Hand-Assisted Laparoscopic Rectal Resection—Experience of a Tertiary Oncology Center
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics
2.2. Study Design
- -
- 18-years or older;
- -
- Biopsy-proven diagnosis of a primary adenocarcinoma of the rectosigmoid junction (RSJ) or the rectum;
- -
- Submitted to HALS RAR.
2.3. Cancer Staging, Treatment, and Follow-Up Protocol
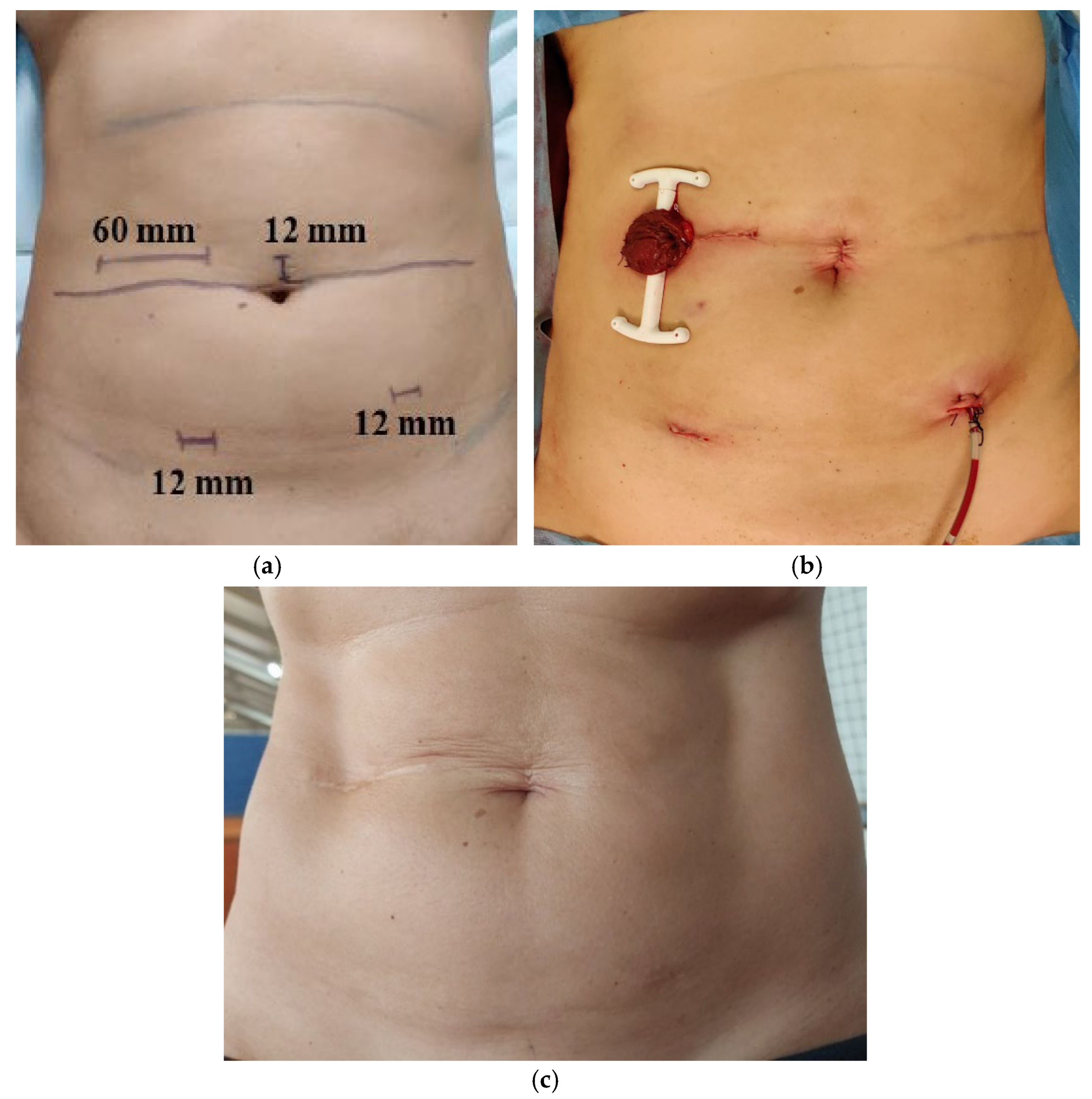
2.4. Surgery Technique
Surgical Protocol
2.5. Variable and Outcome Definitions
- -
- Previous tumors in the past 5 years (with the exception of non-melanoma skin cancer, well-differentiated thyroid tumors);
- -
- Synchronous tumors (colorectal or other);
- -
- Metastatic disease at diagnosis or at time of surgery;
- -
- R2 resection;
- -
- Metachronous tumors until 5 years after surgery.
2.6. Statistical Analysis
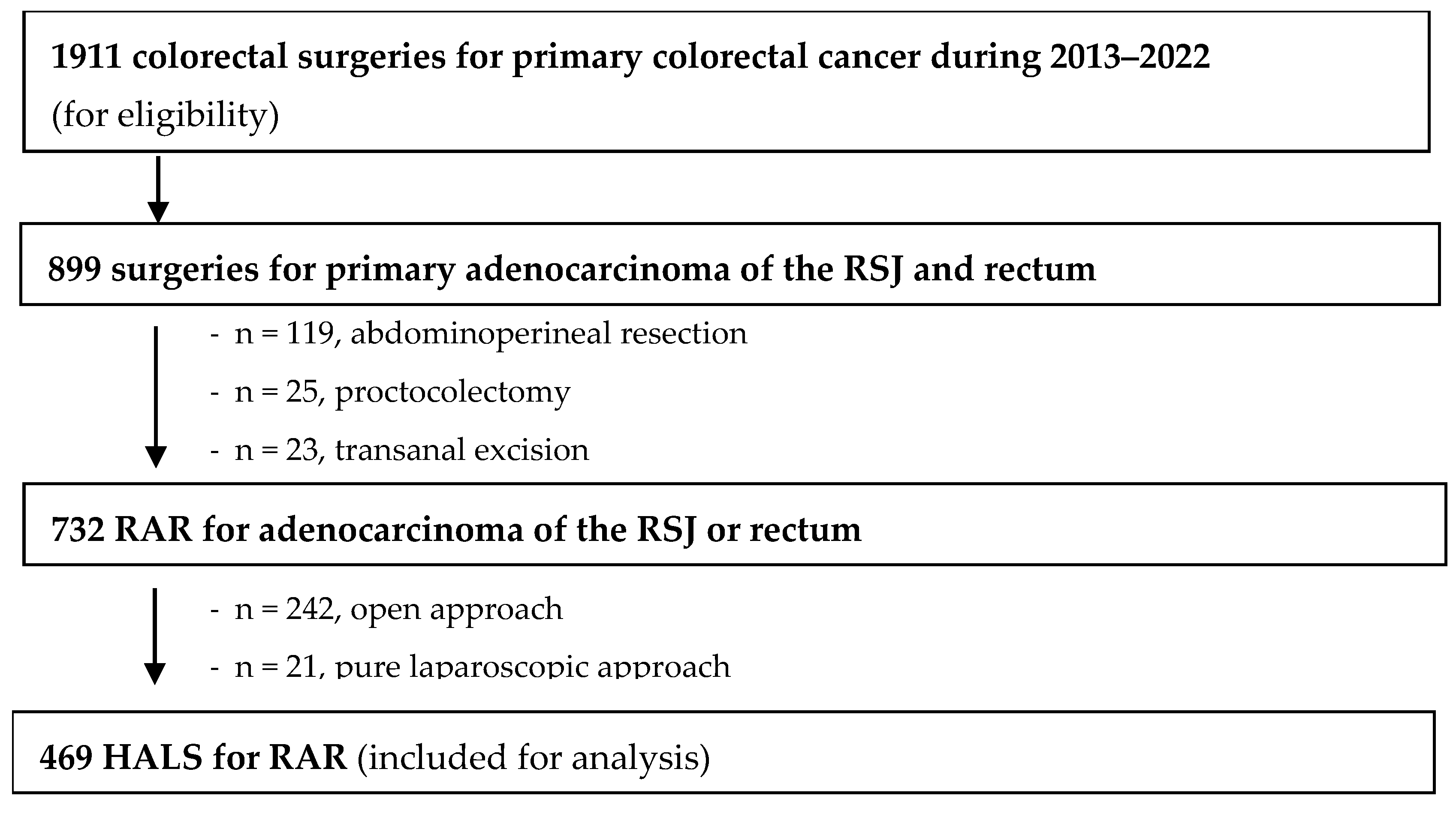
3. Results
3.1. Patients
3.2. Intraoperative Outcomes
3.3. Postoperative Outcomes
3.4. Short-Term Oncological Outcomes
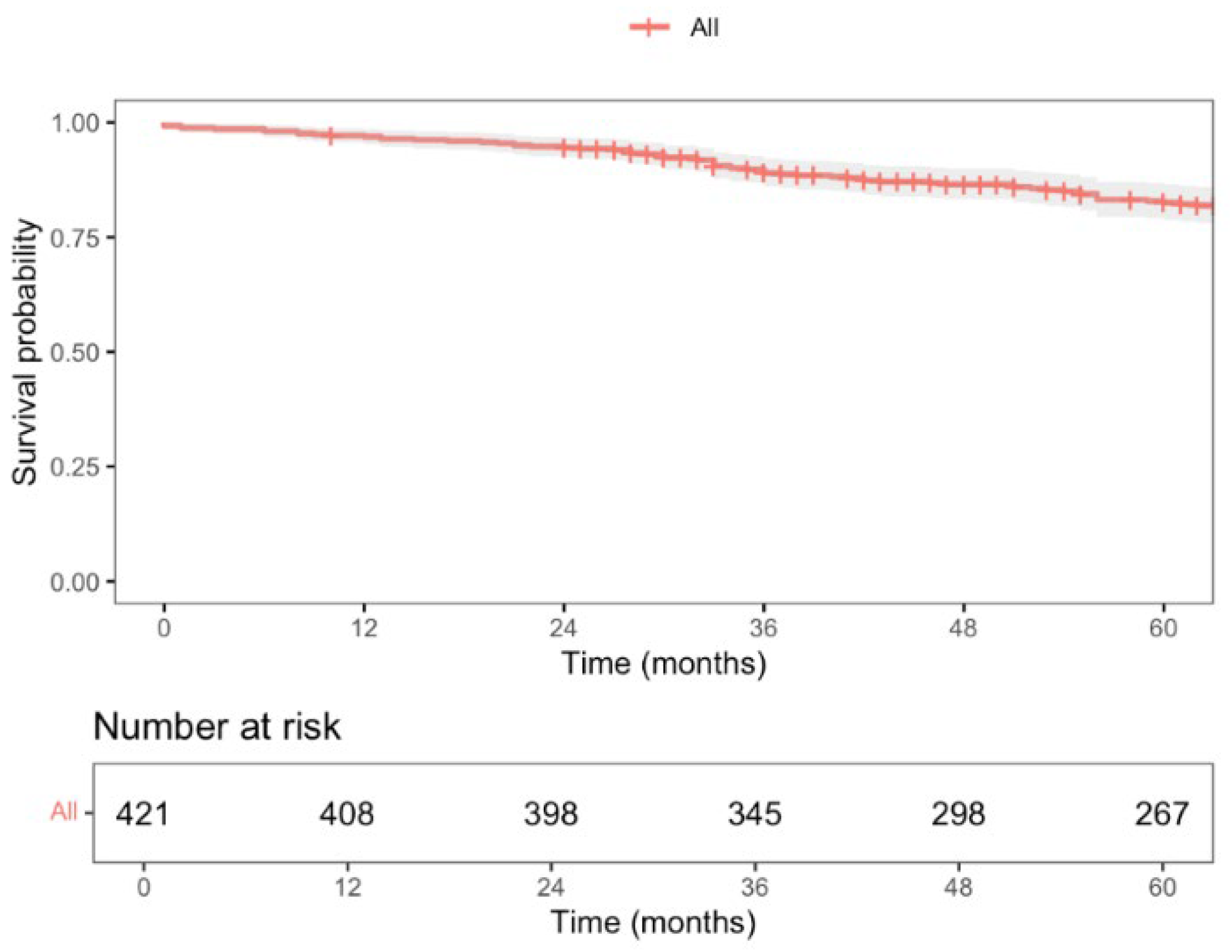
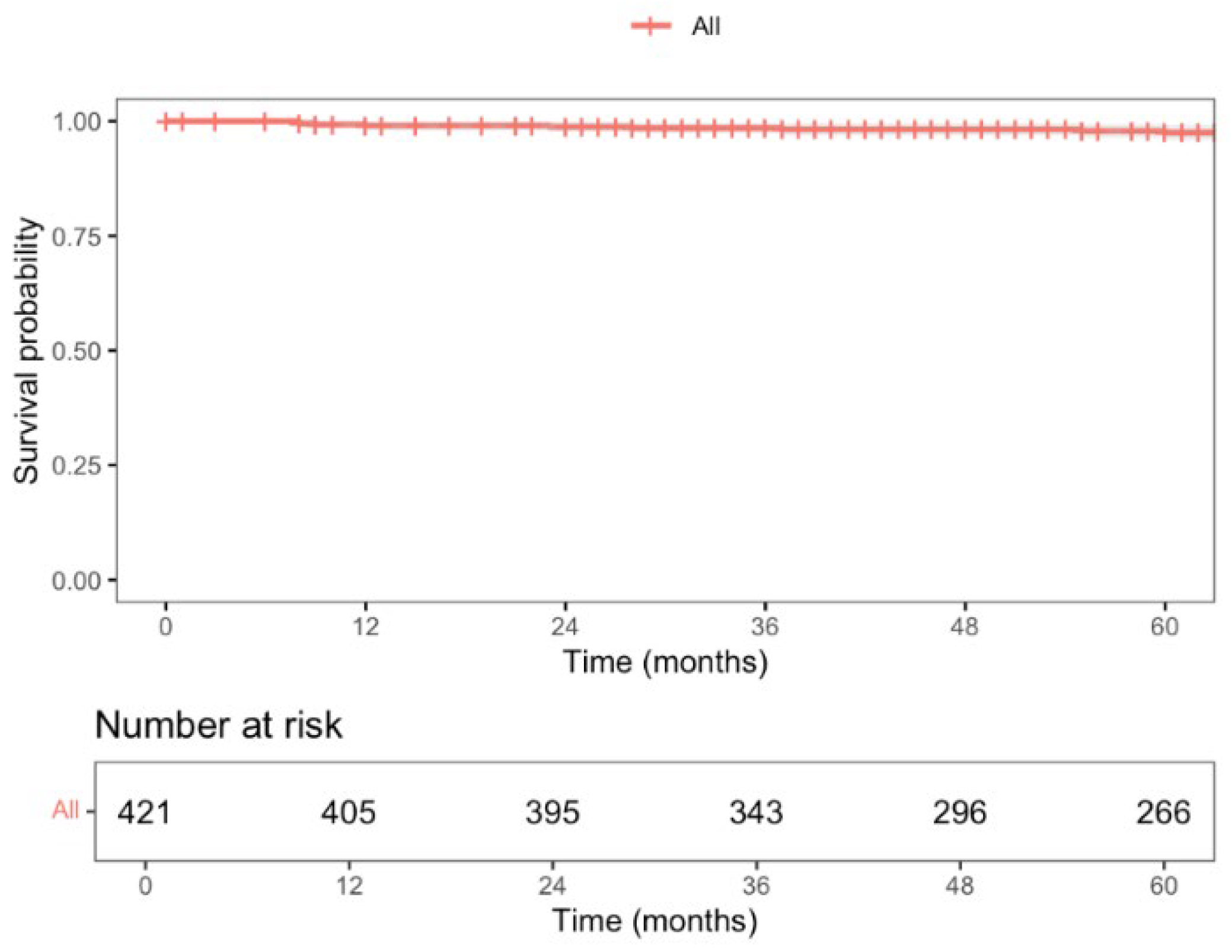
3.5. Long-Term Oncological Outcomes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Habr-Gama, A.; Perez, R.O.; Nadalin, W.; Sabbaga, J.; Ribeiro, U., Jr.; Silva e Sousa, A.H., Jr.; Campos, F.G.; Kiss, D.R.; Gama-Rodrigues, J. Operative Versus Nonoperative Treatment for Stage 0 Distal Rectal Cancer Following Chemoradiation Therapy: Long-term Results. Ann. Surg. 2004, 240, 711–717. [Google Scholar] [CrossRef] [PubMed]
- Donnelly, M.; Ryan, O.K.; Ryan, É.J.; Creavin, B.; O’Reilly, M.; McDermott, R.; Kennelly, R.; Hanly, A.; Martin, S.T.; Winter, D.C. Total neoadjuvant therapy versus standard neoadjuvant treatment strategies for the management of locally advanced rectal cancer: Network meta-analysis of randomized clinical trials. Br. J. Surg. 2023, 110, 1316–1330. [Google Scholar] [CrossRef]
- Heald, R.J.; Ryall, R.D. Recurrence and Survival after Total Mesorectal Excision for Rectal Cancer. Lancet 1986, 1, 1479–1482. [Google Scholar] [CrossRef] [PubMed]
- Bierbaum, V.; Bobeth, C.; Roessler, M.; Gerken, M.; Tol, K.K.; Reissfelder, C.; Fürst, A.; Günster, C.; Dröge, P.; Ruhnke, T.; et al. Treatment in Certified Cancer Centers Is Related to Better Survival in Patients with Colon and Rectal Cancer: Evidence from a Large German Cohort Study. World J. Surg. Oncol. 2024, 22, 11. [Google Scholar] [CrossRef] [PubMed]
- van der Pas, M.H.; Haglind, E.; Cuesta, M.A.; Fürst, A.; Lacy, A.M.; Hop, W.C.; Bonjer, H.J. COlorectal cancer Laparoscopic or Open Resection II (COLOR II) Study Group. Laparoscopic versus open surgery for rectal cancer (COLOR II): Short-term outcomes of a randomised, phase 3 trial. Lancet Oncol. 2013, 14, 210–218. [Google Scholar] [CrossRef]
- Bonjer, H.J.; Deijen, C.L.; Abis, G.A.; Cuesta, M.A.; van der Pas, M.H.; de Lange-de Klerk, E.S.; Lacy, A.M.; Bemelman, W.A.; Andersson, J.; Angenete, E.; et al. A randomized trial of laparoscopic versus open surgery for rectal cancer. N. Engl. J. Med. 2015, 372, 1324–1332. [Google Scholar] [CrossRef]
- Stevenson, A.R.; Solomon, M.J.; Lumley, J.W.; Hewett, P.; Clouston, A.D.; Gebski, V.J.; Davies, L.; Wilson, K.; Hague, W.; Simes, J.; et al. Effect of Laparoscopic-Assisted Resection vs Open Resection on Pathological Outcomes in Rectal Cancer: The ALaCaRT Randomized Clinical Trial. JAMA 2015, 314, 1356–1363. [Google Scholar] [CrossRef] [PubMed]
- Jayne, D.; Pigazzi, A.; Marshall, H.; Croft, J.; Corrigan, N.; Copeland, J.; Quirke, P.; West, N.; Rautio, T.; Thomassen, N.; et al. Effect of Robotic-Assisted vs. Conventional Laparoscopic Surgery on Risk of Conversion to Open Laparotomy Among Patients Undergoing Resection for Rectal Cancer: The ROLARR Randomized Clinical Trial. JAMA 2017, 318, 1569–1580. [Google Scholar] [CrossRef]
- Penna, M.; Hompes, R.; Arnold, S.; Wynn, G.; Austin, R.; Warusavitarne, J.; Moran, B.; Hanna, G.B.; Mortensen, N.J.; Tekkis, P.P. Incidence and Risk Factors for Anastomotic Failure in 1594 Patients Treated by Transanal Total Mesorectal Excision: Results from the International TaTME Registry. Ann. Surg. 2019, 269, 700–711. [Google Scholar] [CrossRef]
- O’Reilly, M.J.; Saye, W.B.; Mullins, S.G.; Pinto, S.E.; Falkner, P.T. Technique of hand-assisted laparoscopic surgery. J. Laparoendosc. Surg. 1996, 6, 239–244. [Google Scholar] [CrossRef]
- Ou, H. Laparoscopic-assisted mini laparatomy with colectomy. Dis. Colon. Rectum. 1995, 38, 324–326. [Google Scholar] [CrossRef] [PubMed]
- Bemelman, W.A.; Ringers, J.; Meijer, D.W.; de Wit, C.W.; Bannenberg, J.J. Laparoscopic-assisted colectomy with the dexterity pneumo sleeve. Dis. Colon. Rectum. 1996, 39 (Suppl. 10), S59–S61. [Google Scholar] [CrossRef]
- Mooney, M.J.; Elliott, P.L.; Galapon, D.B.; James, L.K.; Lilac, L.J.; O’Reilly, M.J. Hand-assisted laparoscopic sigmoidectomy for diverticulitis. Dis. Colon. Rectum. 1998, 41, 630–635. [Google Scholar] [CrossRef] [PubMed]
- Yang, I.; Boushey, R.P.; Marcello, P.W. Hand-assisted laparoscopic colorectal surgery. Tech. Coloproctol. 2013, 17 (Suppl. 1), S23–S27. [Google Scholar] [CrossRef]
- Kang, J.C.; Chung, M.H.; Chao, P.C.; Yeh, C.C.; Hsiao, C.W.; Lee, T.Y.; Jao, S.W. Hand-assisted laparoscopic colectomy vs open colectomy: A prospective randomized study. Surg. Endosc. 2004, 18, 577–581. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wu, Q.; Gu, C.; Hu, T.; Bi, L.; Wang, Z. Hand-assisted laparoscopic surgery versus conventional open surgery in intraoperative and postoperative outcomes for colorectal cancer: An updated systematic review and meta-analysis. Medicine 2017, 96, e7794. [Google Scholar] [CrossRef]
- Orenstein, S.B.; Elliott, H.L.; Reines, L.A.; Novitsky, Y.W. Advantages of the hand-assisted versus the open approach to elective colectomies. Surg. Endosc. 2011, 25, 1364–1368. [Google Scholar] [CrossRef]
- Benlice, C.; Costedio, M.; Stocchi, L.; Abbas, M.A.; Gorgun, E. Hand-assisted laparoscopic vs open colectomy: An assessment from the American College of Surgeons National Surgical Quality Improvement Program procedure-targeted cohort. Am. J. Surg. 2016, 212, 808–813. [Google Scholar] [CrossRef]
- Moloo, H.; Haggar, F.; Coyle, D.; Hutton, B.; Duhaime, S.; Mamazza, J.; Poulin, E.C.; Boushey, R.P.; Grimshaw, J. Hand assisted laparoscopic surgery versus conventional laparoscopy for colorectal surgery. Cochrane Database Syst. Rev. 2010, 6, CD006585. [Google Scholar] [CrossRef]
- HALS Study Group. Hand-assisted laparoscopic surgery vs standard laparoscopic surgery for colorectal disease: A prospective randomized trial. Surg. Endosc. 2000, 14, 896–901. [Google Scholar] [CrossRef]
- Marcello, P.W.; Fleshman, J.W.; Milsom, J.W.; Read, T.E.; Arnell, T.D.; Birnbaum, E.H.; Feingold, D.L.; Lee, S.W.; Mutch, M.G.; Sonoda, T.; et al. Hand-assisted laparoscopic vs. laparoscopic colorectal surgery: A multicenter, prospective, randomized trial. Dis. Colon. Rectum. 2008, 51, 818–826, discussion 826–828. [Google Scholar] [CrossRef] [PubMed]
- Targarona, E.M.; Gracia, E.; Garriga, J.; Martínez-Bru, C.; Cortés, M.; Boluda, R.; Lerma, L.; Trías, M. Prospective randomized trial comparing conventional laparoscopic colectomy with hand-assisted laparoscopic colectomy: Applicability, immediate clinical outcome, inflammatory response, and cost. Surg. Endosc. 2002, 16, 234–239. [Google Scholar] [CrossRef]
- Frois, A.O.; Huang, Y.; Young, C.J. Hand-assisted versus straight laparoscopy for colorectal surgery—A systematic review and meta-analysis. Int. J. Colorectal. Dis. 2022, 37, 2309–2319. [Google Scholar] [CrossRef]
- Cima, R.R.; Pendlimari, R.; Holubar, S.D.; Pattana-Arun, J.; Larson, D.W.; Dozois, E.J.; Wolff, B.G.; Pemberton, J.H. Utility and short-term outcomes of hand-assisted laparoscopic colorectal surgery: A single-institution experience in 1103 patients. Dis. Colon. Rectum. 2011, 54, 1076–1081. [Google Scholar] [CrossRef]
- Samalavicius, N.E.; Kuliesius, Z.; Samalavičius, R.S.; Tikuisis, R.; Smolskas, E.; Gricius, Z.; Kavaliauskas, P.; Dulskas, A. Hand Assisted Laparoscopic Surgery for Colorectal Cancer: Surgical and Oncological Outcomes from a Single Tertiary Referral Centre. J. Clin. Med. 2022, 29, 3781. [Google Scholar] [CrossRef]
- European Union. Regulation (EU) 2016/679 of the European Parliament and of the Council of 27 April 2016 on the Protection of Natural Persons with Regard to the Processing of Personal Data and on the Free Movement of Such Data, and Repealing Directive 95/46/EC (General Data Protection Regulation); European Union: Brussels, Belgium, 2016. [Google Scholar]
- Vandenbroucke, J.P.; von Elm, E.; Altman, D.G.; Gøtzsche, P.C.; Mulrow, C.D.; Pocock, S.J.; Poole, C.; Schlesselman, J.J.; Egger, M.; Strobe Initiative. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE): Explanation and elaboration. Epidemiology 2007, 18, 805–835. [Google Scholar] [CrossRef] [PubMed]
- Limbert, M.; de Almeida, J.M. Colorectal anastomosis after laparoscopic low anterior resection with total mesorectal excision: A difficult problem made simple. Dis. Colon. Rectum. 2009, 52, 2048–2050. [Google Scholar] [CrossRef]
- Charlson, M.; Szatrowski, T.P.; Peterson, J.; Gold, J. Validation of a combined comorbidity index. J. Clin. Epidemiol. 1994, 47, 1245–1251. [Google Scholar] [CrossRef] [PubMed]
- Orive, M.; Barrio, I.; Lázaro, S.; Gonzalez, N.; Bare, M.; de Larrea, N.F.; Redondo, M.; Cortajarena, S.; Bilbao, A.; Aguirre, U.; et al. Five-year follow-up mortality prognostic index for colorectal patients. Int. J. Colorectal. Dis. 2023, 38, 64. [Google Scholar] [CrossRef]
- Amin, M.B.; Greene, F.L.; Edge, S.B.; Compton, C.C.; Gershenwald, J.E.; Brookland, R.K.; Meyer, L.; Gress, D.M.; Byrd, D.R.; Winchester, D.P. The Eighth Edition AJCC Cancer Staging Manual: Continuing to build a bridge from a population-based to a more “personalized” approach to cancer staging. CA Cancer J. Clin. 2017, 67, 93–99. [Google Scholar] [CrossRef]
- Amer, M.H. Multiple neoplasms, single primaries, and patient survival. Cancer Manag. Res. 2014, 5, 119–134. [Google Scholar] [CrossRef] [PubMed]
- Dindo, D.; Demartines, N.; Clavien, P.A. Classification of surgical complications: A new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann. Surg. 2004, 240, 205–213. [Google Scholar] [CrossRef]
- Kulu, Y.; Ulrich, A.; Bruckner, T.; Contin, P.; Welsch, T.; Rahbari, N.N.; Büchler, M.W.; Weitz, J.; International Study Group of Rectal Cancer. Validation of the International Study Group of Rectal Cancer definition and severity grading of anastomotic leakage. Surgery 2013, 153, 753–761. [Google Scholar] [CrossRef] [PubMed]
- Quirke, P.; Steele, R.; Monson, J.; Grieve, R.; Khanna, S.; Couture, J.; O’Callaghan, C.; Myint, A.S.; Bessell, E.; Thompson, L.C.; et al. Effect of the plane of surgery achieved on local recurrence in patients with operable rectal cancer: A prospective study using data from the MRC CR07 and NCIC-CTG CO16 randomised clinical trial. Lancet 2009, 373, 821–828. [Google Scholar] [CrossRef] [PubMed]
- Ryan, R.; Gibbons, D.; Hyland, J.M.; Treanor, D.; White, A.; Mulcahy, H.E.; O’Donoghue, D.P.; Moriarty, M.; Fennelly, D.; Sheahan, K. Pathological response following long-course neoadjuvant chemoradiotherapy for locally advanced rectal cancer. Histopathology 2005, 47, 141–146. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2024; Available online: https://www.R-project.org/ (accessed on 5 June 2025).
- Lee, L.; de Lacy, B.; Gomez Ruiz, M.; Liberman, A.S.; Albert, M.R.; Monson, J.R.T.; Lacy, A.; Kim, S.H.; Atallah, S.B. A Multicenter Matched Comparison of Transanal and Robotic Total Mesorectal Excision for Mid and Low-rectal Adenocarcinoma. Ann. Surg. 2019, 270, 1110–1116. [Google Scholar] [CrossRef]
- Khan, S.; Kaltenmeier, C.; Hrebinko, K.; Nassour, I.; Hoehn, R.S.; Medich, D.S.; Zureikat, A.; Tohme, S. Readmission After Surgical Resection for Colon and Rectal Cancers: A Retrospective Cohort Study. Am. Surg. 2022, 88, 1118–1130. [Google Scholar] [CrossRef]
- Ban, K.A.; Berian, J.R.; Ko, C.Y. Does Implementation of Enhanced Recovery after Surgery (ERAS) Protocols in Colorectal Surgery Improve Patient Outcomes? Clin. Colon. Rectal. Surg. 2019, 32, 109–113. [Google Scholar]
- Gavriilidis, P.; Azoulay, D.; Taflampas, P. Loop transverse colostomy versus loop ileostomy for defunctioning of colorectal anastomosis: A systematic review, updated conventional meta-analysis, and cumulative meta-analysis. Surg. Today 2019, 49, 108–117. [Google Scholar] [CrossRef]
- Yagyu, T.; Hamada, M.; Hatta, M.; Kobayashi, T.; Matsumi, Y.; Inada, R.; Matsumoto, T.; Oishi, M. Impact of the Diverting Stoma on Renal Function. Dis. Colon. Rectum. 2024, 67, 1576–1583. [Google Scholar] [CrossRef]
- Pecorelli, N.; Greco, M.; Amodeo, S.; Braga, M. Small bowel obstruction and incisional hernia after laparoscopic and open colorectal surgery: A meta-analysis of comparative trials. Surg. Endosc. 2017, 31, 85–99. [Google Scholar] [CrossRef] [PubMed]
- Petersson, J.; Koedam, T.W.; Bonjer, H.J.; Andersson, J.; Angenete, E.; Bock, D.; Cuesta, M.A.; Deijen, C.L.; Fürst, A.; Lacy, A.M.; et al. Bowel Obstruction and Ventral Hernia After Laparoscopic Versus Open Surgery for Rectal Cancer in A Randomized Trial (COLOR II). Ann. Surg. 2019, 269, 53–57. [Google Scholar] [CrossRef] [PubMed]
- Park, J.W.; Kang, S.B.; Hao, J.; Lim, S.B.; Choi, H.S.; Kim, D.W.; Chang, H.J.; Kim, D.Y.; Jung, K.H.; Kim, T.Y.; et al. Open versus laparoscopic surgery for mid or low rectal cancer after neoadjuvant chemoradiotherapy (COREAN trial): 10-year follow-up of an open-label, non-inferiority, randomised controlled trial. Lancet Gastroenterol. Hepatol. 2021, 6, 569–577. [Google Scholar] [CrossRef] [PubMed]
- Lacy, A.M.; Tasende, M.M.; Delgado, S.; Fernandez-Hevia, M.; Jimenez, M.; De Lacy, B.; Castells, A.; Bravo, R.; Wexner, S.D.; Heald, R.J. Transanal Total Mesorectal Excision for Rectal Cancer: Outcomes after 140 Patients. J. Am. Coll. Surg. 2015, 221, 415–423. [Google Scholar] [CrossRef]

| Direct Surgery | CRT + CT (TNT) | SCRT + CT (TNT) | |
|---|---|---|---|
| High rectum (>11–15 cm) or RSJ | ≤cT3a-b N0/N1 MRF− EMVI− | cT4b MRF+ (any cN, EMVI) | cT3c-d/T4a cN2 EMVI+ (MRF−) |
| Mid rectum (>6–11 cm) | ≤cT3a-b N0 MRF− EMVI− | cT4b MRF+ (any cN, EMVI) | cT3c-d/T4a cN1/N2 EMVI+ (MRF−) |
| Low rectum * (0–6 cm) | cT1 N0 MRF− EMVI− | ≥cT2 cN+ MRF+ EMVI+ |
| Patient and Tumor Characteristics | n = 469 | |
|---|---|---|
| Age at time of surgery in years, median (IQR) | 66 (57–74) | |
| Male sex, n (%) | 296 (63.1) | |
| ASA score, n (%) | ||
| I–II | 383 (81.6) | |
| III-–V | 86 (18.4) | |
| CCI score | ||
| Median (IQR) | 3 (1–4) | |
| ≥5, n (%) | 52 (11.1) | |
| BMI | ||
| Median (IQR), kg/m2 | 25.7 (23.3–28.1) | |
| ≥30 kg/m2, n (%) | 63 (13.4) | |
| Preoperative anemia, n (%) | 125 (26.7) | |
| Previous abdominal surgery, n (%) | 80 (17.1) | |
| Tumor location, n (%) | ||
| Rectosigmoid junction | 35 (7.5) | |
| High rectum | 111 (23.7) | |
| Mid rectum | 217 (46.2) | |
| Low rectum | 106 (22.6) | |
| Staging, n (%) | ||
| cT | T1–T2 | 99 (21.1) |
| T3–T4 | 370 (78.9) | |
| cN | N0 | 135 (28.8) |
| N+ | 334 (71.2) | |
| cM | M0 | 449 (95.7) |
| M1 | 20 (4.3) | |
| cTNM | I | 70 (14.9) |
| II | 66 (14.1) | |
| III | 313 (66.7) | |
| IV | 21 (4.3) | |
| Neoadjuvant therapy, n (%) | 314 (70.0) | |
| Isolated CRT | 252 (53.7) | |
| Isolated SCRT | 51 (10.9) | |
| TNT | 6 (1.3) | |
| Conversion CT + CRT/SCRT (cM1) | 5 (1.1) | |
| Synchronous tumors, n (%) | 9 (1.9) | |
| Prostate | 2 | |
| Ependymoma | 1 | |
| Gastric adenocarcinoma | 1 | |
| Hepatocarcinoma | 1 | |
| Thymoma | 1 | |
| Pancreatic NET | 1 | |
| Pulmonary NET | 1 | |
| Primary-occult NET | 1 | |
| Intraoperative Outcomes | n = 469 | |
|---|---|---|
| RAR type, n (%) | ||
| High RAR | 126 (26.9) | |
| Low RAR | 343 (73.1) | |
| Anastomosis, n (%) | 446 (95.1) | |
| Type, n (%)—n = 446 | Colorectal | 390 (87.4) |
| Coloanal | 56 (12.6) | |
| Technique, n (%)—n = 446 | Mechanic (circular stapler) | 411 (92.2) |
| Manual | 31 (7.0) | |
| Missing | 4 (0.8) | |
| Configuration, n (%)—n = 446 | End-to-end | 367 (82.3) |
| Lateral-to-end | 78 (17.5) | |
| Missing | 1 (0.2) | |
| Protective stoma, n (%)—n = 446 | 375 (84.0) | |
| Type, n (%)—n = 373 | Transversostomy | 366 (97.6) |
| Ileostomy | 9 (2.4) | |
| By RAR type, n (%) | High RAR—n = 121 | 52 (43.0) |
| Low RAR—n = 325 | 322 (99.1) | |
| Terminal stoma, n (%) | 23 (4.9) | |
| Other visceral resections, n (%) | 13 (2.8) | |
| Appendicectomy | 1 | |
| Enterectomy | 2 | |
| Hepatic excisional biopsy | 2 | |
| Mesenteric implant excision | 1 | |
| Partial cystectomy | 1 | |
| Partial prostatectomy | 1 | |
| Splenectomy | 1 | |
| Uni- or bilateral seminal vesical excision | 2 | |
| Uni- or bilateral oophorectomy | 2 | |
| Operative time in minutes, median (IQR) | 152 (135–180) | |
| Estimated blood loss in mL, median (IQR) | 75 (50–200) | |
| Conversion to open surgery, n (%) | 18 (3.8) | |
| Adhesions (previous surgery) | 3 | |
| Bleeding | 3 | |
| Urinary tract injury (or suspected) | 3 | |
| Tumor invagination or perforation | 2 | |
| Suspected carcinomatosis | 1 | |
| Missing | 5 | |
| Postoperative Outcomes | n = 469 |
|---|---|
| Major morbility (Clavien-Dindo ≥ III), n (%) | 47 (10.0) |
| IIIa | 12 (2.6) |
| IIIb | 26 (5.5) |
| IVa | 2 (0.4) |
| IVb | 1 (0.2) |
| V | 6 (1.3) |
| Reoperation (≤30 days), n (%) | 30 (6.4) |
| Postoperative mortality, n (%) | |
| 30 days | 4 (0.9) |
| 90 days | 6 (1.3) |
| Length of hospital stay in days, median (IQR) | 5 (4–7) |
| Hospital early readmission (≤30 days), n (%) | 25 (5.3) |
| Overall anastomotic leak, n (%)—n = 446 | 54 (12.1) |
| SSI, n (%) | 52 (11.1) |
| Superficial and deep incisional | 34 (7.3) |
| Organ or space | 18 (3.8) |
| Stoma-related complications, n (%)—n = 396 | 12 (3.0) |
| Mucocutaneous dehiscence | 6 |
| Perforation | 3 |
| Stenosis | 2 |
| Prolapse | 1 |
| Incisional hernia related to HALS, n (%) | 57 (12.1) |
| Previous stoma/GelPort® site | 53 |
| GelPort® site without stoma | 1 |
| Trocar site | 3 |
| Anastomotic Leak | n = 446 | p-Value |
|---|---|---|
| Overall, n (%) | 54 (12.1) | |
| Timing of presentation, n (%) | ||
| Early (≤30 days) | 40 (9.0) | |
| Late (>30 days) | 14 (3.1) | |
| Severity grading, n (%)—n = 54 | ||
| A—without active therapeutic intervention | 5 (9.3) | |
| B—nonsurgical intervention | 24 (44.4) | |
| C—C1—surgical intervention, with anastomosis preservation | 14 (25.9) | |
| —C2—surgical intervention, without anastomosis preservation | 11 (20.4) | |
| By surgery type | ||
| High RAR—n = 121 | 6 (5.0) | |
| Low RAR—n = 325 | 48 (14.8) | 0.005 |
| By anastomosis type | ||
| Colorectal—n = 390 | 36 (9.2) | |
| Coloanal—n = 56 | 18 (32.1) | <0.005 |
| Management, n (%)—n = 54 | ||
| No treatment | 5 (9.3) | |
| Conservative (drainage and antibiotic) | 22 (40.7) | |
| Protective stoma | 20 | |
| Endoscopic negative pressure therapy (EndoSponge®) | 13 (24.1) | |
| Protective stoma | 13 | |
| Endoscopic clip | 5 (9.3) | |
| Protective stoma | 2 | |
| Anastomotic takedown | 11 (20.4) | |
| After failure of conservative or endoscopic treatment | 2 | |
| Leak resolution with anastomosis preservation, n (%)—n = 54 | 31 (57.4) | |
| Stoma closure (in those with leak), n (%)—n = 54 | 26 (48.1) |
| Pathologic Outcomes | n = 469 |
|---|---|
| Mesorectum quality, n (%)—n = 387 | |
| Complete | 285 (73.6) |
| Near-complete | 62 (16.0) |
| Incomplete | 40 (10.4) |
| (not described) | (82) |
| Lymph node count, median (IQR) | 15 (12–21) |
| Resection radicality, n (%) | |
| R0 | 451 (96.2) |
| R1 | 18 (3.8) |
| R2 | 0 (0.0) |
| Positive resection margins, n (%) | |
| CRM | 17 (3.6) |
| DRM | 2 (0.4) |
| Tumor regression grade, n (%)—n = 314 | |
| 0 (complete response) | 51 (16.2) |
| 1 (near-complete response) | 67 (21.3) |
| 2 (partial response) | 136 (43.3) |
| 3 (poor/no response) | 53 (16.9) |
| Not described | 7 (2.3) |
| (y)pTNM, n (%) | |
| 0 | 56 (11.9) |
| I | 155 (33.1) |
| II | 112 (23.9) |
| III | 121 (25.8) |
| IV | 25 (5.3) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gonçalves, B.; Costeira, B.; Fonseca, F.; Cabral, F.; Caiado, A.; Cavadas, D.; Maciel, J.; Limbert, M. Hand-Assisted Laparoscopic Rectal Resection—Experience of a Tertiary Oncology Center. J. Clin. Med. 2025, 14, 4097. https://doi.org/10.3390/jcm14124097
Gonçalves B, Costeira B, Fonseca F, Cabral F, Caiado A, Cavadas D, Maciel J, Limbert M. Hand-Assisted Laparoscopic Rectal Resection—Experience of a Tertiary Oncology Center. Journal of Clinical Medicine. 2025; 14(12):4097. https://doi.org/10.3390/jcm14124097
Chicago/Turabian StyleGonçalves, Beatriz, Beatriz Costeira, Filipa Fonseca, Francisco Cabral, André Caiado, Daniela Cavadas, João Maciel, and Manuel Limbert. 2025. "Hand-Assisted Laparoscopic Rectal Resection—Experience of a Tertiary Oncology Center" Journal of Clinical Medicine 14, no. 12: 4097. https://doi.org/10.3390/jcm14124097
APA StyleGonçalves, B., Costeira, B., Fonseca, F., Cabral, F., Caiado, A., Cavadas, D., Maciel, J., & Limbert, M. (2025). Hand-Assisted Laparoscopic Rectal Resection—Experience of a Tertiary Oncology Center. Journal of Clinical Medicine, 14(12), 4097. https://doi.org/10.3390/jcm14124097






