Impact of Surgical Margin Distance on Oncologic Outcomes in Vulvar Squamous Cell Carcinoma
Abstract
1. Introduction
2. Methods
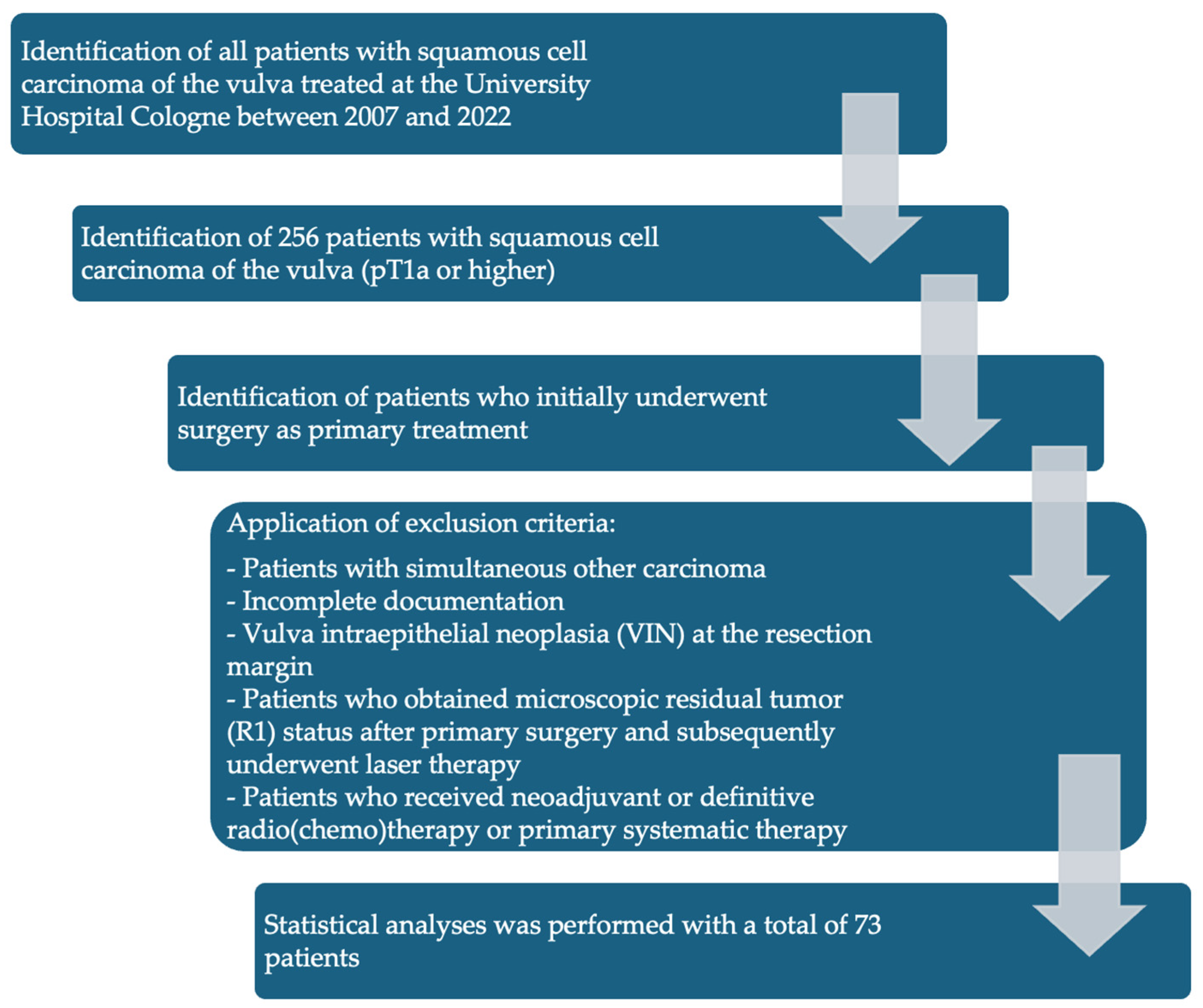
- Patients with SCC of the vulva treated between 2007 and 2022 at the University Hospital Cologne.
- SCC with TNM stage pT1a (pathological tumor invasion ≤ 1 mm and diameter ≤ 2 cm) or higher who initially underwent surgery as primary treatment.
- Patients who did not undergo surgical therapy as primary treatment.
- Patients with simultaneous other carcinoma.
- Incomplete documentation.
- Vulva intraepithelial neoplasia (VIN) at the resection margin.
- Patients who obtained microscopic residual tumor (R1) status after primary surgery and subsequently underwent laser therapy.
- Patients who received neoadjuvant radio (chemo) therapy were excluded from this study.
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dittmer, C.; Katalinic, A.; Mundhenke, C.; Thill, M.; Fischer, D. Epidemiology of vulvar and vaginal cancer in Germany. Arch. Gynecol. Obstet. 2011, 284, 169–174. [Google Scholar] [CrossRef]
- Judson, P.L.; Habermann, E.B.; Baxter, N.N.; Durham, S.B.; Virnig, B.A. Trends in the incidence of invasive and in situ vulvar carcinoma. Obstet. Gynecol. 2006, 107, 1018–1022. [Google Scholar] [CrossRef] [PubMed]
- Chhabra, S.; Bhavani, M.; Deshpande, A. Trends of vulvar cancer. J. Obstet. Gynaecol. 2014, 34, 165–168. [Google Scholar] [CrossRef] [PubMed]
- Robert Koch Institut. Zentrum für Krebsregisterdaten. 2020. Available online: https://www.krebsdaten.de/Krebs/DE/Content/Krebsarten/Vulvakrebs/vulvakrebs_node.html (accessed on 22 January 2025).
- Mix, J.M.; Gopalani, S.V.; Simko, S.; Saraiya, M. Trends in HPV- and non-HPV-associated vulvar cancer incidence, United States, 2001–2017. Prev. Med. 2022, 164, 107302. [Google Scholar] [CrossRef]
- Berenson, A.B.; Chang, M.; Hawk, E.T.; Ramondetta, L.M.; Hoang, T. Vulvar Cancer Incidence in the United States and its Relationship to Human Papillomavirus Vaccinations, 2001–2018. Cancer Prev. Res. 2022, 15, 777–784. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Almadani, N.; Thompson, E.F.; Tessier-Cloutier, B.; Chen, J.; Ho, J.; Senz, J.; McConechy, M.K.; Chow, C.; Ta, M.; et al. Classification of Vulvar Squamous Cell Carcinoma and Precursor Lesions by p16 and p53 Immunohistochemistry: Considerations, Caveats, and an Algorithmic Approach. Mod. Pathol. 2023, 36, 100145. [Google Scholar] [CrossRef] [PubMed]
- AWMF. S2k-Leitlinie Vulvakarzinom und Seine Vorstufen, Diagnostik und Therapie; AWMF: Frankfurt, Germany, 2020. [Google Scholar]
- Magrina, J.F.; Gonzalez-Bosquet, J.; Weaver, A.L.; Gaffey, T.A.; Webb, M.J.; Podratz, K.C.; Cornella, J.L. Primary squamous cell cancer of the vulva: Radical versus modified radical vulvar surgery. Gynecol. Oncol. 1998, 71, 116–121. [Google Scholar] [CrossRef]
- Pahmeyer, C.; Thangarajah, F.; Ratiu, D.; Schultheis, A.M.; Schomig-Markiefka, B.; Mallmann, P.; Morgenstern, B. Preoperative biopsies as predictor for the necessity of inguinal lymph node surgery in squamous cell carcinoma of the vulva-a retrospective tertiary center analysis. J. Cancer Res. Clin. Oncol. 2020, 146, 2709–2712. [Google Scholar] [CrossRef]
- Macchia, G.; Casa, C.; Ferioli, M.; Lancellotta, V.; Pezzulla, D.; Pappalardi, B.; Laliscia, C.; Ippolito, E.; Di Muzio, J.; Huscher, A.; et al. Observational multicenter Italian study on vulvar cancer adjuvant radiotherapy (OLDLADY 1.2): A cooperation among AIRO Gyn, MITO and MaNGO groups. Radiol. Med. 2022, 127, 1292–1302. [Google Scholar] [CrossRef]
- Thangarajah, F.; Rogee, K.; Pahmeyer, C.; Kuhr, K.; Schmidt, M.; Fridrich, C.; Morgenstern, B. Morbidity and quality of life in patients with vulvar cancer after inguinal sentinel lymphadenectomy compared to radical inguinofemoral lymphadenectomy. Nuklearmedizin 2021, 60, 368–374. [Google Scholar] [CrossRef]
- Zafarnia, M.; Kennes, L.N.; Stickeler, E.; Hoff, J.; Najjari, L. Evaluation of urinary continence status and its influence on quality of life after gyneco-oncological treatment of female pelvic malignancies at an oncological center. BMC Women’s Health 2022, 22, 422. [Google Scholar] [CrossRef] [PubMed]
- Tantipalakorn, C.; Robertson, G.; Marsden, D.E.; Gebski, V.; Hacker, N.F. Outcome and patterns of recurrence for International Federation of Gynecology and Obstetrics (FIGO) stages I and II squamous cell vulvar cancer. Obstet. Gynecol. 2009, 113, 895–901. [Google Scholar] [CrossRef]
- Chan, J.K.; Sugiyama, V.; Pham, H.; Gu, M.; Rutgers, J.; Osann, K.; Cheung, M.K.; Berman, M.L.; Disaia, P.J. Margin distance and other clinico-pathologic prognostic factors in vulvar carcinoma: A multivariate analysis. Gynecol. Oncol. 2007, 104, 636–641. [Google Scholar] [CrossRef]
- De Hullu, J.A.; Hollema, H.; Lolkema, S.; Boezen, M.; Boonstra, H.; Burger, M.P.; Aalders, J.G.; Mourits, M.J.; Van Der Zee, A.G. Vulvar carcinoma. The price of less radical surgery. Cancer 2002, 95, 2331–2338. [Google Scholar] [CrossRef]
- Iacoponi, S.; Zapardiel, I.; Diestro, M.D.; Hernandez, A.; De Santiago, J. Prognostic factors associated with local recurrence in squamous cell carcinoma of the vulva. J. Gynecol. Oncol. 2013, 24, 242–248. [Google Scholar] [CrossRef]
- Groenen, S.M.; Timmers, P.J.; Burger, C.W. Recurrence rate in vulvar carcinoma in relation to pathological margin distance. Int. J. Gynecol. Cancer 2010, 20, 869–873. [Google Scholar] [CrossRef]
- Woelber, L.; Choschzick, M.; Eulenburg, C.; Hager, M.; Jaenicke, F.; Gieseking, F.; Kock, L.; Ihnen, M.; Petersen, C.; Schwarz, J.; et al. Prognostic value of pathological resection margin distance in squamous cell cancer of the vulva. Ann. Surg. Oncol. 2011, 18, 3811–3818. [Google Scholar] [CrossRef] [PubMed]
- Nooij, L.S.; van der Slot, M.A.; Dekkers, O.M.; Stijnen, T.; Gaarenstroom, K.N.; Creutzberg, C.L.; Smit, V.T.; Bosse, T.; van Poelgeest, M.I. Tumour-free margins in vulvar squamous cell carcinoma: Does distance really matter? Eur. J. Cancer 2016, 65, 139–149. [Google Scholar] [CrossRef] [PubMed]
- Woelber, L.; Griebel, L.F.; Eulenburg, C.; Sehouli, J.; Jueckstock, J.; Hilpert, F.; de Gregorio, N.; Hasenburg, A.; Ignatov, A.; Hillemanns, P.; et al. Role of tumour-free margin distance for loco-regional control in vulvar cancer—A subset analysis of the Arbeitsgemeinschaft Gynakologische Onkologie CaRE-1 multicenter study. Eur. J. Cancer 2016, 69, 180–188. [Google Scholar] [CrossRef]
- Balega, J.; Butler, J.; Jeyarajah, A.; Oram, D.; Shepherd, J.; Faruqi, A.; Singh, N.; Reynolds, K. Vulval cancer: What is an adequate surgical margin? Eur. J. Gynaecol. Oncol. 2008, 29, 455–458. [Google Scholar]
- Palaia, I.; Bellati, F.; Calcagno, M.; Musella, A.; Perniola, G.; Panici, P.B. Invasive vulvar carcinoma and the question of the surgical margin. Int. J. Gynaecol. Obstet. 2011, 114, 120–123. [Google Scholar] [CrossRef] [PubMed]
- Abu-Rustum, N.R.; Yashar, C.M.; Arend, R.; Barber, E.; Bradley, K.; Brooks, R.; Campos, S.M.; Chino, J.; Chon, H.S.; Crispens, M.A.; et al. Vulvar Cancer, Version 3.2024, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2024, 22, 117–135. [Google Scholar] [CrossRef]
- Oonk, M.H.M.; Planchamp, F.; Baldwin, P.; Mahner, S.; Mirza, M.R.; Fischerova, D.; Creutzberg, C.L.; Guillot, E.; Garganese, G.; Lax, S.; et al. European Society of Gynaecological Oncology Guidelines for the Management of Patients with Vulvar Cancer—Update 2023. Int. J. Gynecol. Cancer 2023, 33, 1023–1043. [Google Scholar] [CrossRef]
- Heaps, J.M.; Fu, Y.S.; Montz, F.J.; Hacker, N.F.; Berek, J.S. Surgical-pathologic variables predictive of local recurrence in squamous cell carcinoma of the vulva. Gynecol. Oncol. 1990, 38, 309–314. [Google Scholar] [CrossRef] [PubMed]
- Viswanathan, A.N.; Pinto, A.P.; Schultz, D.; Berkowitz, R.; Crum, C.P. Relationship of margin status and radiation dose to recurrence in post-operative vulvar carcinoma. Gynecol. Oncol. 2013, 130, 545–549. [Google Scholar] [CrossRef]
- Raimond, E.; Delorme, C.; Ouldamer, L.; Carcopino, X.; Bendifallah, S.; Touboul, C.; Darai, E.; Ballester, M.; Graesslin, O.; Research group FRANCOGYN. Surgical treatment of vulvar cancer: Impact of tumor-free margin distance on recurrence and survival. A multicentre cohort analysis from the francogyn study group. Eur. J. Surg. Oncol. 2019, 45, 2109–2114. [Google Scholar] [CrossRef]
- Nomura, H.; Omi, M.; Netsu, S.; Aoki, Y.; Tanigawa, T.; Kurita, T.; Matoda, M.; Okamoto, S.; Omatsu, K.; Kanao, H. Positive surgical margin is an independent predictor of overall survival of patients with vulvar squamous cell carcinoma. J. Obstet. Gynaecol. Res. 2021, 47, 3990–3997. [Google Scholar] [CrossRef] [PubMed]
- Taran, F.A.; Pasternak, J.; Staebler, A.; Rohner, A.; Neis, F.; Engler, T.; Oberlechner, E.; Schonfisch, B.; Juhasz-Boss, I.; Hartkopf, A.D.; et al. Tumor-Free Resection Margin Distance in the Surgical Treatment of Node-Negative Squamous Cell Cancer of the Vulva Has No Impact on Survival: Analysis of a Large Patient Cohort in a Tertiary Care Center. Cancers 2023, 15, 4110. [Google Scholar] [CrossRef] [PubMed]
- Baiocchi, G.; Mantoan, H.; de Brot, L.; Badiglian-Filho, L.; Kumagai, L.Y.; Faloppa, C.C.; da Costa, A.A. How important is the pathological margin distance in vulvar cancer? Eur. J. Surg. Oncol. 2015, 41, 1653–1658. [Google Scholar] [CrossRef]
- Hockel, M.; Trott, S.; Dornhofer, N.; Horn, L.C.; Hentschel, B.; Wolf, B. Vulvar field resection based on ontogenetic cancer field theory for surgical treatment of vulvar carcinoma: A single-centre, single-group, prospective trial. Lancet Oncol. 2018, 19, 537–548. [Google Scholar] [CrossRef]
- Thangarajah, F.; Hüser, A.; Höckel, M. Operative Therapie des Vulvakarzinoms: Krebsfeldchirurgie vs. Standardtherapie. Die Gynäkologie 2024, 57, 482–489. [Google Scholar] [CrossRef]



| All Patients | Group 1 | Group 2 | Group 3 | |||||
|---|---|---|---|---|---|---|---|---|
| Mean age (in years) | 59.1 (SD = 13.8) | 60.1 (SD = 15.1) | 59.6 (SD = 12.7) | 54.7 (SD = 14.1) | ||||
| Mean BMI (in kg/m2) | 27.6 (SD = 7.3) | 27.9 (SD = 6.9) | 26.8 (SD = 7.7) | 29.4 (SD = 8.0) | ||||
| Mean size of tumor (in cm) | 1.7 (SD = 1.2; 0.1–4.7 cm) | 1.6 (SD = 1.2) | 1.9 (SD = 1.3) | 1.3 (SD = 0.6) | ||||
| Mean depth of infiltration (in cm) | 0.32 (SD = 0.32; 0.01–1.5 cm) | 0.35 (SD = 0.4) | 0.33 (SD = 0.3) | 2.2 (SD = 0.3) | ||||
| n | % | n | % | n | % | n | % | |
| pT1 | 2 | 2.7% | 0 | 0.0% | 1 | 50.0% | 1 | 50.0% |
| pT1a | 15 | 20.6% | 5 | 33.3% | 9 | 60.0% | 1 | 6.7% |
| pT1b | 54 | 74.0% | 22 | 40.7% | 23 | 42.6% | 9 | 16.7% |
| pT2 | 2 | 2.7% | 0 | 0.00% | 2 | 100.0% | 0 | 0.0% |
| pN0 | 50 | 68.5% | 19 | 38.0% | 23 | 46.0% | 8 | 16.0% |
| pNx | 9 | 12.3% | 2 | 22.2% | 6 | 66.7% | 1 | 11.1% |
| pN1a | 8 | 11.0% | 2 | 25.0% | 4 | 50.0% | 2 | 25.0% |
| pN1b | 1 | 1.4% | 0 | 0.0% | 1 | 100.0% | 0 | 0.0% |
| pN1mi | 1 | 1.4% | 1 | 100.0% | 0 | 0.0% | 0 | 0.0% |
| pN2c | 4 | 5.5% | 3 | 75.0% | 1 | 25.0% | 0 | 0.0% |
| G1 | 2 | 2.7% | 0 | 0.0% | 0 | 0.0% | 2 | 100.0% |
| G2 | 58 | 79.5% | 26 | 44.8% | 25 | 43.1% | 7 | 12.1% |
| G3 | 13 | 17.8% | 1 | 7.7% | 10 | 76.9% | 2 | 15.4% |
| Recurrence | Death | Local Recurrence | LN Recurrence | Metastasis | Local and LN Recurrence | |||
|---|---|---|---|---|---|---|---|---|
| Yes | No | Yes | No | |||||
| All patients (n = 73) | 19 (26.0%) | 54 (74.0%) | 5 (6.9%) | 68 (93.2%) | 13 (17.8%) | 4 (5.5%) | 1 (1.4%) | 1 (1.4%) |
| Group 1 (n = 27) | 6 (31.5%) | 21 (38.9%) | 0 (0.0%) | 27 (100.0%) | 3 (11.1%) | 2 (7.4%) | 1 (3.7%) | 0 (0.0%) |
| Group 2 (n = 35) | 8 (42.1%) | 27 (50.0%) | 4 (11.4%) | 31 (88.6%) | 8 (22.9%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) |
| Group 3 (n = 11) | 5 (26.3%) | 6 (11.1%) | 1 (9.1%) | 10 (90.9%) | 2 (18.2%) | 2 (18.2%) | 0 (0.0%) | 1 (9.1%) |
| Nodal negative patients (n = 59) | 14 (23.7%) | 45 (76.3%) | 3 (5.1%) | 56 (94.9%) | ||||
| N0 Group 1 (n = 21) | 4 (28.6%) | 17 (37.8%) | 0 (0.0%) | 21 (37.5%) | ||||
| N0 Group 2 (n = 25) | 6 (42.9) | 23 (51.1%) | 3 (100.%) | 26 (46.4%) | ||||
| N0 Group 3 (n = 8) | 4 (28.6%) | 5 (11.1%) | 0 (0.0%) | 9 (16.1%) | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lenz, C.; Lyu, S.I.; Mallmann, P.; Morgenstern, B.; Thangarajah, F. Impact of Surgical Margin Distance on Oncologic Outcomes in Vulvar Squamous Cell Carcinoma. J. Clin. Med. 2025, 14, 4057. https://doi.org/10.3390/jcm14124057
Lenz C, Lyu SI, Mallmann P, Morgenstern B, Thangarajah F. Impact of Surgical Margin Distance on Oncologic Outcomes in Vulvar Squamous Cell Carcinoma. Journal of Clinical Medicine. 2025; 14(12):4057. https://doi.org/10.3390/jcm14124057
Chicago/Turabian StyleLenz, Caroline, Su Ir Lyu, Peter Mallmann, Bernd Morgenstern, and Fabinshy Thangarajah. 2025. "Impact of Surgical Margin Distance on Oncologic Outcomes in Vulvar Squamous Cell Carcinoma" Journal of Clinical Medicine 14, no. 12: 4057. https://doi.org/10.3390/jcm14124057
APA StyleLenz, C., Lyu, S. I., Mallmann, P., Morgenstern, B., & Thangarajah, F. (2025). Impact of Surgical Margin Distance on Oncologic Outcomes in Vulvar Squamous Cell Carcinoma. Journal of Clinical Medicine, 14(12), 4057. https://doi.org/10.3390/jcm14124057






