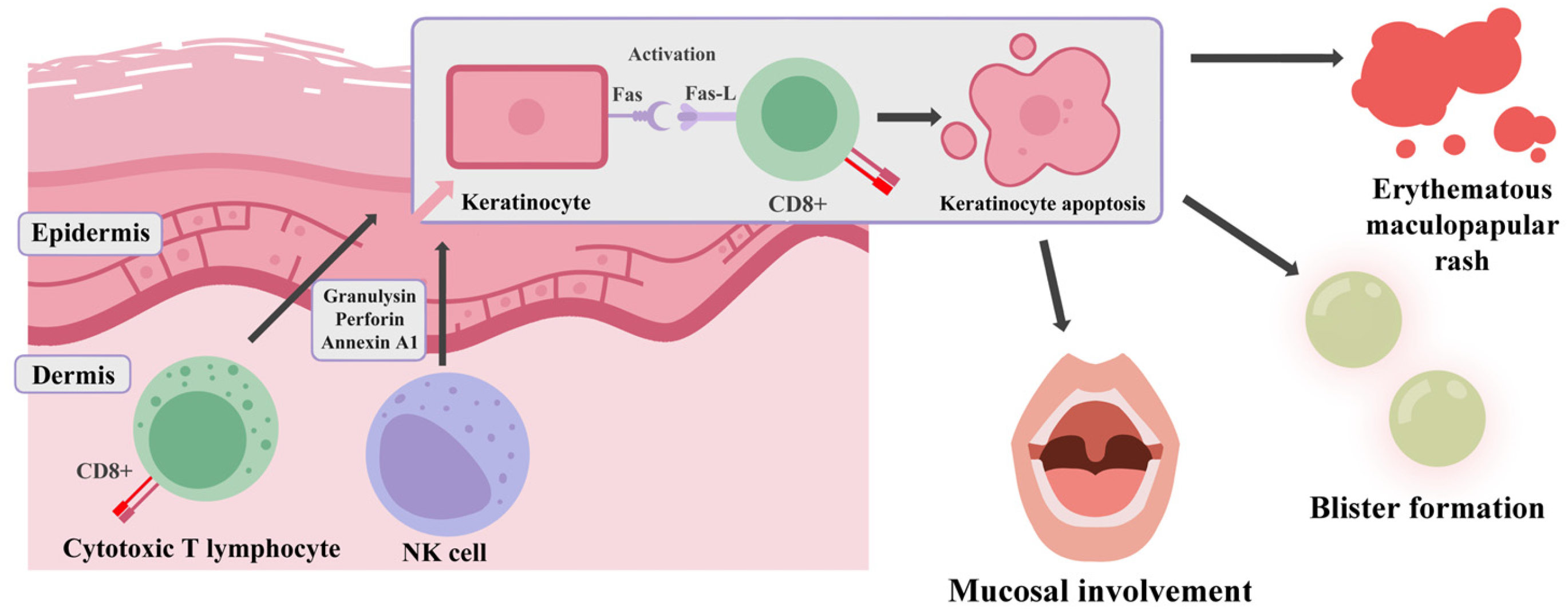
5.1. Pathogenesis
5.2. Clinical Features
5.3. SJS Differential Diagnosis
5.4. Lamotrigine-Induced SJS/TEN and Genetics
5.5. SCORETEN Scale
5.6. SJS Pharmacotherapy
5.7. Pharmacovigilance Data on Lamotrigine and Stevens–Johnson Syndrome
5.8. Supportive Management of SJS/TEN
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hashimoto, Y.; Kotake, K.; Watanabe, N.; Fujiwara, T.; Sakamoto, S. Lamotrigine in the maintenance treatment of bipolar disorder. Cochrane Database Syst. Rev. 2021, 9, CD013575. [Google Scholar] [CrossRef] [PubMed]
- McIntyre, R.S.; Berk, M.; Brietzke, E.; Goldstein, B.I.; López-Jaramillo, C.; Kessing, L.V.; Malhi, G.S.; Nierenberg, A.A.; Rosenblat, J.D.; Majeed, A.; et al. Bipolar disorders. Lancet 2020, 396, 1841–1856. [Google Scholar] [CrossRef] [PubMed]
- Kesebir, S.; Kesebir, S.; Koc, M.I.; Yosmaoglu, A. Bipolar Spectrum Disorder May Be Associated with Family History of Diseases. J. Clin. Med. Res. 2020, 12, 251–254. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Nierenberg, A.A.; Agustini, B.; Köhler-Forsberg, O.; Cusin, C.; Katz, D.; Sylvia, L.G.; Peters, A.; Berk, M. Diagnosis and Treatment of Bipolar Disorder: A Review. JAMA 2023, 330, 1370–1380. [Google Scholar] [CrossRef]
- Miller, J.N.; Black, D.W. Bipolar Disorder and Suicide: A Review. Curr. Psychiatry Rep. 2020, 22, 6. [Google Scholar] [CrossRef]
- Parker, G.; Ricciardi, T.; Tavella, G.; Hadzi-Pavlovic, D. A Single-Blind Randomized Comparison of Lithium and Lamotrigine as Maintenance Treatments for Managing Bipolar II Disorder. J. Clin. Psychopharmacol. 2021, 41, 381–388. [Google Scholar] [CrossRef]
- Baldessarini, R.J.; Vázquez, G.H.; Tondo, L. Bipolar depression: A major unsolved challenge. Int. J. Bipolar Disord. 2020, 8, 1. [Google Scholar] [CrossRef]
- Wood, K.E.; Palmer, K.L.; Krasowski, M.D. Correlation of elevated lamotrigine and levetiracetam serum/plasma levels with toxicity: A long-term retrospective review at an academic medical center. Toxicol. Rep. 2021, 8, 1592–1598. [Google Scholar] [CrossRef]
- Greil, W.; de Bardeci, M.; Nievergelt, N.; Erfurth, A.; Hasler, G.; Bridler, R.; Toto, S.; Grohmann, R.; Seifert, J.; Schoretsanitis, G. Twenty-four years of prescription patterns in bipolar disorder in patients with vs. without lithium: A pharmacoepidemiological analysis of 8707 cases in German-speaking countries. Int. J. Bipolar Disord. 2025, 13, 3. [Google Scholar] [CrossRef]
- Rybakowski, J.K. Mood Stabilizers of First and Second Generation. Brain Sci. 2023, 13, 741. [Google Scholar] [CrossRef]
- Charlton, O.A.; Harris, V.; Phan, K.; Mewton, E.; Jackson, C.; Cooper, A. Toxic Epidermal Necrolysis and Steven–Johnson Syndrome: A Comprehensive Review. Adv. Wound Care 2020, 9, 426–439. [Google Scholar] [CrossRef] [PubMed]
- Liang, C.; An, P.; Zhang, Y.; Liu, X.; Zhang, B. Fatal outcome related to drug reaction with eosinophilia and systemic symptoms: A disproportionality analysis of FAERS database and a systematic review of cases. Front. Immunol. 2024, 15, 1490334. [Google Scholar] [CrossRef] [PubMed]
- Shlobin, N.A.; Li, J.; Sander, J.W.; Keezer, M.R.; Thijs, R.D. Cardiac Conduction Delay for Sodium Channel Antagonist Antiseizure Medications. Neurology 2025, 104, e210302. [Google Scholar] [CrossRef] [PubMed]
- Mayer, J.; Mbizvo, G.K.; Bucci, T.; Marson, A.; Lip, G.Y.H. Association of antiseizure medications and adverse cardiovascular events: A global health federated network analysis. Epilepsia 2024, 65, 1264–1274. [Google Scholar] [CrossRef]
- Tu, L.; Xiao, J.; Hong, Q.; Ouyang, A.; Tu, Y.; Wang, S. Assessment of adverse events of the novel antiepileptic drug lamotrigine: A real-world pharmacovigilance study based on FAERS. Expert Opin. Drug Saf. 2025, 25, 1–10. [Google Scholar] [CrossRef]
- Cyrkler, M.; Drabik, A.; Czerwiak, K.Z.; Soroka, E. Lamotrigine: A safe and effective mood stabilizer for bipolar disorder in reproductive-age adults. Med. Sci. Monit. 2024, 30, e945464. [Google Scholar] [CrossRef]
- Ho, A.M.; Coombes, B.J.; Nguyen, T.T.L.; Liu, D.; McElroy, S.L.; Singh, B.; Nassan, M.; Colby, C.L.; Larrabee, B.R.; Weinshilboum, R.M.; et al. Mood-stabilizing antiepileptic treatment response in bipolar disorder: A genome-wide association study. Clin. Pharmacol. Ther. 2020, 108, 1233–1242. [Google Scholar] [CrossRef]
- Costa, B.; Vale, N. Understanding Lamotrigine’s Role in the CNS and Possible Future Evolution. Int. J. Mol. Sci. 2023, 24, 6050. [Google Scholar] [CrossRef]
- Mitra-Ghosh, T.; Callisto, S.P.; Lamba, J.K.; Remmel, R.P.; Birnbaum, A.K.; Barbarino, J.M.; Klein, T.E.; Altman, R.B. PharmGKB summary: Lamotrigine pathway, pharmacokinetics and pharmacodynamics. Pharmacogenet. Genom. 2020, 30, 81–90. [Google Scholar] [CrossRef]
- Buyan, A.; Whitfield, A.A.; Corry, B. Differences in local anaesthetic and antiepileptic binding in the inactivated state of human sodium channel Nav1.4. Biophys. J. 2021, 120, 5553–5563. [Google Scholar] [CrossRef]
- Kowalczyk, E.; Koziej, S.; Soroka, E. Advances in mood disorder pharmacotherapy: Evaluating new antipsychotics and mood stabilizers for bipolar disorder and schizophrenia. Med. Sci. Monit. 2024, 30, e945412. [Google Scholar] [CrossRef] [PubMed]
- Martens, M.A.G.; Zghoul, T.; Watson, E.; Rieger, S.W.; Capitão, L.P.; Harmer, C.J. Acute neural effects of the mood stabiliser lamotrigine on emotional processing in healthy volunteers: A randomised control trial. Transl. Psychiatry 2024, 14, 211. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-Y.; Vuong, T.A.; So, H.-K.; Kim, H.-J.; Bin Kim, Y.; Kang, J.-S.; Kwon, I.; Cho, H. PRMT7 deficiency causes dysregulation of the HCN channels in the CA1 pyramidal cells and impairment of social behaviors. Exp. Mol. Med. 2020, 52, 604–614. [Google Scholar] [CrossRef]
- Guo, B.; Liu, T.; Choi, S.; Mao, H.; Wang, W.; Xi, K.; Jones, C.; Hartley, N.D.; Feng, D.; Chen, Q.; et al. Restoring thalamocortical circuit dysfunction by correcting HCN channelopathy in Shank3 mutant mice. Cell Rep. Med. 2024, 21, 5. [Google Scholar] [CrossRef]
- Filiz, A.K.; Gumus, E.; Karabulut, S.; Tastemur, Y.; Taskiran, A.S. Protective effects of lamotrigine and vitamin B12 on pentylenetetrazole-induced epileptogenesis in rats. Epilepsy Behav. 2021, 118, 107915. [Google Scholar] [CrossRef]
- Onishi, K.; Kamida, T.; Fujiki, M.; Momii, Y.; Sugita, K. Anticonvulsant and antioxidant effects of lamotrigine on pilocarpine-induced status epilepticus in mice. Neuroreport 2023, 34, 61–66. [Google Scholar] [CrossRef]
- Chávez-Castillo, C.E.; Medellín-Garibay, S.E.; Milán-Segovia, R.D.C.; Rodríguez-Leyva, I.; Romano-Moreno, S. Dosing recommendations based on population pharmacokinetics of lamotrigine in Mexican adult patients with epilepsy. J. Pharm. Sci. 2020, 109, 2902–2908. [Google Scholar] [CrossRef]
- Yang, J.; Wang, J.; Ning, L.; Wu, C.; Liu, Y.; Xia, J.; Guan, Y.; Liu, Q.; Zheng, J. Influence of UGT2B7, UGT1A4 and ABCG2 Polymorphisms on the Pharmacokinetics and Therapeutic Efficacy of Lamotrigine in Patients with Epilepsy. Eur. J. Drug Metab. Pharmacokinet. 2024, 49, 437–447. [Google Scholar] [CrossRef]
- Li, Y.; Ang, H.S.; Fatehi, P.; Htet, N. The roller coaster of lamotrigine levels: Successful treatment of massive lamotrigine overdose with continuous veno-venous hemodiafiltration and rifampin. Cureus 2024, 16, e65637. [Google Scholar] [CrossRef]
- Schoretsanitis, G.; Deligiannidis, K.M.; Kasperk, N.; Schmidt, C.T.; Kittel-Schneider, S.; Ter Horst, P.; Berlin, M.; Kohn, E.; Poels, E.M.; Zutshi, D.; et al. The impact of pregnancy on the pharmacokinetics of antiseizure medications: A systematic review and meta-analysis of data from 674 pregnancies. Prog. Neuropsychopharmacol. Biol. Psychiatry 2024, 133, 111030. [Google Scholar] [CrossRef]
- Reddy, D.S. Clinical pharmacokinetic interactions between antiepileptic drugs and hormonal contraceptives. Expert Rev. Clin. Pharmacol. 2010, 3, 183–192. [Google Scholar] [CrossRef] [PubMed]
- Sabers, A.; Buchholt, J.M.; Uldall, P.; Hansen, E.L. Lamotrigine plasma levels reduced by oral contraceptives. Epilepsy Res. 2001, 47, 151–154. [Google Scholar] [CrossRef] [PubMed]
- King, A.; Bachman, E.; Macken, M.P.; Lee, J.; Gerard, E.E. Contraceptive vaginal ring reduces lamotrigine levels. Epilepsy Behav. 2020, 111, 107162. [Google Scholar] [CrossRef]
- Nebert, D.W.; Russell, D.W. Clinical importance of the cytochromes P450. Lancet 2002, 360, 1155–1162. [Google Scholar] [CrossRef]
- Pedersen, S.; Kverneland, M.; Rudi, K.; Gervin, K.; Landmark, C.J.; Iversen, P.O.; Selmer, K.K. Decreased serum concentrations of antiseizure medications in children with drug resistant epilepsy following treatment with ketogenic diet. Epilepsia Open 2023, 8, 858–866. [Google Scholar] [CrossRef]
- Miziak, B.; Błaszczyk, B.; Chrościńska-Krawczyk, M.; Czuczwar, S.J. Caffeine and Its Interactions with Antiseizure Medications—Is There a Correlation between Preclinical and Clinical Data? Int. J. Mol. Sci. 2023, 24, 17569. [Google Scholar] [CrossRef]
- Treiman, D.M. Management of refractory complex partial seizures: Current state of the art. Neuropsychiatr. Dis. Treat. 2010, 6, 297–308. [Google Scholar] [CrossRef]
- Mazurkiewicz-Bełdzińska, M. New antiepileptic drugs in pediatric epilepsy–did they fulfill the expectation? Neurol. Dziec. 2014, 23, 11–19. Available online: http://www.neurologia-dziecieca.pl/nd00.php?id=32 (accessed on 25 April 2025).
- Chen, J.; Huang, L.; Zeng, L.; Jiang, Z.; Xiong, M.; Jia, Z.-J.; Cheng, G.; Miao, L.; Zhao, L.; Zhang, L. The reference range of lamotrigine in the treatment of epilepsy in children: A systematic review. Eur. J. Clin. Pharmacol. 2023, 80, 1–10. [Google Scholar] [CrossRef]
- Cross, J.H.; Auvin, S.; Falip, M.; Striano, P.; Arzimanoglou, A. Expert Opinion on the Management of Lennox-Gastaut Syndrome: Treatment Algorithms and Practical Considerations. Front. Neurol. 2017, 8, 505. [Google Scholar] [CrossRef]
- Panebianco, M.; Bresnahan, R.; Ramaratnam, S.; Marson, A.G. Lamotrigine add-on therapy for drug-resistant focal epilepsy. Cochrane Database Syst. Rev. 2023, 12, CD001909. [Google Scholar] [CrossRef] [PubMed]
- Köhler-Forsberg, O.; Sylvia, L.G.; Thase, M.; Calabrese, J.R.; Tohen, M.; Bowden, C.L.; McInnis, M.; Iosifescu, D.V.; Kocsis, J.H.; Friedman, E.S.; et al. Lithium plus antipsychotics or anticonvulsants for bipolar disorder: Comparing clinical response and metabolic changes. Aust. N. Z. J. Psychiatry. 2023, 57, 93–103. [Google Scholar] [CrossRef] [PubMed]
- Besag, F.M.C.; Vasey, M.J.; Sharma, A.N.; Lam, I.C.H. Efficacy and safety of lamotrigine in the treatment of bipolar disorder across the lifespan: A systematic review. Ther. Adv. Psychopharmacol. 2021, 11, 20451253211045870. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.J.; Fersh, M.E.; Ernst, C.; Klipstein, K.; Albertini, E.S.; Lusskin, S.I. Bipolar Disorder in Pregnancy and Postpartum: Principles of Management. Curr. Psychiatry Rep. 2016, 18, 13. [Google Scholar] [CrossRef]
- Pariente, G.; Leibson, T.; Shulman, T.; Adams-Webber, T.; Barzilay, E.; Nulman, I. Pregnancy Outcomes Following In Utero Exposure to Lamotrigine: A Systematic Review and Meta-Analysis. CNS Drugs 2017, 31, 439–450. [Google Scholar] [CrossRef]
- Crawford, M.J.; Sanatinia, R.; Barrett, B.; Cunningham, G.; Dale, O.; Ganguli, P.; Lawrence-Smith, G.; Leeson, V.; Lemonsky, F.; Lykomitrou, G.; et al. The Clinical Effectiveness and Cost-Effectiveness of Lamotrigine in Borderline Personality Disorder: A Randomized Placebo-Controlled Trial. Am. J. Psychiatry 2018, 175, 756–764. [Google Scholar] [CrossRef]
- Ade, R.M.; Patil, P.S.; Pathade, A. The Therapeutic Role of Lamotrigine in Borderline Personality Disorder: A Comprehensive Review of Outcomes, Mechanisms, and Treatment Strategies. Cureus 2024, 16, e67362. [Google Scholar] [CrossRef]
- Reich, D.B.; Zanarini, M.C.; Bieri, K.A. A preliminary study of lamotrigine in the treatment of affective instability in borderline personality disorder. Int. Clin. Psychopharmacol. 2009, 24, 270–275. [Google Scholar] [CrossRef]
- Tritt, K.; Nickel, C.; Lahmann, C.; Leiberich, P.K.; Rother, W.K.; Loew, T.H.; Nickel, M.K. Lamotrigine treatment of aggression in female borderline-patients: A randomized, double-blind, placebo-controlled study. J. Psychopharmacol. 2005, 19, 287–291. [Google Scholar] [CrossRef]
- Rissardo, J.P.; Caprara, A.L.F. Lamotrigine-Associated Movement Disorder: A Literature Review. Neurol. India 2021, 69, 1524–1538. [Google Scholar] [CrossRef]
- Lambru, G.; Zakrzewska, J.; Matharu, M. Trigeminal neuralgia: A practical guide. Pract. Neurol. 2021, 21, 392–402. [Google Scholar] [CrossRef] [PubMed]
- Wiffen, P.J.; Derry, S.; Moore, R.A. Lamotrigine for chronic neuropathic pain and fibromyalgia in adults. Cochrane Database Syst. Rev. 2013, 12, CD006044. [Google Scholar] [CrossRef] [PubMed]
- Solmi, M.; Veronese, N.; Zaninotto, L.; van der Loos, M.L.M.; Gao, K.; Schaffer, A.; Reis, C.; Normann, C.; Anghelescu, I.-G.; Correll, C.U. Lamotrigine compared to placebo and other agents with antidepressant activity in patients with unipolar and bipolar depression: A comprehensive meta-analysis of efficacy and safety outcomes in short-term trials. CNS Spectr. 2016, 21, 403–418. [Google Scholar] [CrossRef] [PubMed]
- Fountoulakis, K.N.; Kontis, D.; Gonda, X.; Yatham, L.N. A systematic review of the evidence on the treatment of rapid cycling bipolar disorder. Bipolar Disord. 2013, 15, 115–137. [Google Scholar] [CrossRef]
- Parker, G.; Mccraw, S. The “disconnect” between initial judgments of lamotrigine vs. its real-world effectiveness in managing bipolar disorder. A tale with wider ramifications. Acta Psychiatr. Scand. 2015, 132, 345–354. [Google Scholar] [CrossRef]
- Prabhavalkar, K.S.; Poovanpallil, N.B.; Bhatt, L.K. Management of bipolar depression with lamotrigine: An antiepileptic mood stabilizer. Front. Pharmacol. 2015, 23, 242. [Google Scholar] [CrossRef]
- Rybakowski, J. Choroby afektywne. In Standardy Leczenia Farmakologicznego Niektórych Zaburzeń Psychicznych, 2nd ed.; Jarema, M., Ed.; Via Medica: Gdańsk, Poland, 2015; pp. 55–133. [Google Scholar]
- Yatham, L.N.; Kennedy, S.H.; Parikh, S.V.; Schaffer, A.; Bond, D.J.; Frey, B.N.; Sharma, V.; Goldstein, B.I.; Rej, S.; Beaulieu, S.; et al. Canadian Network for Mood and Anxiety Treatments (CANMAT) and International Society for Bipolar Disorders (ISBD) 2018 guidelines for the management of patients with bipolar disorder. Bipolar Disord. 2018, 20, 97–170. [Google Scholar] [CrossRef]
- Chakrabarti, S. Bipolar disorder in the International Classification of Diseases-Eleventh version: A review of the changes, their basis, and usefulness. World J. Psychiatry 2022, 12, 1335–1355. [Google Scholar] [CrossRef]
- Roosen, L.; Sienaert, P. Evidence-based treatment strategies for rapid cycling bipolar disorder, a systematic review. J. Affect. Disord. 2022, 15, 69–77. [Google Scholar] [CrossRef]
- Zhihan, G.; Fengli, S.; Wangqiang, L.; Dong, S.; Weidong, J. Lamotrigine and Lithium Combination for Treatment of Rapid Cycling Bipolar Disorder: Results From Meta-Analysis. Front. Psychiatry 2022, 14, 913051. [Google Scholar] [CrossRef]
- Muzina, D.J.; Calabrese, J.R. Maintenance Therapies in Bipolar Disorder: Focus on Randomized Controlled Trials. Aust. N. Z. J. Psychiatry 2005, 39, 652–661. [Google Scholar] [CrossRef] [PubMed]
- Goldsmith, D.R.; Wagstaff, A.J.; Ibbotson, T.; Perry, C.M. Lamotrigine: A review of its use in bipolar disorder. Drugs 2003, 63, 2029–2050. [Google Scholar] [CrossRef] [PubMed]
- Clark, C.T.; Wisner, K.L. Treatment of Peripartum Bipolar Disorder. Obstet. Gynecol. Clin. N. Am. 2018, 45, 403–417. [Google Scholar] [CrossRef] [PubMed]
- Ruberto, V.L.; Jha, M.K.; Murrough, J.W. Pharmacological Treatments for Patients with Treatment-Resistant Depression. Pharmaceuticals 2020, 13, 116. [Google Scholar] [CrossRef]
- McInerney, S.J.; Kennedy, S.H. Review of evidence for use of antidepressants in bipolar depression. Prim. Care Companion CNS Disord. 2014, 16, 5. [Google Scholar] [CrossRef][Green Version]
- Strzelecki, D. Mood stabilizers in the treatment of bipolar disorder. Psychoter. Uzależnienia 2017, 2, 1–7. Available online: https://www.researchgate.net/publication/323116332 (accessed on 11 December 2017).
- Shah, N.; Grover, S.; Rao, G. Clinical Practice Guidelines for Management of Bipolar Disorder. Indian J. Psychiatry 2017, 59, S51–S66. [Google Scholar] [CrossRef]
- Lembke, A. Optimal Dosing of Lithium, Valproic Acid, and Lamotrigine in the Treatment of Mood Disorders. Clin. Focus Prim. Psychiatry 2009, 16, 37–42. [Google Scholar]
- Lovrić, M.; Čajić, I.; Gadže, Ž.P.; Domjanović, I.K.; Božina, N. Effect of antiepileptic drug comedication on lamotrigine concentrations. Croat. Med. J. 2018, 59, 13–19. [Google Scholar] [CrossRef]
- Kumar, R.; Nuñez, N.A.; Prokop, L.J.; Veldic, M.; Betcher, H.; Singh, B. Association of Optimal Lamotrigine Serum Levels and Therapeutic Efficacy in Mood Disorders: A Systematic Review. J. Clin. Psychopharmacol. 2021, 41, 681–686. [Google Scholar] [CrossRef]
- Shirzadi, M.; Saunes, M.; Reimers, A.; Brodtkorb, E. Rash during lamotrigine treatment is not always drug hypersensitivity: A retrospective cohort study among children and adults. Seizure 2021, 89, 12–18. [Google Scholar] [CrossRef] [PubMed]
- Suleman, N.; Ozdemirli, M.; Weisman, D. Lamotrigine-associated hemophagocytic lymphohistiocytosis. BMJ Case Rep. 2021, 14, e238183. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Bairam, A.; Liu, M.C.; Uetrecht, J. Potential involvement of sulfotransferase in the mechanism of lamotrigine-induced skin rash. Chem. Res. Toxicol. 2023, 36, 1711–1716. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.K.; Jang, K.S.; Kim, D.W. Comparative analysis of adverse drug reactions associated with new antiseizure medications from the Korea Adverse Event Reporting System database. Epilepsy Behav. 2024, 154, 109784. [Google Scholar] [CrossRef]
- Liang, D.; Gardella, E.; Kragholm, K.; Polcwiartek, C.; Sessa, M. The relationship between valproate and lamotrigine/levetiracetam use and prognosis in patients with epilepsy and heart failure: A Danish register-based study. J. Card. Fail. 2022, 28, 630–638. [Google Scholar] [CrossRef]
- Rohde, C.; Köhler-Forsberg, O.; Nierenberg, A.A.; Østergaard, S.D. Pharmacological treatment of bipolar disorder and risk of diabetes mellitus: A nationwide study of 30,451 patients. Bipolar Disord. 2023, 25, 323–334. [Google Scholar] [CrossRef]
- Vieta, E.; Ghorpade, S.; Biswas, A.; Sarkar, A.; Phansalkar, A.; Cooper, J. Lamotrigine efficacy, safety, and tolerability for women of childbearing age with bipolar I disorder: Meta-analysis from four randomized, placebo-controlled maintenance studies. Eur. Neuropsychopharmacol. 2024, 78, 81–92. [Google Scholar] [CrossRef]
- Abou-Taleb, D.A.E.; El-Sayed, A.M.; Ghabesha, A.A.; Hassan, S.B. Severe cutaneous adverse drug reactions: Incidence, clinical patterns, causative drugs and modalities of treatment in Assiut University Hospital, Upper Egypt. Dermatol. Ther. 2020, 33, e14176. [Google Scholar] [CrossRef]
- Abtahi-Naeini, B.; Dehghan, M.-S.; Paknazar, F.; Shahmoradi, Z.; Faghihi, G.; Sabzghabaee, A.M.; Akbari, M.; Hadian, M.; Momen, T. Clinical and Epidemiological Features of Patients with Drug-Induced Stevens-Johnson Syndrome and Toxic Epidermal Necrolysis in Iran: Different Points of Children from Adults. Int. J. Pediatr. 2022, 2022, 8163588. [Google Scholar] [CrossRef]
- Huang, P.-W.; Chiou, M.-H.; Chien, M.-Y.; Chen, W.-W.; Chu, C.-Y. Analysis of severe cutaneous adverse reactions (SCARs) in Taiwan drug-injury relief system: 18-year results. J. Formos. Med. Assoc. 2022, 121, 1397–1405. [Google Scholar] [CrossRef]
- Glahn, J.Z.; Almeida, M.N.; Kochen, A.; Noel, O.; Stogner, V.; Hsia, H.C.; Savetamal, A. Lamotrigine Emerging as a Driver of Stevens-Johnson Syndrome and Toxic Epidermal Necrolysis: An 8-Year Retrospective Study. Burns 2024, 50, 2114–2123. [Google Scholar] [CrossRef] [PubMed]
- Moshayedi, M.A.; Asilian, A.; Mokhtari, F. Evaluation of severe adverse cutaneous drug reactions in patients admitted to tertiary care center: A cross-sectional study. Health Sci. Rep. 2024, 7, e1969. [Google Scholar] [CrossRef] [PubMed]
- Bataille, P.; Lebrun-Vignes, B.; Bettuzzi, T.; Ingen-Housz-Oro, S.; Hadj-Rabia, S.; Welfringer-Morin, A.; Bodemer, C. Drugs associated with epidermal necrolysis in children: A World Health Organization pharmacovigilance database analysis. J. Eur. Acad. Dermatol. Venereol. 2024, 38, 1681–1682. [Google Scholar] [CrossRef]
- Sassolas, B.; Haddad, C.; Mockenhaupt, M.; Dunant, A.; Liss, Y.; Bork, K.; Haustein, U.F.; Vieluf, D.; Roujeau, J.C.; Louet, H.L. ALDEN, an algorithm for assessment of drug causality in Stevens-Johnson Syndrome and toxic epidermal necrolysis: Comparison with case-control analysis. Clin. Pharmacol. Ther. 2010, 88, 60–68. [Google Scholar] [CrossRef]
- Fukasawa, T.; Takahashi, H.; Takahashi, K.; Tanemura, N.; Urushihara, M.A.H. Risk of Stevens-Johnson syndrome and toxic epidermal necrolysis associated with anticonvulsants in a Japanese population: Matched case–control and cohort studies. Allergol. Int. 2021, 70, 335–342. [Google Scholar] [CrossRef]
- Gronich, N.; Maman, D.; Stein, N.; Saliba, W. Culprit Medications and Risk Factors Associated with Stevens-Johnson Syndrome and Toxic Epidermal Necrolysis: Population-Based Nested Case-Control Study. Am. J. Clin. Dermatol. 2022, 23, 257–266. [Google Scholar] [CrossRef]
- Pierre, A.B.; Jernigan, A.M.; Castellano, T. SJS/TEN immune-related dermatologic reaction secondary to immune checkpoint inhibitor pembrolizumab in skin of color. Gynecol. Oncol. Rep. 2023, 50, 101290. [Google Scholar] [CrossRef]
- Zhu, J.; Chen, G.; He, Z.; Zheng, Y.; Gao, S.; Li, J.; Ling, Y.; Yu, X.; Qiu, K.; Wu, J. Stevens-Johnson syndrome/toxic epidermal necrolysis in patients treated with immune checkpoint inhibitors: A safety analysis of clinical trials and FDA pharmacovigilance database. eClinicalMedicine 2021, 37, 100951. [Google Scholar] [CrossRef]
- Frantz, R.; Huang, S.; Are, A.; Motaparthi, K. Stevens–johnson syndrome and toxic epidermal necrolysis: A review of diagnosis and management. Medicina 2021, 57, 895. [Google Scholar] [CrossRef]
- Neill, B.C.; Seger, E.W.; Ferguson, J.E.; Hooton, T.; Rickstrew, J.J.; Rajpara, A. SJS/TENN: A Mnemonic for Early Clinical Diagnosis of Stevens-Johnson Syndrome and Toxic Epidermal Necrolysis. Kans. J. Med. 2021, 14, 114–115. [Google Scholar] [CrossRef]
- Goh, S.J.R.; Tuomisto, J.E.E.; Purcell, A.W.; Mifsud, N.A.; Illing, P.T. The complexity of T cell–mediated penicillin hypersensitivity reactions. Allergy 2021, 76, 150–167. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, A.; Abe, R. Stevens–Johnson syndrome and toxic epidermal necrolysis: Updates in pathophysiology and management. Chin. Med. J. 2024, 137, 2294–2307. [Google Scholar] [CrossRef] [PubMed]
- Eskandari, E.; Eaves, C.J. Paradoxical roles of caspase-3 in regulating cell survival, proliferation, and tumorigenesis. J. Cell Biol. 2022, 221, e202201159. [Google Scholar] [CrossRef] [PubMed]
- Hoetzenecker, W.; Nägeli, M.; Mehra, E.T.; Jensen, A.N.; Saulite, I.; Schmid-Grendelmeier, P.; Guenova, E.; Cozzio, A.; French, L.E. Adverse cutaneous drug eruptions: Current understanding. Semin Immunopathol. 2016, 38, 75–86. [Google Scholar] [CrossRef]
- Bellón, T. Mechanisms of Severe Cutaneous Adverse Reactions: Recent Advances. Drug Saf. 2019, 42, 973–992. [Google Scholar] [CrossRef]
- Line, J.; Saville, E.; Meng, X.; Naisbitt, D. Why drug exposure is frequently associated with T-cell mediated cutaneous hypersensitivity reactions. Front. Toxicol. 2023, 5, 1268107. [Google Scholar] [CrossRef]
- Maity, S.; Banerjee, I.; Sinha, R.; Jha, H.; Ghosh, P.; Mustafi, S. Nikolsky’s sign: A pathognomic boon. J. Fam. Med. Prim. Care 2020, 9, 526–530. [Google Scholar] [CrossRef]
- Wetter, D.A.; Camilleri, M.J. Clinical, Etiologic, and Histopathologic Features of Stevens-Johnson Syndrome During an 8-Year Period at Mayo Clinic. Mayo Clin Proc. 2010, 85(2), 131–138. [Google Scholar] [CrossRef]
- Lagrew, M.; Perryman, K.L.; Walker, A.; Hahn, P. Stevens-Johnson Syndrome (SJS) As the Initial Presentation in a Patient With a New Diagnosis of HIV. Cureus 2023, 15, e5027. [Google Scholar] [CrossRef]
- de Bustros, P.; Baldea, A.; Sanford, A.; Joyce, C.; Adams, W.; Bouchard, C. Review of culprit drugs associated with patients admitted to the burn unit with the diagnosis of Stevens-Johnson Syndrome and Toxic Epidermal Necrolysis Syndrome. Burns 2022, 48, 1561–1573. [Google Scholar] [CrossRef]
- Park, J.S.; Hamilton, C.D.; Patel, S.; Lee, J.B.; Hsu, S. Linear Immunoglobulin A (IgA) Bullous Dermatosis Mimicking Stevens-Johnson Syndrome. Cureus 2022, 14, e30309. [Google Scholar] [CrossRef] [PubMed]
- Grünwald, P.; Mockenhaupt, M.; Panzer, R.; Emmert, S. Erythema multiforme, Stevens-Johnson syndrome/toxic epidermal necrolysis–diagnosis and treatment. J. Dtsch. Dermatol. Ges. 2020, 18, 547–553. [Google Scholar] [CrossRef] [PubMed]
- de Moraes Souza, R.; Bellón, T.; Fiz Benito, E.; Sendagorta Cudós, E. Flow Cytometric Analysis of Skin Blister Fluid Cells: A Promising Tool in the Differential Diagnosis of Acute Cutaneous Graft-versus-host Disease and Stevens-Johnson Syndrome/Toxic Epidermal Necrolysis. Actas Dermosifiliogr. 2024, 115, 1042–1044. [Google Scholar] [CrossRef] [PubMed]
- Gungor, T.; Gumru, S.; Gumru, B. Erythema multiforme: A retrospective study of etiologies, clinical manifestations, and treatments. J. Dent. Sci. 2024, 19, 2295–2304. [Google Scholar] [CrossRef]
- Brazel, M.; Desai, A.; Are, A.; Motaparthi, K. Staphylococcal Scalded Skin Syndrome and Bullous Impetigo. Medicina 2021, 57, 1157. [Google Scholar] [CrossRef]
- Khan, M.; Park, L.; Skopit, S. Management Options for Linear Immunoglobulin A (IgA) Bullous Dermatosis: A Literature Review. Cureus 2023, 15, e36481. [Google Scholar] [CrossRef]
- Shah, H.; Parisi, R.; Mukherjee, E.; Phillips, E.J.; Dodiuk-Gad, R.P. Update on Stevens–Johnson Syndrome and Toxic Epidermal Necrolysis: Diagnosis and Management. Am. J. Clin. Dermatol. 2024, 25, 891–908. [Google Scholar] [CrossRef]
- Nusman, C.M.; Blokhuis, C.; Pajkrt, D.; Visser, D.H. Staphylococcal Scalded Skin Syndrome in Neonates: Case Series and Overview of Outbreaks. Antibiotics 2022, 12, 38. [Google Scholar] [CrossRef]
- Lammer, J.; Hein, R.; Roenneberg, S.; Biedermann, T.; Volz, T. Drug-induced linear IgA bullous dermatosis: A case report and review of the literature. Acta Derm. Venereol. 2019, 99, 508–515. [Google Scholar] [CrossRef]
- Hama, N.; Nishimura, K.; Hasegawa, A.; Yuki, A.; Kume, H.; Adachi, J.; Kinoshita, M.; Ogawa, Y.; Nakajima, S.; Nomura, T.; et al. Galectin-7 as a potential biomarker of Stevens-Johnson syndrome/toxic epidermal necrolysis: Identification by targeted proteomics using causative drug-exposed peripheral blood cells. J. Allergy Clin. Immunol. Pract. 2019, 7, 2894–2897.e7. [Google Scholar] [CrossRef]
- Hasegawa, A.; Abe, R. Recent advances in managing and understanding Stevens-Johnson syndrome and toxic epidermal necrolysis. F1000Research 2020, 9, 612. [Google Scholar] [CrossRef] [PubMed]
- Edinoff, A.N.; Nguyen, L.H.; Fitz-Gerald, M.J.; Crane, E.; Lewis, K.; Pierre, S.S.; Kaye, A.D.; Kaye, A.M.; Kaye, J.S.; Kaye, R.J.; et al. Lamotrigine and Stevens-Johnson Syndrome Prevention. Psychopharmacol. Bull. 2021, 51, 96–114. [Google Scholar] [PubMed]
- Biswas, M.; Ershadian, M.; Shobana, J.; Nguyen, A.H.; Sukasem, C. Associations of HLA genetic variants with carbamazepine-induced cutaneous adverse drug reactions: An updated meta-analysis. Clin. Transl. Sci. 2022, 15, 1887–1905. [Google Scholar] [CrossRef] [PubMed]
- Zimmerman, D.; Dang, N.H. Stevens–Johnson Syndrome (SJS) and Toxic Epidermal Necrolysis (TEN). Oncol. Crit. Care 2019, 9, 267–280. [Google Scholar] [CrossRef]
- Jongkhajornpong, P.; Ueta, M.; Lekhanont, K.; Puangsricharern, V.; Prabhasawat, P.; Chantaren, P.; Pisuchpen, P.; Kinoshita, S. Association of HLA polymorphisms and acetaminophen-related Steven-Johnson syndrome with severe ocular complications in Thai population. Br. J. Ophthalmol. 2022, 106, 884–888. [Google Scholar] [CrossRef]
- Koomdee, N.; Pratoomwun, J.; Jantararoungtong, T.; Theeramoke, V.; Tassaneeyakul, W.; Klaewsongkram, J.; Rerkpattanapipat, T.; Santon, S.; Puangpetch, A.; Intusoma, U.; et al. Association of HLA-A and HLA-B Alleles with Lamotrigine-Induced Cutaneous Adverse Drug Reactions in the Thai Population. Front. Pharmacol. 2017, 8, 879. [Google Scholar] [CrossRef]
- Ramírez, E.; Bellón, T.; Tong, H.Y.; Borobia, A.M.; de Abajo, F.J.; Lerma, V.; Hidalgo, M.A.M.; Castañer, J.L.; Cabañas, R.; Fiandor, A.; et al. Significant HLA class I type associations with aromatic antiepileptic drug (AED)-induced SJS/TEN are different from those found for the same AED-induced DRESS in the Spanish population. Pharmacol. Res. 2017, 115, 168–178. [Google Scholar] [CrossRef]
- Lonjou, C.; Borot, N.; Sekula, P.; Ledger, N.; Thomas, L.; Halevy, S.; Naldi, L.; Bouwes-Bavinck, J.-N.; Sidoroff, A.; de Toma, C.; et al. A European study of HLA-B in Stevens-Johnson syndrome and toxic epidermal necrolysis related to five high-risk drugs. Pharmacogenet. Genom. 2008, 18, 99–107. [Google Scholar] [CrossRef]
- Shi, Y.-W.; Min, F.-L.; Zhou, D.; Qin, B.; Wang, J.; Hu, F.-Y.; Cheung, Y.-K.; Zhou, J.-H.; Hu, X.-S.; Zhou, J.-Q.; et al. HLA-A*24:02 as a common risk factor for antiepileptic drug-induced cutaneous adverse reactions. Neurology 2017, 88, 2183–2191. [Google Scholar] [CrossRef]
- Kim, B.-K.; Jung, J.-W.; Kim, T.-B.; Chang, Y.-S.; Park, H.-S.; Moon, J.; Lee, S.-T.; Jung, K.-H.; Jung, K.-Y.; Chu, K.; et al. HLA-A*31:01 and lamotrigine-induced severe cutaneous adverse drug reactions in a Korean population. Ann. Allergy Asthma Immunol. 2017, 118, 629–630. [Google Scholar] [CrossRef]
- Strużyna, J.; Surowiecka, A.; Korzeniowski, T.; Korulczyk, P.; Drozd, L.; Stachura, A.; Torres, K.; Krajewski, A. Accuracy of SCORTEN in predicting mortality in toxic epidermal necrolysis. BMC Med. Inform. Decis. Mak. 2022, 22, 273. [Google Scholar] [CrossRef] [PubMed]
- Torres-Navarro, I.; Briz-Redón, A.; Botella-Estrada, R. Systemic therapies for Stevens-Johnson Syndrome and Toxic Epidermal Necrolysis: A SCORTEN-based systematic review and meta-analysis. J. Eur. Acad. Dermatol. Venereol. 2021, 35, 159–171. [Google Scholar] [CrossRef] [PubMed]
- Suo, H.; Jiang, B.; Sun, X.; Dong, J.; Alamgir, M.; Guan, X.; Su, H.; Liu, Y.; Xia, Y.; Zhou, N.; et al. Comparing the Accuracy of ABCD-10 and SCORTEN in Predicting the In-Hospital Mortality of Stevens-Johnson Syndrome/Toxic Epidermal Necrolysis: A Multi-Institutional Study from Central China. Dermatology 2022, 238, 736–744. [Google Scholar] [CrossRef] [PubMed]
- Kanagarajan, A.; Murthy, A.B.; Moni, P.K.; Palanivel, N. Clinicoetiological Study of Stevens-Johnson Syndrome and Toxic Epidermal Necrolysis Spectrum and the Correlation of SCORTEN with Prognosis. Indian J. Dermatol. 2023, 68, 25–33. [Google Scholar] [CrossRef]
- Koh, H.K.; Fook-Chong, S.; Lee, H.Y. Assessment and Comparison of Performance of ABCD-10 and SCORTEN in Prognostication of Epidermal Necrolysis. JAMA Dermatol. 2020, 156, 1294–1299. [Google Scholar] [CrossRef]
- Koh, H.K.; Fook-Chong, S.M.C.; Lee, H.Y. Improvement of Mortality Prognostication in Patients With Epidermal Necrolysis: The Role of Novel Inflammatory Markers and Proposed Revision of SCORTEN (Re-SCORTEN). JAMA Dermatol. 2022, 158, 160–166. [Google Scholar] [CrossRef]
- Baaklini, G.T.; Mitchell, T.; Davis, J.; Cindass, R.; McGovern, K.; Aden, J.; Cancio, L. Genitourinary management and follow-up for patients with Stevens-Johnson syndrome/toxic epidermal necrolysis. Burns Open 2023, 7, 33–36. [Google Scholar] [CrossRef]
- Griffith, V.; Ramnot, A.; Resnik, S.R.; Resnik, B.I. A case of Stevens-Johnson syndrome treated with oral cyclosporine. J. Fam. Med. Prim. Care 2023, 12, 3425–3428. [Google Scholar] [CrossRef]
- Muntyanu, A.; Netchiporouk, E.; Gerstein, W.; Gniadecki, R.; Litvinov, I.V. Cutaneous Immune-Related Adverse Events (irAEs) to Immune Checkpoint Inhibitors: A Dermatology Perspective on Management. J. Cutan. Med. Surg. 2021, 25, 59–76. [Google Scholar] [CrossRef]
- Nordmann, T.M.; Anderton, H.; Hasegawa, A.; Schweizer, L.; Zhang, P.; Stadler, P.-C.; Sinha, A.; Metousis, A.; Rosenberger, F.A.; Zwiebel, M.; et al. Spatial proteomics identifies JAKi as treatment for a lethal skin disease. Nature 2024, 635, 1001–1009. [Google Scholar] [CrossRef]
- Almadfaa, A.O.; Alattas, M.K.; Aleissa, A.I.; Bormah, A.W.; Alqurashi, H.M.; Felemban, A.A.M.; Assiri, S.H.; Alshehri, M.A.A.; Alharbi, B.A. An Overview on the Evaluation and Management of Stevens-Johnson Syndrome/Toxic Epidermal Necrolysis. Int. J. Pharm. Res. Allied Sci. 2022, 11, 29–34. [Google Scholar] [CrossRef]
- Ingen-Housz-Oro, S.; Matei, I.; Gaillet, A.; Gueudry, J.; Zaghbib, K.; Assier, H.; Hua, C.; Bensaid, B.; Colin, A.; Ouedraogo, R.; et al. French national protocol for diagnosis and management of epidermal necrolysis in adults (Stevens-Johnson syndrome and toxic epidermal necrolysis). Ann. Dermatol. Venereol. 2024, 151, 103282. [Google Scholar] [CrossRef] [PubMed]
- Alomar, M.; Palaian, S.; Al-tabakha, M.M. Pharmacovigilance in perspective: Drug withdrawals, data mining and policy implications. F1000Research 2019, 8, 2109. [Google Scholar] [CrossRef] [PubMed]
- Baftiu, A.; Lima, M.H.; Svendsen, K.; Larsson, P.G.; Johannessen, S.I.; Landmark, C.J. Safety aspects of antiepileptic drugs—A population-based study of adverse effects relative to changes in utilisation. Eur. J. Clin. Pharmacol. 2019, 75, 1153–1160. [Google Scholar] [CrossRef]
- Castellana, E.; Budau, P.M.; Chiappetta, M.R. Pharmacovigilance and Stevens-Johnson Syndrome (SJS)/Toxic Epidermal Necrolysis (TEN): 55 Years of Retrospective Analysis of the FDA Adverse Event Reporting System (FAERS). Database Hosp. Pharm. 2025, 1–9. [Google Scholar] [CrossRef]
- Vogel, U.; van Stekelenborg, J.; Dreyfus, B.; Garg, A.; Habib, M.; Hosain, R.; Wisniewski, A. Investigating Overlap in Signals from EVDAS, FAERS, and VigiBase®. Drug Saf. 2020, 43, 351–362. [Google Scholar] [CrossRef]
- European Medicines Agency. Lamictal and Associated Names–Article 30 Referral: Annex I, II, III. 2008. Available online: https://www.ema.europa.eu/en/medicines/human/referrals/lamictal (accessed on 3 June 2025).
- European Medicines Agency. EudraVigilance Data Analysis System (EVDAS). 2025. Available online: https://www.ema.europa.eu/en/human-regulatory-overview/research-development/pharmacovigilance-research-development/eudravigilance (accessed on 3 June 2025).
- Costa, C.; Abeijon, P.; Rodrigues, D.A.; Figueiras, A.; Herdeiro, M.T.; Torre, C. Factors associated with underreporting of adverse drug reactions by patients: A systematic review. Int. J. Clin. Pharm. 2023, 45, 1349–1358. [Google Scholar] [CrossRef]

| Risk Factors for SCORETEN | Number of Risk Factors | Mortality Rate (%) |
|---|---|---|
| Age above 40 years | 0–1 | 3.2 |
| Heart rate higher than 120 beats per minute | 2 | 12.1 |
| History or a present malignancy | 3 | 35.3 |
| Epidermal detachment area involving body surface area higher than 10% | 4 | 58.3 |
| Blood urea nitrogen higher than 28 mg/dL (10 mmol/L) | 5 | 90 |
| Blood glucose higher than 252 mg/dL (14 mmol/L) | 6 | 90 |
| Bicarbonate lower than 20 mEq/L | 7 | 90 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Żełabowski, K.; Wojtysiak, K.; Ratka, Z.; Biedka, K.; Chłopaś-Konowałek, A. Lamotrigine Therapy: Relation Between Treatment of Bipolar Affective Disorder and Incidence of Stevens–Johnson Syndrome—A Narrative Review of the Existing Literature. J. Clin. Med. 2025, 14, 4103. https://doi.org/10.3390/jcm14124103
Żełabowski K, Wojtysiak K, Ratka Z, Biedka K, Chłopaś-Konowałek A. Lamotrigine Therapy: Relation Between Treatment of Bipolar Affective Disorder and Incidence of Stevens–Johnson Syndrome—A Narrative Review of the Existing Literature. Journal of Clinical Medicine. 2025; 14(12):4103. https://doi.org/10.3390/jcm14124103
Chicago/Turabian StyleŻełabowski, Kacper, Kacper Wojtysiak, Zuzanna Ratka, Kamil Biedka, and Agnieszka Chłopaś-Konowałek. 2025. "Lamotrigine Therapy: Relation Between Treatment of Bipolar Affective Disorder and Incidence of Stevens–Johnson Syndrome—A Narrative Review of the Existing Literature" Journal of Clinical Medicine 14, no. 12: 4103. https://doi.org/10.3390/jcm14124103
APA StyleŻełabowski, K., Wojtysiak, K., Ratka, Z., Biedka, K., & Chłopaś-Konowałek, A. (2025). Lamotrigine Therapy: Relation Between Treatment of Bipolar Affective Disorder and Incidence of Stevens–Johnson Syndrome—A Narrative Review of the Existing Literature. Journal of Clinical Medicine, 14(12), 4103. https://doi.org/10.3390/jcm14124103








